Abstract
The polyamines spermidine and spermine are positively charged aliphatic molecules. They are critical in the regulation of nucleic acid and protein structures, protein synthesis, protein and nucleic acid interactions, oxidative balance, and cell proliferation. Cellular polyamine levels are tightly controlled through their import, export, de novo synthesis, and catabolism. Enzymes and enzymatic cascades involved in polyamine metabolism have been well characterized. This knowledge has been used for the development of novel compounds for research and medical applications. Furthermore, studies have shown that disturbances in polyamine levels and their metabolic pathways, as a result of spontaneous mutations in patients, genetic engineering in mice or experimentally induced injuries in rodents, are associated with multiple maladaptive changes. The adverse effects of altered polyamine metabolism have also been demonstrated in in vitro models. These observations highlight the important role these molecules and their metabolism play in the maintenance of physiological normalcy and the mediation of injury. This review will attempt to cover the extensive and diverse knowledge of the biological role of polyamines and their metabolism in the maintenance of physiological homeostasis and the mediation of tissue injury.
1. Introduction
Polyamines are aliphatic polycations that are present in all cells and play an important role in the mediation of biological functions that are necessary for growth and survival [1,2]. Their discovery dates back to 1678, when spermine was first identified as crystals in cooled human sperm [3]. It was not until 1926 that the chemical structure of spermine (Spm) was elucidated [4]. Polyamines are abundant in all organisms and perform important biological functions that are essential for life [5]. The biologically significant polyamines in mammals (Figure 1A), Spm (N-N’-bisaminopropyl-1,4-diaminobutane) and spermidine (Spd; N-aminopropyl-1,4-diaminobutane), are derived by sequential enzymatic addition of aminopropyl groups to putrescine (Put; 1,4-diaminobutane) and are required for growth and development [5,6]. Intracellular levels of polyamines are regulated through their transport, synthesis, and degradation (Figure 1B). The transporters of polyamines have been identified in mammalian cells and organs [7], while polyamine synthetic and degradative pathways have been elucidated in bacteria, yeast and higher organisms [8]. Disturbances in cellular polyamine levels and metabolism are observed in many injuries and in cancer [5,9,10,11,12]. The adverse effects of elevated polyamine metabolism have also been demonstrated in vitro [13,14,15,16]. These observations point to the importance of polyamines and their metabolism in the maintenance of physiological homeostasis and mediation of tissue injury. In this review, we will cover the important aspects of polyamine biology and polyamine metabolism in health and disease.
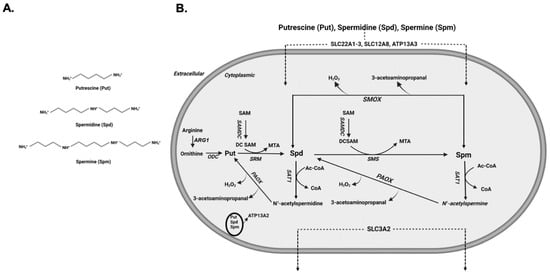
Figure 1.
Polyamine molecular structure and polyamine regulation. (A) Chemical structure of Put, Spd and Spm. In physiological pH all three molecules are positively charged. (B) Schematic depiction of polyamine metabolic pathway and polyamine transporters. Arginase 1 (ARG1), Ornithine decarboxylase (ODC), SAM decarboxylase (SAMDC), Spermidine synthase (SRM), Spermine synthase (SMS), Spermidine/Spermine N1-acetyltransferase (SAT1), acetylpolyamine oxidase (PAOX) and Spermine oxidase (SMOX).
2. Polyamines and Regulation of Their Cellular Content in Mammals
In mammals, Spm and Spd are the naturally occurring polyamines, while Put is the primary diamine (Figure 1A). At physiological pH, these aliphatic cations contain at least two positively charged amino groups and serve a variety of biologically important functions. Because of their important biological role, the cellular content of polyamines is tightly regulated via their import, export, synthesis or degradation [5].
2.1. Polyamine Metabolism
Polyamine synthesis is initiated by the Arginase 1 (ARG1) catalyzed conversion of arginine to ornithine followed by the decarboxylation of ornithine by ornithine decarboxylase (ODC), the rate-limiting enzyme in polyamine synthetic pathway, to generate putrescine (Put). The activity of ODC is regulated at multiple levels. The transcription of ODC is regulated by MYC [17], while the stability of the ODC mRNA is regulated through binding of HuR, an RNA binding protein, to AU rich regions in its 3′ untranslated region [18]. Additional regulatory mechanisms of ODC expression that affect the translation of its transcript have been also described. These involve: (1) using alternative upstream open reading frames that reduce the translated ODC levels, and (2) a cap-independent translation mechanism for the synthesis of ODC that uses an alternative ribosomal binding site and is most likely used to maintain polyamine synthesis through G2/M transition phase, the transition between the second growth phase (growth phase after S phase) and mitosis in the cell cycle [19,20]. ODC levels are also post-translationally regulated by antizyme 1, which binds to ODC, prevents its dimerization, and targets it for proteosomal degradation [21]. The sequential addition of aminopropyl groups, derived from decarboxylated S-adenosyl methionine (dcSAM), to Put and Spd in reactions that are catalyzed by spermidine synthase (SRM) and spermine synthase (SMS) generates Spd and Spm, respectively.
