Hepatoprotective and Antioxidant Potential of Phenolics-Enriched Fraction of Anogeissus acuminata Leaf against Alcohol-Induced Hepatotoxicity in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Kits
2.2. Extract Preparation and Phytochemical Profiling
2.3. In-Vitro Antioxidant Activity
2.4. Experimental Animals
2.5. Determination of Biochemical Parameters
2.6. Determination of Antioxidant Enzymes and Stress Markers
2.7. Estimation of Interleukins and TNF-α in Hepatic Tissue
2.8. Gene Expression Analysis via Quantitative Real-Time PCR (qRT-PCR)
2.9. Statistical Analysis
3. Results
3.1. GC–MS Analysis of Ethyl Acetate Fraction of A. acuminata Identified Bioactive Phytochemicals
3.2. Total Phenolic and Flavonoid Content
3.3. In Vitro Antioxidant Studies
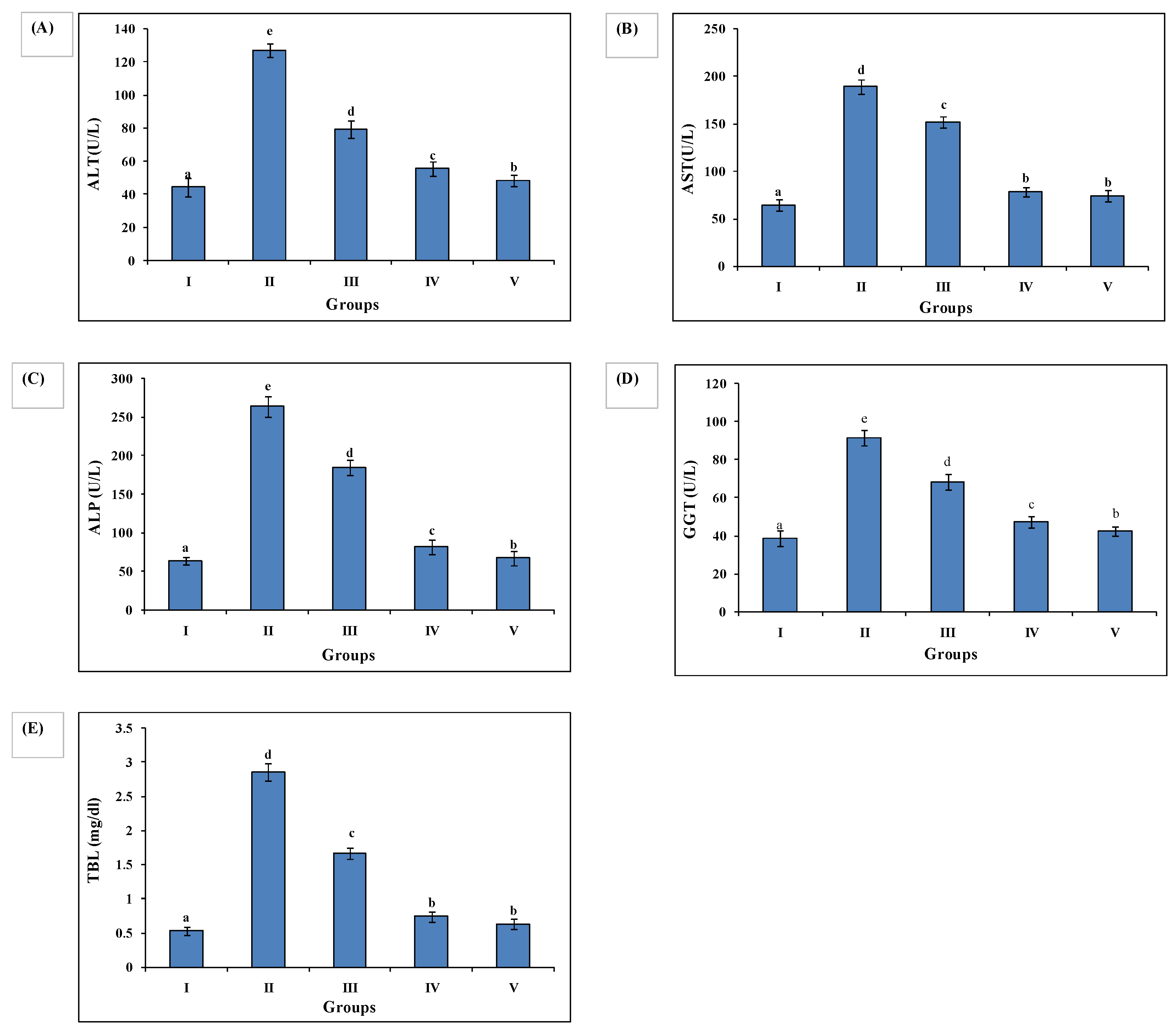
3.4. Effect of AAE on Biochemical Parameters
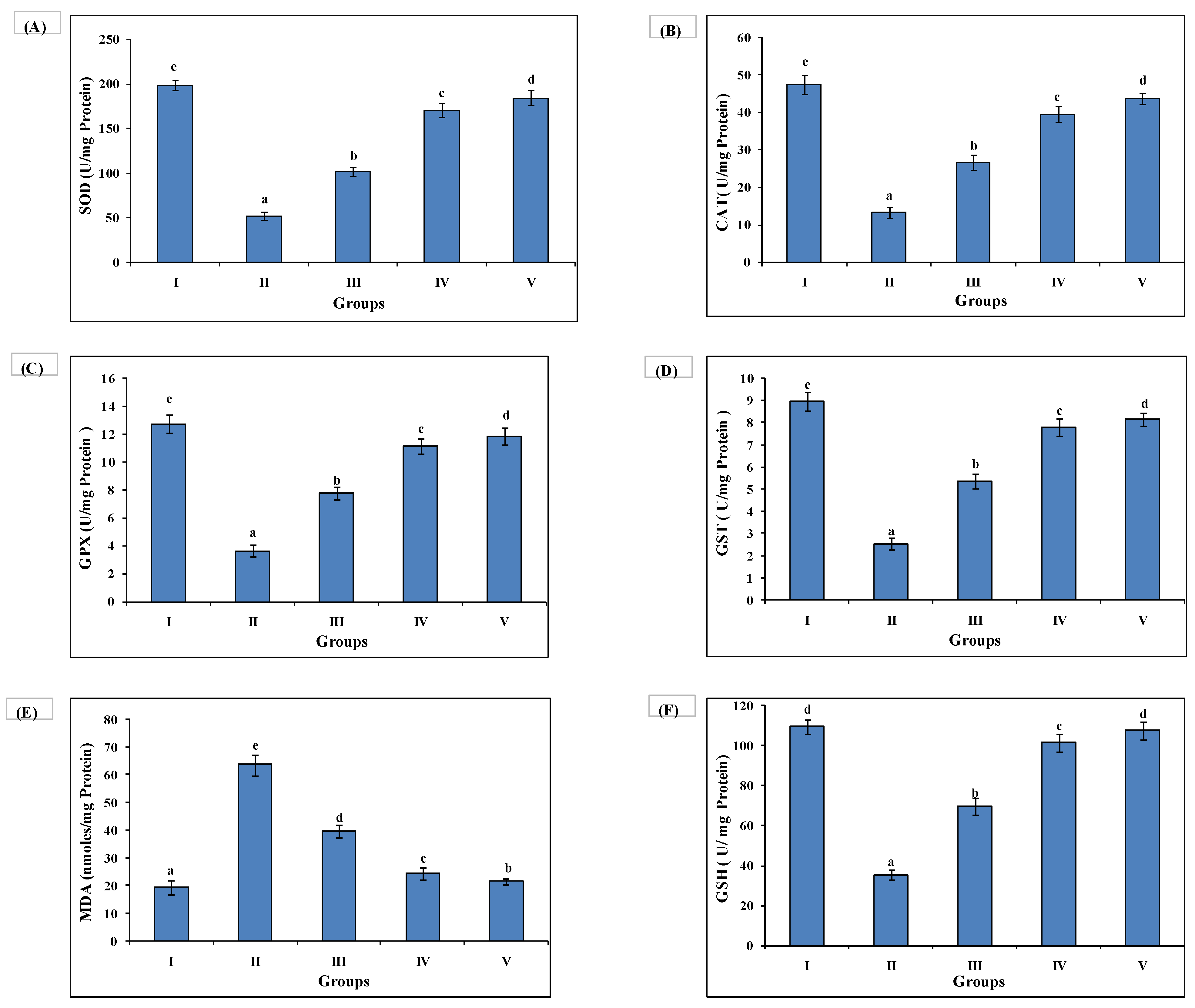
3.5. AAE Treatment Improves Antioxidant Enzymes Activity and Lowers Lipid Peroxidation
3.6. ELISA Quantified Interleukins and TNF-α
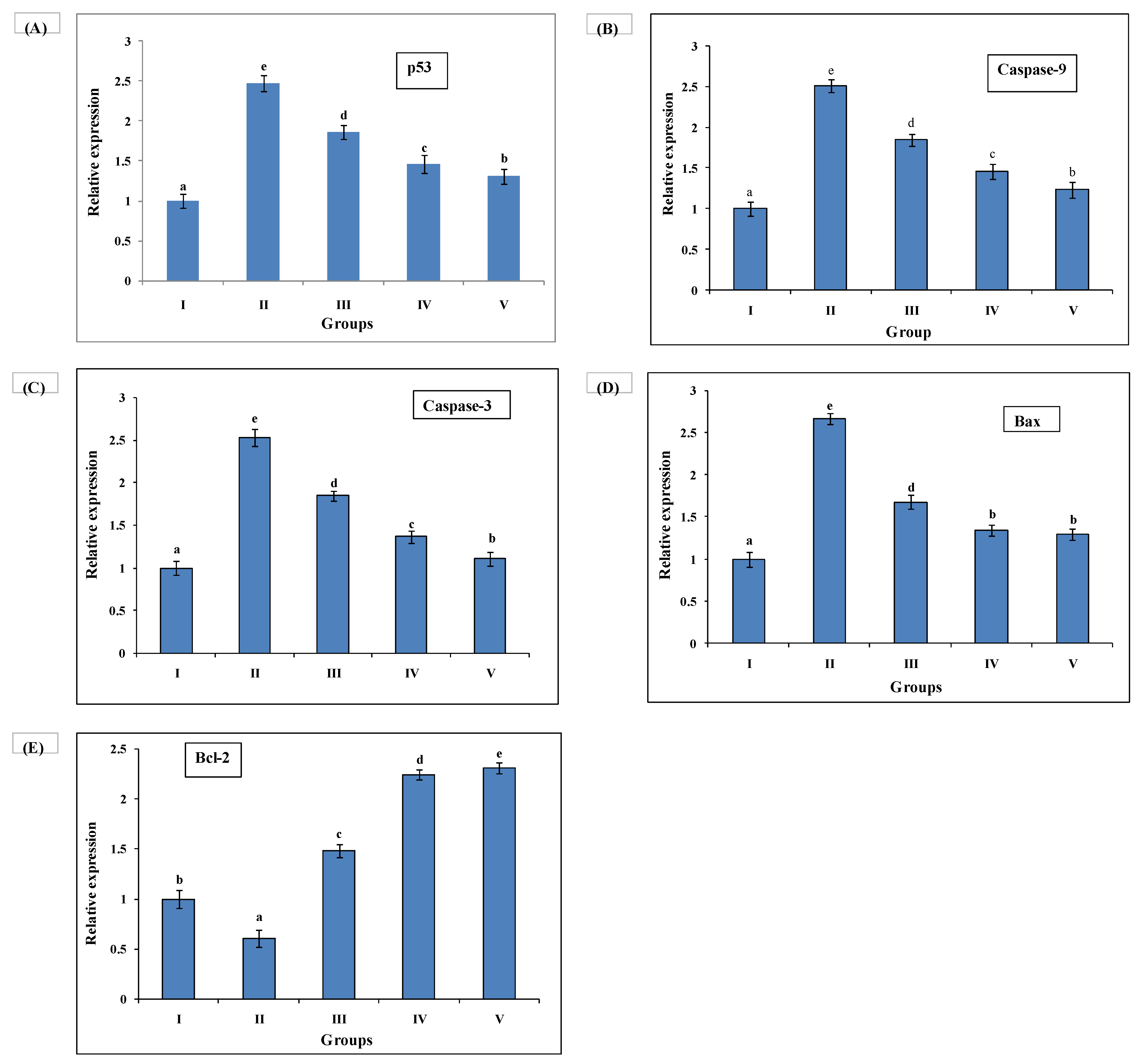
3.7. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Investigation
3.8. Histopathological Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Nagappan, A.; Kim, J.-H.; Jung, D.Y.; Jung, M.H. Cryptotanshinone from the Salvia miltiorrhiza bunge attenuates ethanol-induced liver injury by activation of AMPK/SIRT1 and Nrf2 signaling pathways. Int. J. Mol. Sci. 2020, 21, 265. [Google Scholar] [CrossRef] [PubMed]