The catabolism of polyamines is mediated by their back conversion via spermidine/spermine N1-acetyltransferase (SAT1)/acetylpolyamine oxidase (PAOX) cascade. The back conversion of polyamines depends on their acetylation by SAT1 in a reaction that utilizes acetyl coenzyme A (Acetyl-CoA) followed by the oxidation of acetylated polyamines by PAOX. The SAT1-mediated acetylation of polyamines is a rate limiting step in their catabolism, and the expression and activity of SAT1 are regulated at multiple levels. The transcription of SAT1 mRNA and its induction by polyamines or their analogs is regulated by Nuclear factor erythroid 2-related factor 2 (Nrf-2), which binds to the polyamine response element in the promoter region of the SAT1 gene, and its co-factor polyamine modulated factor 1 (PMF-1) [22,23,24]. Another important factor in the regulation of SAT1 gene is a SP1 binding site that is essential for its basal transcription [25]. The SP1 and factors that bind to PRE (Nrf-2 and PMF-1) are important in the increased transcription of SAT1 gene in response to radiation damage [25]. The activation of P53, a DNA-damage-activated gene, has also been implicated in the upregulation of SAT1 gene transcription in the process of ferroptosis [26]. Tumor necrosis-α (TNF-α), a known activator of NFκB and p53 [27,28,29], also can induce the expression of SAT1 transcription [30,31]. The cross talk between the various mechanisms of transcriptional regulation of the SAT1 gene remains to be elucidated. Post-transcriptionally, SAT1 expression is regulated via a number of mechanisms including: (1) the unproductive splicing of SAT1 transcript in response to cellular polyamine levels [32]; (2) the polyamine-mediated override of translational repression caused by the usage of false upstream open reading frames, allowing the use of the proper translation start site [33]; and (3) the translational repression by nucleolin binding to a potential stem loop structure in the 5′ region of SAT1 mRNA coding region that is relieved by elevated cellular polyamine levels and consequent degradation of nucleolin [34]. At the post-translational level, the regulation of SAT1 activity is mediated via its dimerization to form the active enzyme [35]. The SAT1/PAOX cascade generates H2O2, 3-acetylaminopropanal, and acrolein that is generated through the spontaneous deamination of 3-acetylaminopropanal. The generation of these compounds may account for the adverse effect of increased SAT1 expression in cultured cells [14,15].
The oxidation of Spm by spermine oxidase (SMOX), a highly inducible enzyme, is another major mode of polyamine catabolism (Figure 1B). The expression of SMOX is elevated in response to tissue injury and inflammation [36,37,38,39,40]. In injured tissue, increased oxidation of Spm by SMOX generates cytotoxic H2O2, 3-aminopropanal, and acrolein molecules, which can further exacerbate the severity of tissue damage [5,9,36,39,41]. The expression of SMOX can be induced by treatment with polyamine analogs, as well as pro-inflammatory cytokines such as interleukin-6 or TNF-α [42]. Tissue culture studies indicate that the increased expression of SMOX in response to treatment with the Spm analog, CPENSpm, is dependent on de novo synthesis and increased stability of the SMOX transcript [43]. Examination of the SMOX gene promoter region indicates that its polyamine response elements are active in constructs spanning the residues −1117 to −55 of its 5′ untranslated region [43]. These results also suggest that there are multiple responsive elements present in this region that regulate the basal activity of the gene and its responsiveness to polyamines. An additional layer of regulation consists of miR-124 modulation of SMOX expression through its interaction with the 3′ untranslated region of the SMOX mRNA [44]. In vitro studies indicate that increased expression of SAT1 in cultured cells enhances the expression of the SMOX mRNA. They further demonstrate that the enhanced expression of the SMOX transcript in response to SAT1 induction is dependent on the activity SAT1, suggesting the existence of crosstalk between SAT1 expression and activity and the expression of SMOX [45].
2.2. Polyamine Transport
Polyamine transport is important in the regulation of cellular polyamine levels [7]. The polyamine transporters identified thus far include members of solute carrier (SLC) and p-type ATPase families. Multiple members of the organic cation transporter (OTC/SLC22A) family, such as SLC22A1-3, have been implicated in the transport of Put and Agmatine [46,47,48]. The role of SLC3A2 as a transporter that favors Put and acetylated polyamines has also been well characterized [49,50]. Another member of the SLC family, SLC18B1, that functions as a vesicular transporter was also identified as a transporter of Spd and Spm in neurons and astrocytes [51,52]. Recent studies demonstrated the role of ATP13A3, a P5B-ATPase, which is linked to pulmonary arterial hypertension, in the cellular transport of polyamines in CHO and pancreatic duct carcinoma cells [53,54]. A member of the same family of transporters, ATP13A2, that is associated with an atypical form of juvenile onset Parkinson disease is also implicated in the lysosomal export of polyamine to the cytosol [55].
3. Biological Functions of Polyamines and Their Potential Physiological Role in Health and Disease
Spm and Spd are polycations that are ubiquitous in all mammalian cells and perform a variety of biologically significant functions [1,2]. Because of their positive charge at physiological pH, the majority of the cellular functions attributed to polyamines are due to their interactions with negatively charged biomolecules (e.g., phosphate back bone of nucleic acids, negatively charged amino acid residues in proteins, and negatively charged membrane phospholipids) [6,56].
3.1. Interaction of Polyamines and Nucleic Acids
In mammalian cells, the highest proportion of polyamines is found in association with RNA and DNA, while only about 10% are found as free molecules [57]. Polyamine binding stabilizes transfer- and mRNA molecules and enhances the translation of the latter [6,58,59]. Interaction of polyamines with antizyme mRNA is required for the +1 programmed ribosomal frameshift that leads to an increased production of antizyme and proteasomal degradation of ODC, the rate-limiting enzyme in polyamine synthesis [60]. Studies examining the effect of polyamine on DNA structure indicated a B to Z transition upon incubation with polyamines (Spm and Spd), as well as Put. The structural transition was dependent on the concentration of polyamines and Put, with the former being more effective than Put in the induction of B to Z conformational transition. Furthermore, the long-term incubation with Spm enhanced the aggregation of DNA [61]. The conformational transition of DNA from B to Z may also occur in response to polyamine/diamine complexes known as nuclear aggregates of polyamines (NAP) [62]. This structural transition may be important in the development of anti-Z-DNA antibodies in autoimmune diseases such as systemic lupus erythematosus (SLE) and rheumatoid arthritis [63]. NAPs also have high affinity for G-C rich Alu elements [63,64], which are found in primates and through their transposition can have deleterious genetic effects [65,66]. Polyamines in mM concentration can destabilize the telomeric G-quadruplex structures [67]. With higher polyamine content in actively proliferating cells, the polyamine-mediated destabilization of the G-quadruplex may be important in the process of carcinogenesis [67].
3.2. Antioxidant Role of Polyamines
Polyamines can not only directly regulate DNA structure and function [61,63] but can also act as free-radical scavengers or modulators of the Fenton reaction that protect nucleic acids and cells against oxidative damage [68,69,70]. Studies in cell-free systems indicate that the free radical scavenging activity of polyamines is dependent on the concentration of transition metals (e.g., Spm is protective in the presence of low concentrations and can induce DNA strand break in the presence of high concentrations of transition metals) [71]. Additionally, free-radical scavenging activity of polyamines is primarily directed against hydroxyl radicals formed by Fenton-like reactions and against singlet oxygen; however, polyamines are not effective scavengers of super oxide radicals [72]. The free radical scavenging function of Spm has been confirmed in yeast cells and Gy11 cultured mouse embryonic fibroblast cells that lack SMS [73,74]. While GY11 fibroblasts were highly susceptible to H2O2, normalization of their intracellular polyamine levels protected them against H2O2-induced injury, thereby confirming the antioxidant role of polyamines [74].
3.3. Interaction with Proteins
As positively charged molecules, polyamines interact with a variety of proteins and affect the structure, function, interaction, and localization of these proteins [75,76,77]. While these reactions are charge-dependent, many are further stabilized through enzymatic polyamination of targeted residues [77,78,79]. The predominant enzymatic reaction that drives the polyamination of proteins is catalyzed by transglutaminases [77]. The transglutaminase-mediated polyamination of phospholipase A2 increases its enzymatic activity by nearly threefold compared to its non-polyaminated counterpart [78]. Transglutaminase-mediated polyamination of Tau, a microtubule-associated protein that is found in amyloid deposits of Alzheimer disease, prevents its proteolysis by calpain and may play a role in plaque formation [80]. Spd and Spm were also shown to promote the aggregation and fibril formation by α-synuclein, with Spm being more efficient than Spd as a driver of this reaction [75,81,82,83]. The addition of polyamines increased the rate of nucleation and monomer addition in α-synuclein aggregates [81]. Polyamination of α-synuclein was also demonstrated in a mouse model of combined SMOX and SAT1 deficiency [84]. These animals develop severe cerebellar damage and ataxia [84]. The cerebellar damage is associated with increased expression of transglutaminase 2; as well as expression, polyamination, and aggregation of α-synuclein [84]. Treatment of Smox/Sat-1 double knock-out mice (Smox/Sat1-dKO) with transglutaminase inhibitors decreased the expression and aggregation of polyamines, reduced the severity of cerebellar damage, and significantly delayed the onset of ataxia [84].
Depending on the applied dosage, intracerebroventricular administration of polyamines leads to hypothermia, sedation, and convulsions [85]. The function of polyamines in neurotransmission has been demonstrated in single neurons studies, where these molecules had either depressive or excitatory functions depending on the type of the neuron [86,87]. Such observations suggest that polyamines are important regulators of neuronal function and their activity may vary based on their source (intracellular vs. extracellular), nature of the channel molecules, auxiliary factors associated with the channel protein, and more specifically, the target cell type [86,87]. These studies led to the discovery of multiple channels that are affected by polyamines including ionotropic glutamate receptors and potassium channels [86,88]. Polyamines have been established as modulators of glutamate receptors, such as calcium dependent AMPA and kainite receptors [86,89,90]. The regulation of these channels is mediated through blocking their ion channel activity, in addition to their regulation by auxiliary molecules that interact with the channel proteins [86]. Activity of NMDA receptors that contain the GluN2B subunit are also regulated by polyamines, where polyamines are thought to stabilize the receptor in its active form [91]. Polyamines also control the activity of inward rectifier potassium channels, which is likely mediated through steric blocking of their ion channel pore [92,93].
3.4. Spd and Hypusination of Eukaryotic Translation Elongation Factor 5A (eIF5A)
Hypusine is an amino acid derived through modification of a lysine residue. It is found only in the lysine 50 residue of eIF5A [94,95,96]. Hypusination of eIF5A is initiated when the aminobutyl group of Spd is transferred, in an NAD+ dependent reaction catalyzed by deoxyhupusine synthase (DHPS), to the ε-amino group of its lysine residue 50 to generate a deoxyhypusine residue [97]. The hypusination process of eIF5A is then completed in a reaction catalyzed by deoxyhypusine hydroxylase that adds a hydroxyl group to carbon 9 of the deoxyhypusine residue, forming hypusine residue [97]. Although originally thought to be involved in the ease of processing proline residues during translation [98,99], there are now other functions and physiological roles attributed to eIF5A [96]. The depletion of eIF5A can stall protein synthesis and lead to the formation of stress granules (translationally stalled messenger ribonucleoprotein complexes) and the onset of endoplasmic reticulum stress [100,101]. The targeting of eIF5A can also impart tolerance to ischemia and endotoxic acute kidney injuries, enhance metabolic adaptation in diabetes, alter macrophage and immune cell functions, and affect embryonic and neuronal development [96,102,103,104,105]. The physiological importance of hypusination of eIF5A was also observed in heterozygote Dhps-KO mice and in individuals with mutations in the DHPS gene [106,107]. Examination of the mutant proteins in vitro revealed that the mutated DHPS proteins have either reduced or no enzymatic activity [106]. The reduced or absent activity of DHPS enzyme in these individuals led to neurodevelopmental phenotypes including seizures and delayed development [106].
3.5. Polyamines: Toxic or Panacea?
The effects of exogenous administration of Spm and Spd have been the subject of many in vitro and in vivo studies. While a number of studies showed the administration of Spm, and to a lesser extent Spd, to be toxic others suggest that exogenous polyamine administration may be beneficial [11,108,109,110]. The identification of bovine serum amine oxidase-like enzymes and other extracellular amine oxidases that target diamines and polyamines is now considered as the likely mechanism behind the toxicity of polyamines [111,112]. The degradation of polyamines by extracellular amino oxidases generates H2O2 and reactive aldehydes, including acrolein that is spontaneously formed via conversion of aldehydes generated when polyamines are oxidized. These are thought to be the instigating factors that mediate the toxicity of exogenous polyamines [111,112,113,114]. The ubiquitous presence of extracellular amino oxidases and the importance of their activity in the mediation of tissue injury are supported by studies that show increased polyamine oxidation in the plasma of patients after cerebral stroke, in patients with kidney failure, and in the vitreous humor of patients with diabetic retinopathy [115,116,117,118]. Furthermore, in a rat model of cerebral ischemic reperfusion injury (IRI), treatment with aminoguanidine, an amino oxidase inhibitor, was found to reduce the production of 3-aminopropanal and to protect against neuronal and glial cell death [119]. Additional studies confirmed the role of aminoaldehydes in the mediation of tissue damage in this model of cerebral IRI [120]. The results presented above point to the potential role of extracellular amino oxidases and their cytotoxic products as mediators of injury in many studies where toxicity of exogenous polyamines was documented.