- Shukla, I.; Azmi, L.; Gupta, S.S.; Upreti, D.K.; Rao, C.V. Amelioration of anti-hepatotoxic effect by Lichen rangiferinus against alcohol induced liver damage in rats. J. Ayurveda Integr. Med. 2019, 10, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Akter, R.; Kwak, G.Y.; Ahn, J.C.; Mathiyalagan, R.; Ramadhania, Z.M.; Yang, D.C.; Kang, S.C. Protective Effect and Potential Antioxidant Role of Kakadu Plum Extracts on Alcohol-Induced Oxidative Damage in HepG2 Cells. Appl. Sci. 2022, 12, 236. [Google Scholar] [CrossRef]
- Pal, L.C.; Gautam, A.; Pande, V.; Rao, C. Anticancer property of Selaginella bryopteris (L.) Bak. against hepatocellular carcinoma in vitro and in vivo. Phytomedicine Plus 2021, 2, 100201. [Google Scholar] [CrossRef]
- Sharma, U.K.; Kumar, R.; Gupta, A.; Ganguly, R.; Singh, A.K.; Ojha, A.K.; Pandey, A.K. Ameliorating efficacy of eugenol against metanil yellow induced toxicity in albino Wistar rats. Food Chem. Toxicol. 2019, 126, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.M.; Lidon, F.C. An overview on applications and side effects of antioxidant food additives. Emir. J. Food Agric. 2016, 28, 823–832. [Google Scholar] [CrossRef]
- Sarangarajan, R.; Meera, S.; Rukkumani, R.; Sankar, P.; Anuradha, G. Antioxidants: Friend or foe? Asian Pac. J. Trop. Med. 2017, 10, 1111–1116. [Google Scholar] [CrossRef]
- Mendoza-Wilson, A.M.; Castro-Arredondo, S.I.; Espinosa-Plascencia, A.; del Refugio Robles-Burgueño, M.; Balandrán-Quintana, R.R.; del Carmen Bermúdez-Almada, M. Chemical composition and antioxidant-prooxidant potential of a polyphenolic extract and a proanthocyanidin-rich fraction of apple skin. Heliyon 2016, 2, e00073. [Google Scholar] [CrossRef]