The protective role of polyamines and their derivatives has also been demonstrated in a number of studies [11,108,109,121]. Spm was shown to reduce the lesion size in a model of cerebral hypoxia-ischemia in rat pups, where neuroprotection imparted by Spm was due to increased generation of neuroprotective nitric oxide and reduced mitochondrial damage [108]. Similarly, exogenously administered Spm was shown to protect against manganese-induced neurodegeneration in α-synuclein-expressing Caenorhabditis elegans (C. elegans) [122]. Exogenously administered Spm, as well as elevated intracellular polyamine levels, also protected the α-synuclein-expressing SK-MEL-28 cell line against manganese-induced injury [122]. The protective role of Spm has also been demonstrated in cardiomyocytes exposed to in vitro conditions that mimic ischemic IRI [123]. This protection was conferred through inhibition of the mTOR pathway and the enhancement of autophagic flux [123].
The protective effects of Spd and its derivatives are also extensively studied [11]. Dietary Spd supplementation suppressed the age dependent loss of neuronal plasticity and impairment of memory formation in Drosophila melanogaster (D. melanogaster) [124]. Dietary provision of Spd also increased the longevity of yeast, C. elegans and D. melanogaster [125]. Similar to studies conducted with Spm, dietary Spd also reduced the neurotoxicity of α-synuclein in C. elegans and D. melanogaster [126]. The above, as well as other neuroprotective activities of Spd including the preservation of locomotor activity and promotion of stress resistance in D. melanogaster, were shown to be mediated through the induction of autophagy [125,126,127,128]. In mice, provision of Spd in their diet provided protection against cardiac damage caused by age. This was achieved through protecting the cardimoyocytes against injury via the induction of autophagy, enhanced mitochondrial respiration, and improved cardiomyocyte function [129]. Oral administration of Spd prior to induction of renal IRI in mice also reduced the oxidative injury to DNA, the activation of poly(ADP-ribose) polymerase1 (PARP1), and the severity of renal injury [130]. In Staurosporine-induced cell injury, Spd was shown to protect PC12 and cortical neurons against damage via prevention of Becli1 cleavage and induction of autophagy [131]. In autoimmune encephalomyelitis, a model of multiple sclerosis, intraperitoneal administration of Spd was shown to reduce the severity of injury by modifying the macrophage response toward an inhibitory phenotype, which was associated with reduced expression of inflammatory mediators and enhanced expression of Arg1 [132]. A catabolically stable functional mimetic of Spd, α-methylspermidine, exhibited protection against CCl4-induced liver and pancreatic injury in spermidine/spermine N1-acetyltransferase transgenic rats [133].
4. Polyamine Metabolism and Its Role in Health and Disease
The balance of polyamine synthesis and catabolism is critical to the regulation of cellular polyamine levels. Alterations in polyamine metabolism in vitro, in genetically engineered animals and in patients due to spontaneous mutations in polyamine pathway genes, can have severe consequences [5,9,12].
4.1. Effect of Dysregulation of Polyamine Synthetic Pathway
The de novo synthesis of polyamines is catalyzed by ODC, the rate limiting enzyme responsible for the generation of Put, followed by sequential addition of aminopropyl groups from dcSAM to Put by SRM and SMS to generate Spd and Spm, respectively (Figure 1B).
The importance of ODC in the regulation of cell growth, differentiation, and function has been well established [134,135]. The physiological importance of ODC was further established in knock-out mice, where the homozygote ablation of its alleles was shown to be lethal and to have adverse developmental effects in heterozygote mice [136,137]. ODC expression and regulation is altered in response to injuries and in cancer [16,17,138,139]. ODC is a transcriptional target of Myc [17], and its expression is increased in Myc-amplified tumors [140,141]. The adverse role of ODC in tumorigenesis is supported by the observation that its over-expression promotes skin tumorigenesis, and that ODC over-expressing cells when injected into immunocompromised mice can enhance tumor formation [140,142,143].
Genetic polymorphisms in ODC genes are also associated with a number of cancers including neuroblastomas, as well as gastric, colorectal, breast and prostate cancers [144,145,146,147,148]. Yet, maintaining physiological levels of ODC is vital for cell growth and function, as demonstrated through in vitro studies [134,135]. Intestinal epithelial cells displayed reduced growth resulting from inhibition of ODC and subsequent polyamine depletion leading to activation of the Transforming growth factor-β mediated SMAD signaling pathway [149,150,151]. Using keratinocytes from normal and ODC over-expressing mice, it was demonstrated that the over-expression of ODC was associated with the induction of DNA damage, activation of the DNA damage checkpoint protein kinase, ataxia telangiectasia mutated (ATM), and cell cycle arrest [16]. DNA damage in this cell model was attributed to the induction of the polyamine catabolic enzyme SMOX and the toxic products resulting from its enzymatic activity [16].
The expression and activity of ODC increases following acute ischemic injuries in the targeted organs [138,139]. The role of ODC in the mediation of tissue injury was most closely studied in cerebral IRI in rats. ODC-expressing transgenic rats were protected against cerebral ischemia, while the inhibition or knockdown of ODC exacerbated the severity of injury in multiple studies indicating a protective role for ODC [152,153,154,155]. These results suggest that ODC plays a protective role in cerebral IRI.
Although the physiological importance of polyamine metabolism has been well documented, it was not until recently that mutations in polyamine biosynthetic pathway enzymes, ODC and SMS, were identified. Description of multiple individuals with gain of function ODC mutations revealed that such mutations lead to macrosomia, macrocephaly, delayed visual maturation, sensorineural hearing loss, and ectodermal abnormalities (e.g., alopecia) along with other presentations [156,157]. In a single case of ODC gain of function mutation, eflornithine normalized the polyamine levels while improving alopecia and neurological symptoms [158]. A loss of function mutation of ODC (gly84Arg) was also recently described in South Asian individuals [137]. This missense variant reduces the activity of ODC by nearly 60% and is associated with psychiatric and neurological traits [137].
In male mice, the mutations in SMS and the absence of tissue Spm leads to neurological abnormalities, growth defects and a shortened lifespan [159]. In humans, Snyder–Robinson Syndrome (SRS) is a recessive X-linked disorder that is attributed to loss of function mutations in the SMS gene [160,161]. SRS is characterized by intellectual disability, skeletal and muscular defects, as well as seizures [161]. Similarly, lymphoblast cell lines derived from individuals with SRS present with significant derangements in cellular polyamine levels. Levels of Put and Spm are considerably reduced, while Spd levels are elevated. Further examination of these cells revealed increased content of tissue transglutaminase 2, an enzyme associated with neurodegenerative diseases [162]. The interplay of increased cellular Spd content and elevated TGM2 levels in the mediation of tissue abnormalities in SRS remain to be elucidated.
4.2. Polyamine Catabolism in Health and Disease
Catabolism of polyamines through their back conversion via the SAT1/PAOX cascade and oxidation of Spm by SMOX is important in maintaining homeostasis of these important molecules [12]. Polyamine back conversion/oxidation and Spm oxidation while contributing to the maintenance of physiological levels of polyamines also generate H2O2 and reactive aldehydes that promote tissue damage in conditions where polyamine catabolism is elevated.
4.2.1. Polyamine Catabolism and Tissue Damage
The expression of SAT1 and SMOX increases in the brain, cardiac, liver, kidney, and GI tract in response to a variety of injuries [9,40,45,163,164,165,166]. The induction of polyamine catabolism in the liver after renal IRI or partial nephrectomy suggests that heightened polyamine catabolism contributes to tissue damage in organs that are not the direct target of the injurious insult [167]. In the kidney and liver, injuries caused by toxic compounds (cisplatin or CCl4), sepsis (bacterial lipopolysaccharide), or IRI enhance the expression of both SAT1 and SMOX [167]. The ablation of SAT1, as well as its inhibition, reduced the severity of tissue damage in renal IRI [139,168]. In cisplatin-induced acute kidney injury, the deficiency or inhibition of polyamine catabolic enzymes, as well as the neutralization of their toxic by-products (e.g., reactive aldehydes and H2O2), reduces the severity of acute tubular damage [39]. The protective effect of the ablation or inhibition of polyamine catabolism was revealed to result from the reduction in oxidative injury, modulation of the innate immune response, and down-regulation of endoplasmic reticulum stress/unfolded protein response [39,164,165,168].
The expression of SAT1 and SMOX is increased in the brain after IRI and traumatic injury [40]. The inhibition of polyamine oxidation by MDL72525, as well as the neutralization of reactive aldehydes generated as a result of polyamine oxidation by N-2-mercaptopropionyl glycine (N-2-MPG), reduce the severity of ischemic damage to the brain [120,123]. The importance of SMOX in the mediation of brain injury is further demonstrated in SMOX transgenic mice that show hypersensitivity to kainic acid, pentylentetrazole-induced seizures, enhanced neurotoxicity, and reactive astrocytosis [169,170,171,172].
Increased expression and activity of SMOX has been documented in response to bacterial endotoxin and in bacterial infections [36,38,164]. In Helicobacter pylori infections, the expression of SMOX is associated with chronic inflammation and increased risk of developing gastric cancer [173]. Biopsies of H. pylori infected individuals with gastritis demonstrated that elevated levels of SMOX expression in the samples were associated with increased risk of developing gastric cancer [38,174]. The potential correlation between SMOX and gastric cancer was further established in a Mongolian gerbil model of H. pylori. In these studies, the expression of SMOX, induction of DNA damage, and development of cancerous lesions was associated with a strain of H. pylori that was more likely to lead to gastric cancer vs. that of the low-cancer-risk strain [174]. The role of SMOX in tumorigenesis was demonstrated in a model of Bacteroides fragilis (B. fragilis)-induced colon cancer [36]. In these studies, B. fragilis was shown to induce the expression of SMOX and lead to DNA damage in infected culture cells. Furthermore, B. fragilis infection in wildtype mice led to increased expression of SMOX, while its inhibition reduced the severity of colitis [36]. Moreover, the induction of colon tumorigenesis by B. fragilis was reduced when animals were treated with MDL72527, an inhibitor of FAD-dependent polyamine oxidases such as PAOX and SMOX [36].
4.2.2. Polyamine Catabolism and Cell Injury
Enhanced catabolism of polyamines causes cell injury through the disruption of polyamine homeostasis and generation of toxic metabolites. Treatment with polyamine analogs such as N1,N11-bis(ethyl)norspermine (BENSpm) activates the polyamine catabolic pathways in cultured tumor cells [175,176]. The over-expression of SAT1 in HEK293 cells led to reductions in Spm and Spd levels, disruption of cell cytoskeleton and adhesion, and reduced cell proliferation caused by cell cycle arrest at the G2 to M transition [15,177]. Growth arrest was in part due to severe DNA damage (single- and double-stranded breaks), which activated DNA damage/repair response proteins (e.g., ataxia telangiectasia mutated; ATM and ataxia telangiectasia and Rad3-related; ATR) effectively halting cell cycle progression [177]. Similarly, HEK293 cells combined with an adenovirus expression system demonstrated that enhanced expression of SAT1 leads to the depletion of Spm and Spd, growth arrest, and loss of cell viability [13,14]. The disruption of protein synthesis was another hallmark of increased SAT1 over-expression [13,14]. The reduction in polysomes and accumulation of monosomes, reduced eIF5A levels, and the onset of ERS/UPR underscored how the activation of this response can contribute to cell death in SAT1-over-expressing cells [39,99]. In both systems, the enhanced expression of SAT1 eventually led to mitochondrial alterations and apoptosis [14,168]. It is not completely clear what the contribution of each maladaptive pathway plays in inducing cell death in SAT1-expressing cells; however, all of the described cellular changes whether in isolation or in concert can be mediators of cell injury and tissue damage.
4.2.3. The Role of Polyamine Catabolic Pathways in Normal Physiology
Although multiple studies have demonstrated the maladaptive role of elevated polyamine catabolism in disease conditions, there are few studies that address the importance of these pathways in normal physiological states. The harmful role of SMOX and its products in the mediation of acute and chronic tissue injury are well documented; however, the ablation of the Smox gene while protective in injuries does not seem to have an overt effect in normal physiological conditions other than the induction of a mild increase in tissue Spm and a significant reduction in tissue Spd levels. The baseline effect of SAT1 deficiency is more pronounced in that through a reduction in acetyl-coenzynme A (Acetyl-CoA) consumption for polyamine acetylation; it leads to increased availability of Acetyl-CoA for fatty acid synthesis, decreased oxidative phosphorylation, and ultimately an increase in total body fat accumulation over time [178,179].