- Pal, L.C.; Prateeksha; Singh, B.N.; Pande, V.; Rao, C.V. Phenolics-Enriched Fraction of Pterospermum Lanceifolium Roxb. efficiently Reverses the Hepatocellular Carcinoma in NDEA-Induced HCC Rats. Nutr. Cancer 2021, 21, 1–16. [Google Scholar] [CrossRef]
- Singh, D.; Baghel, U.S.; Gautam, A.; Baghel, D.S.; Yadav, D.; Malik, J.; Yadav, R. The genus Anogeissus: A review on ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 2016, 194, 30–56. [Google Scholar] [CrossRef]
- Ragazzi, E.; Veronese, G. Quantitative analysis of phenolic compounds after thin-layer chromatographic separation. J. Chromatogr. A 1973, 77, 369–375. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Bhatia, A.; Bharti, S.K.; Tripathi, T.; Mishra, A.; Sidhu, O.P.; Roy, R.; Nautiyal, C.S. Metabolic profiling of Commiphora wightii (guggul) reveals a potential source for pharmaceuticals and nutraceuticals. Phytochemistry 2015, 110, 29–36. [Google Scholar] [CrossRef]
- Yen, G.C.; Duh, P.D. Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. J. Agric. Food Chem. 1994, 42, 629–632. [Google Scholar] [CrossRef]
- Apati, P.; Szentmihalyi, K.; Kristo, S.T.; Papp, I.; Vinkler, P.; Szoke, E.; Kery, A. Herbal remedies of Solidago—correlation of phytochemical characteristics and antioxidative properties. J. Pharm. Biomed. Anal. 2003, 32, 1045–1053. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- OECD. OECD Guidelines for the Testing of Chemical; Organization for Economic: Paris, France, 1994. [Google Scholar]