Although individual ablation of either SAT1 and SMOX produces a mild phenotype, the combined deletion of both genes creates a severe condition in the organism. Examination of polyamine catabolism’s role in allergic airway inflammation (AAI—a model of asthma) in mice revealed that reductions in SAT1 or SMOX levels increase the severity of AAI [180]. In these studies, impeding SAT1 and SMOX expression by a combination of Berenil (diminazene aceturate) and MDL72527 also increased the severity of AAI providing additional evidence that the inhibition of both genes produces detrimental outcomes [180]. The physiological importance of polyamine catabolism is further confirmed through studies that utilized deletions in both genes to generate Smox/Sat1-dKO mice [84]. These animals, while normal at birth, went on to develop progressive cerebellar damage and ataxia. Cerebellar injury was associated with Purkinje cell loss and gliosis, leading to neuroinflammation and white matter demyelination [84]. The onset of tissue damage in Smox/Sat1-dKO mice was restricted to the cerebellum while other tissues and organs such as the spinal cord, lung, liver, and kidney were not affected [84]. These results led to the conclusion that the cerebellar injury was not solely dependent on changes in polyamine levels as it was highly selective [84]. The studies outlined in this section point out the important role of polyamine catabolic pathway in the maintenance of physiological homeostasis.
5. Conclusions
Polyamines (Figure 1A) are important regulators of a variety of molecular interactions and biological processes [1,2]. The intracellular content of these molecules is tightly regulated by an elaborate network of mechanisms including their import, export, de novo biosynthesis, and degradation (Figure 1B). These mechanisms constitute an exquisite control network that allows the cells and tissues to regulate the levels of polyamines within a physiological range in a manner that quickly responds to the prevailing conditions. The strict control of polyamine levels is necessary, as imbalances in their cellular pools can have many detrimental outcomes. For example, alterations in ODC and SMS functions can cause abnormalities in physical and mental development [137,156,157,159,160,161]. Potential alteration in lysosomal transport of polyamines caused by mutations in ATP13A2 are also associated with a form of juvenile onset Parkinsonism [55]. The depletion of polyamines can adversely affect molecular structures and interactions, while their elevated levels can also lead to chronic derangements that are harmful [1,2].
Likewise, polyamine metabolism can be a double-edged sword. The role of enhanced polyamine synthesis (ODC induction) in the prevention of tissue injury (e.g., cerebral IRI) [152,153,154,155], the development of neoplasia (e.g., MYC amplified tumors) [140,141], as well as developmental abnormalities (e.g., Bachmann–Bupp and Robinson–Snyder Syndromes) [156,161] has been documented. Increased polyamine catabolism has also been implicated in the mediation of acute (e.g., acute kidney injury, cerebral ischemic and traumatic injuries, and hepatic injuries) and chronic tissue injuries and the development of cancer (H. pylori- and B. fragilis-induced inflammation and GI tract cancers) [36,40,45,163,165,174]. Although not as prevalent, the adverse effects of disruptions in polyamine catabolism have also been documented in experimental settings [84,178,179,180]. These range from the propensity to develop late onset obesity to increased severity of AAI to the development of severe cerebellar damage and ataxia [84,178,179,180].
The cogent point of what has been discovered about polyamines and their catabolism is that they have important biological functions and that imbalance in their homeostasis and regulatory pathways can have adverse effects. In conclusion, as ongoing studies increase our knowledge of the biological significance of polyamines and their metabolism, our enhanced understanding of how to utilize polyamine and manipulate their metabolism will enable the development of new interventional and treatment modalities that can be used in a variety of diseases and genetic conditions.
Author Contributions
K.Z., S.B. and M.S. wrote and edited the manuscript. S.B. generated the figures. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Merit Review Award 2 I01 BX001000-10 from the Department of Research Services, Veterans Health Administration and funds from the Biomedical Research Institute of New Mexico (BRINM). Manoocher Soleimani is a Senior Clinician Scientist Investigator with the Department of Veterans Health Administration.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Figures were created using BioRender Application (BioRender.com, accessed on 21 June 2022).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Pegg, A.E.; McCann, P.P. Polyamine metabolism and function. Am. J. Physiol. 1982, 243, C212–C221. [Google Scholar] [CrossRef] [PubMed]
- Tabor, C.W.; Tabor, H. Polyamines in microorganisms. Microbiol. Rev. 1985, 49, 81–99. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwenhoek, A.; Observationes, D. Anthonii Leeuwenhoek, de Natis e seminegenitali Animalculis. Philos. Trans. R. Soc. London 1678, 12, 1040–1043. [Google Scholar] [CrossRef]
- Dudley, H.W.; Rosenheim, O.; Starling, W.W. The Chemical Constitution of Spermine: Structure and Synthesis. Biochem. J. 1926, 20, 1082–1094. [Google Scholar] [CrossRef]
- Pegg, A.E. Toxicity of polyamines and their metabolic products. Chem. Res. Toxicol. 2013, 26, 1782–1800. [Google Scholar] [CrossRef]
- Igarashi, K.; Kashiwagi, K. Modulation of cellular function by polyamines. Int. J. Biochem. Cell Biol. 2010, 42, 39–51. [Google Scholar] [CrossRef]
- Abdulhussein, A.A.; Wallace, H.M. Polyamines and membrane transporters. Amino Acids 2014, 46, 655–660. [Google Scholar] [CrossRef]
- Miller-Fleming, L.; Olin-Sandoval, V.; Campbell, K.; Ralser, M. Remaining Mysteries of Molecular Biology: The Role of Polyamines in the Cell. J. Mol. Biol. 2015, 427, 3389–3406. [Google Scholar] [CrossRef]
- Casero, R.A.; Pegg, A.E. Polyamine catabolism and disease. Biochem. J. 2009, 421, 323–338. [Google Scholar] [CrossRef]
- Igarashi, K.; Kashiwagi, K. Polyamines: Mysterious modulators of cellular functions. Biochem. Biophys. Res. Commun. 2000, 271, 559–564. [Google Scholar] [CrossRef]
- Madeo, F.; Eisenberg, T.; Pietrocola, F.; Kroemer, G. Spermidine in health and disease. Science 2018, 359, eaan2788. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, S.; Cleveland, J.L. Polyamine Homeostasis in Development and Disease. Med. Sci. 2021, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Mandal, A.; Johansson, H.E.; Orjalo, A.V.; Park, M.H. Depletion of cellular polyamines, spermidine and spermine, causes a total arrest in translation and growth in mammalian cells. Proc. Natl. Acad. Sci. USA 2013, 110, 2169–2174. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Mandal, A.; Park, M.H. Depletion of the polyamines spermidine and spermine by overexpression of spermidine/spermine N(1)-acetyltransferase 1 (SAT1) leads to mitochondria-mediated apoptosis in mammalian cells. Biochem. J. 2015, 468, 435–447. [Google Scholar] [CrossRef]
- Wang, Z.; Zahedi, K.; Barone, S.; Tehrani, K.; Rabb, H.; Matlin, K.; Casero, R.A.; Soleimani, M. Overexpression of SSAT in kidney cells recapitulates various phenotypic aspects of kidney ischemia-reperfusion injury. J. Am. Soc. Nephrol. 2004, 15, 1844–1852. [Google Scholar] [CrossRef]
- Wei, G.; DeFeo, K.; Hayes, C.S.; Woster, P.M.; Mandik-Nayak, L.; Gilmour, S.K. Elevated ornithine decarboxylase levels activate ataxia telangiectasia mutated-DNA damage signaling in normal keratinocytes. Cancer Res. 2008, 68, 2214–2222. [Google Scholar] [CrossRef]
- Bello-Fernandez, C.; Packham, G.; Cleveland, J.L. The ornithine decarboxylase gene is a transcriptional target of c-Myc. Proc. Natl. Acad. Sci. USA 1993, 90, 7804–7808. [Google Scholar] [CrossRef]
- Origanti, S.; Nowotarski, S.L.; Carr, T.D.; Sass-Kuhn, S.; Xiao, L.; Wang, J.Y.; Shantz, L.M. Ornithine decarboxylase mRNA is stabilized in an mTORC1-dependent manner in Ras-transformed cells. Biochem. J. 2012, 442, 199–207. [Google Scholar] [CrossRef]
- Ivanov, I.P.; Loughran, G.; Atkins, J.F. uORFs with unusual translational start codons autoregulate expression of eukaryotic ornithine decarboxylase homologs. Proc. Natl. Acad. Sci. USA 2008, 105, 10079–10084. [Google Scholar] [CrossRef]
- Pyronnet, S.; Pradayrol, L.; Sonenberg, N. A cell cycle-dependent internal ribosome entry site. Mol. Cell. 2000, 5, 607–616. [Google Scholar] [CrossRef]
- Murakami, Y.; Matsufuji, S.; Tanaka, K.; Ichihara, A.; Hayashi, S. Involvement of the proteasome and antizyme in ornithine decarboxylase degradation by a reticulocyte lysate. Biochem. J. 1993, 295, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Devereux, W.; Stewart, T.M.; Casero, R.A., Jr. Cloning and characterization of human polyamine-modulated factor-1, a transcriptional cofactor that regulates the transcription of the spermidine/spermine N(1)-acetyltransferase gene. J. Biol. Chem. 1999, 274, 22095–22101. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Devereux, W.; Stewart, T.M.; Casero, R.A., Jr. Characterization of the interaction between the transcription factors human polyamine modulated factor (PMF-1) and NF-E2-related factor 2 (Nrf-2) in the transcriptional regulation of the spermidine/spermine N1-acetyltransferase (SSAT) gene. Biochem. J. 2001, 355, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, L.; Thiagalingam, A.; Nelkin, B.D.; Casero, R.A., Jr. The identification of a cis-element and a trans-acting factor involved in the response to polyamines and polyamine analogues in the regulation of the human spermidine/spermine N1-acetyltransferase gene transcription. J. Biol. Chem. 1998, 273, 34623–34630. [Google Scholar] [CrossRef]
- Tomitori, H.; Nenoi, M.; Mita, K.; Daino, K.; Igarashi, K.; Ichimura, S. Functional characterization of the human spermidine/spermine N(1)-acetyltransferase gene promoter. Biochim. Biophys. Acta 2002, 1579, 180–184. [Google Scholar] [CrossRef]
- Ou, Y.; Wang, S.J.; Li, D.; Chu, B.; Gu, W. Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc. Natl. Acad. Sci. USA 2016, 113, E6806–E6812. [Google Scholar] [CrossRef]
- Goretsky, T.; Dirisina, R.; Sinh, P.; Mittal, N.; Managlia, E.; Williams, D.B.; Posca, D.; Ryu, H.; Katzman, R.B.; Barrett, T.A. p53 mediates TNF-induced epithelial cell apoptosis in IBD. Am. J. Pathol. 2012, 181, 1306–1315. [Google Scholar] [CrossRef]
- Rokhlin, O.W.; Gudkov, A.V.; Kwek, S.; Glover, R.A.; Gewies, A.S.; Cohen, M.B. p53 is involved in tumor necrosis factor-alpha-induced apoptosis in the human prostatic carcinoma cell line LNCaP. Oncogene 2000, 19, 1959–1968. [Google Scholar] [CrossRef]
- Schutze, S.; Wiegmann, K.; Machleidt, T.; Kronke, M. TNF-induced activation of NF-kappa B. Immunobiology 1995, 193, 193–203. [Google Scholar] [CrossRef]
- Babbar, N.; Hacker, A.; Huang, Y.; Casero, R.A., Jr. Tumor necrosis factor alpha induces spermidine/spermine N1-acetyltransferase through nuclear factor kappaB in non-small cell lung cancer cells. J. Biol. Chem. 2006, 281, 24182–24192. [Google Scholar] [CrossRef]
- Smirnova, O.A.; Isaguliants, M.G.; Hyvonen, M.T.; Keinanen, T.A.; Tunitskaya, V.L.; Vepsalainen, J.; Alhonen, L.; Kochetkov, S.N.; Ivanov, A.V. Chemically induced oxidative stress increases polyamine levels by activating the transcription of ornithine decarboxylase and spermidine/spermine-N1-acetyltransferase in human hepatoma HUH7 cells. Biochimie 2012, 94, 1876–1883. [Google Scholar] [CrossRef] [PubMed]