- Sapakal, V.; Shikalgar, T.; Ghadge, R.; Adnaik, R.; Naikwade, N.; Magdum, C. In vivo screening of antioxidant profile: A review. J. Herb. Med. Toxicol. 2008, 2, 1–8. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kakkar, P.; Das, B.; Viswanathan, P. A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. 1984, 21, 130–132. [Google Scholar]
- Aebi, H. Catalase. Methods of Enzymatic Analysis; Elsevier: Amsterdam, The Netherlands, 1974; pp. 673–684. [Google Scholar]
- Rotruck, J.; Pope, A.; Ganther, H.E.; Swanson, A.; Hafeman, D.G.; Hoekstra, W. Selenium: Biochemical role as a component of glutathione peroxidase. Science 1973, 179, 588–590. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres Jr, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Baliga, M.S.; Shivashankara, A.R.; Venkatesh, S.; Bhat, H.P.; Palatty, P.L.; Bhandari, G.; Rao, S. Phytochemicals in the prevention of ethanol-induced hepatotoxicity: A revisit. Diet. Interv. Liver Dis. 2019, 1, 79–89. [Google Scholar]
- Jananie, R.; Priya, V.; Vijayalakshmi, K. Determination of bioactive components of Cynodon dactylon by GC-MS analysis. NY Sci. J. 2011, 4, 1–5. [Google Scholar]
- Chetia, B.; Gogoi, S. Antibacterial activity of the methanolic extract of stem bark of Spondias pinnata, Moringa oleifera and Alstonia scholaris. Asian J. Tradit. Med. 2011, 6, 163–167. [Google Scholar]
- Arif, M.; Sheeba Fareed, M.; Rahman, A. Stress relaxant and antioxidant activities of acid glycoside from Spondias mangifera fruit against physically and chemically challenged albino mice. J. Pharm. Bioallied Sci. 2016, 8, 58. [Google Scholar] [CrossRef]
- Hoskeri, H.J.; Krishna, V.; Kumar, B.V.; Shridar, A.; Babu, K.R.; Sudarshana, M. In vivo prophylactic effects of oleanolic acid isolated from chloroform extract of Flaveria trinervia against ethanol induced liver toxicity in rats. Arch. Pharmacal. Res. 2012, 35, 1803–1810. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Drabu, S.; Sharma, M. Anti-inflammatory and analgesic activity of Tamarix gallica. Int. J. Pharm. Sci 2012, 4, 653–658. [Google Scholar]
- Özdemir, Ö. Bis-azo-linkage Schiff bases—Part (II): Synthesis, characterization, photoluminescence and DPPH radical scavenging properties of their novel luminescent mononuclear Zn (II) complexes. J. Photochem. Photobiol. A Chem. 2020, 392, 112356. [Google Scholar] [CrossRef]
- Agrawal, S.; Barrow, C.J.; Adholeya, A.; Deshmukh, S.K. Unveiling the dermatological potential of marine fungal species components: Antioxidant and inhibitory capacities over tyrosinase. Biotechnol. Appl. Biochem. 2021, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kamath, B.R.; Kizhedath, S. In vitro study on antioxidant activity of methanolic leaf extract of Cassia fistula Linn. Int. J. Basic Clin. Pharm. 2018, 7, 849–853. [Google Scholar] [CrossRef][Green Version]
- Lee, J.; Yang, J.; Jeon, J.; Jeong, H.S.; Lee, J.; Sung, J. Hepatoprotective effect of esculetin on ethanol-induced liver injury in human HepG2 cells and C57BL/6J mice. J. Funct. Foods 2018, 40, 536–543. [Google Scholar] [CrossRef]
- Carrero, R.J.; Husain, K. Renal oxidative stress in chronic alcohol-induced hypertension. Ethn. Dis. 2005, 15, S4. [Google Scholar]
- Jiang, W.; Gao, M.; Sun, S.; Bi, A.; Xin, Y.; Han, X.; Wang, L.; Yin, Z.; Luo, L. Protective effect of L-theanine on carbon tetrachloride-induced acute liver injury in mice. Biochem. Biophys. Res. Commun. 2012, 422, 344–350. [Google Scholar] [CrossRef]
- Tak, P.; Rigby, W.; Rubbert-Roth, A.; Peterfy, C.; Van Vollenhoven, R.; Stohl, W.; Hessey, E.; Chen, A.; Tyrrell, H.; Shaw, T. Inhibition of joint damage and improved clinical outcomes with rituximab plus methotrexate in early active rheumatoid arthritis: The IMAGE trial. Ann. Rheum. Dis. 2011, 70, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Chang, H.H.; Wang, G.J.; Chiu, J.H.; Yang, Y.Y.; Lin, H.C. Water-soluble extract of Salvia miltiorrhiza ameliorates carbon tetrachloride-mediated hepatic apoptosis in rats. J. Pharm. Pharmacol. 2006, 58, 659–665. [Google Scholar] [CrossRef]
- Naseri, M.H.; Mahdavi, M.; Davoodi, J.; Tackallou, S.H.; Goudarzvand, M.; Neishabouri, S.H. Up regulation of Bax and down regulation of Bcl2 during 3-NC mediated apoptosis in human cancer cells. Cancer Cell Int. 2015, 15, 1–9. [Google Scholar] [CrossRef]
- Liu, Y.; Zuo, H.; Wang, Y.; Tian, L.; Xu, X.; Xiong, J.; Pei, X. Ethanol promotes apoptosis in rat ovarian granulosa cells via the Bcl-2 family dependent intrinsic apoptotic pathway. Cell. Mol. Biol. 2018, 64, 118–125. [Google Scholar] [CrossRef]
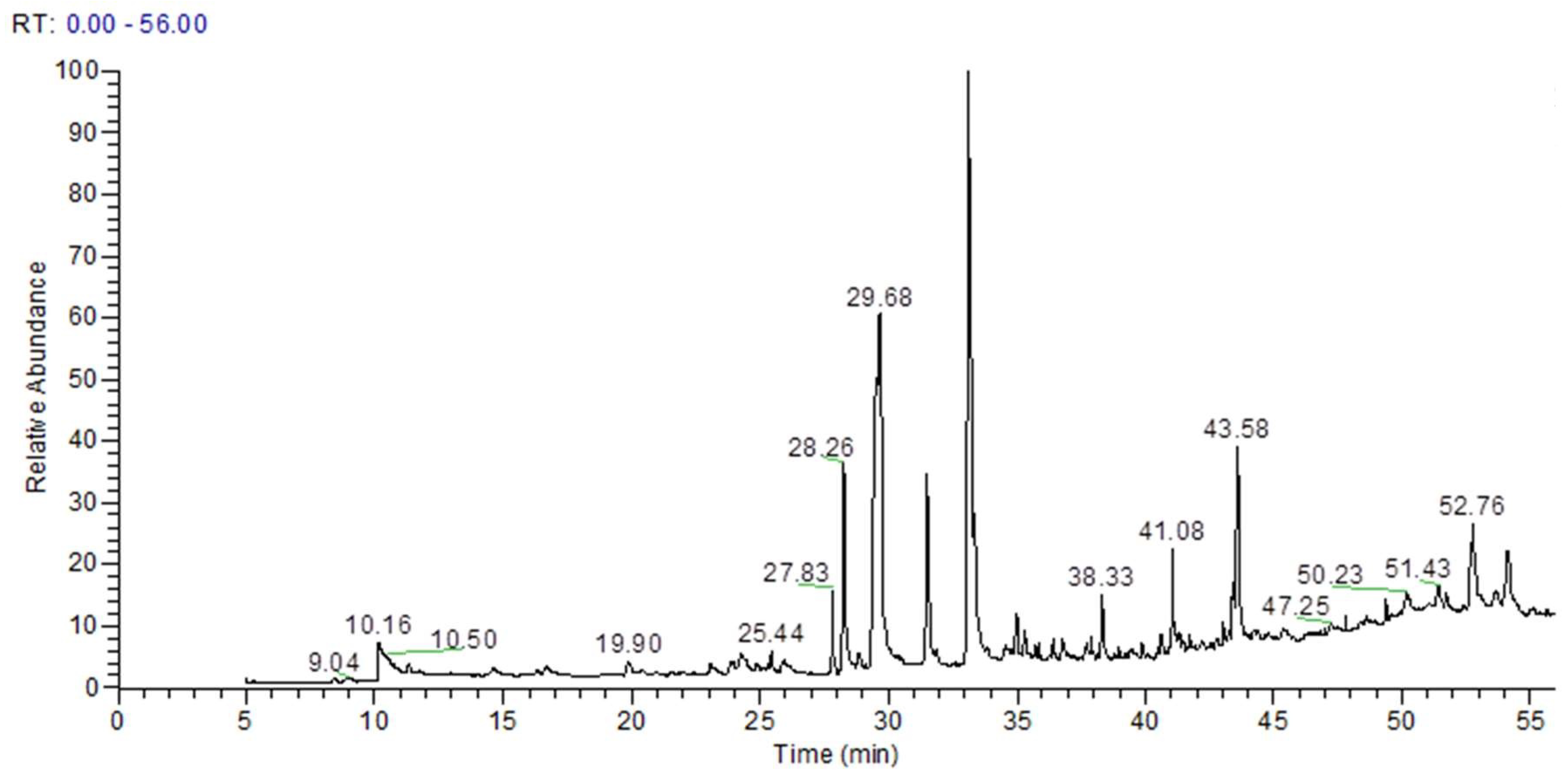


| RT(min) | Metabolites | Formula | Area | %Area |
|---|---|---|---|---|
| 10.16 | 1H-Imidazole | C3H4N2 | 4.27 × 108 | 2.74 |
| 14.56 | Butanedioic acid, 2TMS | C10H22O4Si2 | 83,274,987 | 0.53 |
| 16.66 | Tartaric acid (2R,3R)-, 3TMS | C13H30O6Si3 | 91,428,233 | 0.59 |
| 24.87 | Shikimic acid, 4TMS derivative | C19H42O5Si4 | 23,304,997 | 0.15 |
| 25.44 | Methyl galactoside (1S,2R,3S,4S,5R)-, 4TMS | C19H46O6Si4 | 82,226,063 | 0.53 |
| 27.83 | 2,5-Dihydroxyacetophenone, 2TMS derivative | C14H24O3Si2 | 1.98 × 108 | 1.27 |
| 28.26 | Gallic acid, 4TMS derivative | C19H38O5Si4 | 7.27 × 108 | 4.66 |
| 28.87 | Gibberellic acid, methyl ester | C20H24O6 | 79,478,987 | 0.51 |
| 29.68 | Palmitic Acid, TMS derivative | C19H40O2Si | 3.09 × 109 | 19.78 |
| 31.52 | cis-10-Heptadecenoic acid, trimethylsilyl ester | C20H40O2Si | 8.39 × 108 | 5.38 |
| 33.11 | 9-Octadecenoic acid, (E)-, TMS derivative | C21H42O2Si | 3.49 × 109 | 22.39 |
| 34.98 | 10-Nonadecenoic acid, (Z)-, TMS derivative | C22H44O2Si | 1.42 × 108 | 0.91 |
| 35.29 | Linoleic acid trimethylsilyl ester | C21H40O2Si | 1.15 × 108 | 0.74 |
| 36.41 | Arachidic acid, TMS derivative | C23H48O2Si | 92,205,575 | 0.59 |
| 38.33 | 1-Monopalmitin, 2TMS derivative | C25H54O4Si2 | 2.02 × 108 | 1.29 |
| 40.65 | 2-Palmitoylglycerol, 2TMS derivative | C25H54O4Si2 | 1.14 × 108 | 0.73 |
| 41.08 | 1-Monooleoylglycerol, 2TMS derivative | C27H56O4Si2 | 4.39 × 108 | 2.81 |
| 43.07 | 13-Docosenoic acid, (Z)-, TMS derivative | C25H50O2Si | 59,210,641 | 0.38 |
| 43.58 | Epigallocatechin (6TMS) | C33H62O7Si6 | 9.89 × 108 | 6.34 |
| 44.24 | Catechine (2R-E)-, 5TMS derivative | C30H54O6Si5 | 63,753,701 | 0.41 |
| 47.8 | β-Sitosterol, TMS derivative | C32H58OSi | 32,528,905 | 0.21 |
| 48.66 | (+)-Prostaglandin F2α, 4TMS derivative | C20H34O5 · C4H11NO3 | 1.59 × 108 | 1.02 |
| 51.43 | 1,3-Dipalmitin, TMS derivative | C38H76O5Si | 1.42 × 108 | 0.91 |
| 52.76 | 1,2-Dipalmitin, TMS derivative | C38H76O5Si | 6.17 × 108 | 3.91 |
| 54.12 | 1,3-Dipalmitin, TMS derivative | C38H76O5Si | 4.51 × 108 | 2.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pal, L.C.; Agrawal, S.; Gautam, A.; Chauhan, J.K.; Rao, C.V. Hepatoprotective and Antioxidant Potential of Phenolics-Enriched Fraction of Anogeissus acuminata Leaf against Alcohol-Induced Hepatotoxicity in Rats. Med. Sci. 2022, 10, 17. https://doi.org/10.3390/medsci10010017
Pal LC, Agrawal S, Gautam A, Chauhan JK, Rao CV. Hepatoprotective and Antioxidant Potential of Phenolics-Enriched Fraction of Anogeissus acuminata Leaf against Alcohol-Induced Hepatotoxicity in Rats. Medical Sciences. 2022; 10(1):17. https://doi.org/10.3390/medsci10010017
Chicago/Turabian StylePal, Lal Chand, Shivankar Agrawal, Arti Gautam, Jayhind Kumar Chauhan, and Chandana Venkateswara Rao. 2022. "Hepatoprotective and Antioxidant Potential of Phenolics-Enriched Fraction of Anogeissus acuminata Leaf against Alcohol-Induced Hepatotoxicity in Rats" Medical Sciences 10, no. 1: 17. https://doi.org/10.3390/medsci10010017
APA StylePal, L. C., Agrawal, S., Gautam, A., Chauhan, J. K., & Rao, C. V. (2022). Hepatoprotective and Antioxidant Potential of Phenolics-Enriched Fraction of Anogeissus acuminata Leaf against Alcohol-Induced Hepatotoxicity in Rats. Medical Sciences, 10(1), 17. https://doi.org/10.3390/medsci10010017