- Hyvonen, M.T.; Uimari, A.; Keinanen, T.A.; Heikkinen, S.; Pellinen, R.; Wahlfors, T.; Korhonen, A.; Narvanen, A.; Wahlfors, J.; Alhonen, L.; et al. Polyamine-regulated unproductive splicing and translation of spermidine/spermine N1-acetyltransferase. RNA 2006, 12, 1569–1582. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ivanov, I.P.; Atkins, J.F.; Michael, A.J. A profusion of upstream open reading frame mechanisms in polyamine-responsive translational regulation. Nucleic Acids Res. 2010, 38, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Perez-Leal, O.; Barrero, C.A.; Clarkson, A.B.; Casero, R.A., Jr.; Merali, S. Polyamine-regulated translation of spermidine/spermine-N1-acetyltransferase. Mol. Cell. Biol. 2012, 32, 1453–1467. [Google Scholar] [CrossRef]
- Bewley, M.C.; Graziano, V.; Jiang, J.; Matz, E.; Studier, F.W.; Pegg, A.E.; Coleman, C.S.; Flanagan, J.M. Structures of wild-type and mutant human spermidine/spermine N1-acetyltransferase, a potential therapeutic drug target. Proc. Natl. Acad. Sci. USA 2006, 103, 2063–2068. [Google Scholar] [CrossRef]
- Goodwin, A.C.; Destefano Shields, C.E.; Wu, S.; Huso, D.L.; Wu, X.; Murray-Stewart, T.R.; Hacker-Prietz, A.; Rabizadeh, S.; Woster, P.M.; Sears, C.L.; et al. Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis-induced colon tumorigenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 15354–15359. [Google Scholar] [CrossRef]
- Patel, C.; Xu, Z.; Shosha, E.; Xing, J.; Lucas, R.; Caldwell, R.W.; Caldwell, R.B.; Narayanan, S.P. Treatment with polyamine oxidase inhibitor reduces microglial activation and limits vascular injury in ischemic retinopathy. Biochim. Biophys. Acta 2016, 1862, 1628–1639. [Google Scholar] [CrossRef]
- Xu, H.; Chaturvedi, R.; Cheng, Y.; Bussiere, F.I.; Asim, M.; Yao, M.D.; Potosky, D.; Meltzer, S.J.; Rhee, J.G.; Kim, S.S.; et al. Spermine oxidation induced by Helicobacter pylori results in apoptosis and DNA damage: Implications for gastric carcinogenesis. Cancer Res. 2004, 64, 8521–8525. [Google Scholar] [CrossRef]
- Zahedi, K.; Barone, S.; Destefano-Shields, C.; Brooks, M.; Murray-Stewart, T.; Dunworth, M.; Li, W.; Doherty, J.R.; Hall, M.A.; Smith, R.D.; et al. Activation of endoplasmic reticulum stress response by enhanced polyamine catabolism is important in the mediation of cisplatin-induced acute kidney injury. PLoS ONE 2017, 12, e0184570. [Google Scholar] [CrossRef]
- Zahedi, K.; Huttinger, F.; Morrison, R.; Murray-Stewart, T.; Casero, R.A.; Strauss, K.I. Polyamine catabolism is enhanced after traumatic brain injury. J. Neurotrauma 2010, 27, 515–525. [Google Scholar] [CrossRef]
- Pichavaram, P.; Palani, C.D.; Patel, C.; Xu, Z.; Shosha, E.; Fouda, A.Y.; Caldwell, R.B.; Narayanan, S.P. Targeting Polyamine Oxidase to Prevent Excitotoxicity-Induced Retinal Neurodegeneration. Front. Neurosci. 2018, 12, 956. [Google Scholar] [CrossRef] [PubMed]
- Babbar, N.; Casero, R.A., Jr. Tumor necrosis factor-alpha increases reactive oxygen species by inducing spermine oxidase in human lung epithelial cells: A potential mechanism for inflammation-induced carcinogenesis. Cancer Res. 2006, 66, 11125–11130. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hacker, A.; Murray-Stewart, T.; Fleischer, J.G.; Woster, P.M.; Casero, R.A., Jr. Induction of human spermine oxidase SMO(PAOh1) is regulated at the levels of new mRNA synthesis, mRNA stabilization and newly synthesized protein. Biochem. J. 2005, 386, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Murray-Stewart, T.; Sierra, J.C.; Piazuelo, M.B.; Mera, R.M.; Chaturvedi, R.; Bravo, L.E.; Correa, P.; Schneider, B.G.; Wilson, K.T.; Casero, R.A. Epigenetic silencing of miR-124 prevents spermine oxidase regulation: Implications for Helicobacter pylori-induced gastric cancer. Oncogene 2016, 35, 5480–5488. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, K.; Barone, S.; Soleimani, M. Polyamine Catabolism in Acute Kidney Injury. Int. J. Mol. Sci. 2019, 20, 4790. [Google Scholar] [CrossRef]
- Busch, A.E.; Quester, S.; Ulzheimer, J.C.; Waldegger, S.; Gorboulev, V.; Arndt, P.; Lang, F.; Koepsell, H. Electrogenic properties and substrate specificity of the polyspecific rat cation transporter rOCT1. J. Biol. Chem. 1996, 271, 32599–32604. [Google Scholar] [CrossRef]
- Grundemann, D.; Hahne, C.; Berkels, R.; Schomig, E. Agmatine is efficiently transported by non-neuronal monoamine transporters extraneuronal monoamine transporter (EMT) and organic cation transporter 2 (OCT2). J. Pharmacol. Exp. Ther. 2003, 304, 810–817. [Google Scholar] [CrossRef]
- Winter, T.N.; Elmquist, W.F.; Fairbanks, C.A. OCT2 and MATE1 provide bidirectional agmatine transport. Mol. Pharm. 2011, 8, 133–142. [Google Scholar] [CrossRef]
- Roy, U.K.; Rial, N.S.; Kachel, K.L.; Gerner, E.W. Activated K-RAS increases polyamine uptake in human colon cancer cells through modulation of caveolar endocytosis. Mol. Carcinog. 2008, 47, 538–553. [Google Scholar] [CrossRef]
- Uemura, T.; Yerushalmi, H.F.; Tsaprailis, G.; Stringer, D.E.; Pastorian, K.E.; Hawel, L., 3rd; Byus, C.V.; Gerner, E.W. Identification and characterization of a diamine exporter in colon epithelial cells. J. Biol. Chem. 2008, 283, 26428–26435. [Google Scholar] [CrossRef]
- Hiasa, M.; Miyaji, T.; Haruna, Y.; Takeuchi, T.; Harada, Y.; Moriyama, S.; Yamamoto, A.; Omote, H.; Moriyama, Y. Identification of a mammalian vesicular polyamine transporter. Sci. Rep. 2014, 4, 6836. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, Y.; Hatano, R.; Moriyama, S.; Uehara, S. Vesicular polyamine transporter as a novel player in amine-mediated chemical transmission. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183208. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, N.N.; Van den Haute, C.; Vanhoutte, R.; Sannerud, R.; Azfar, M.; Mayer, R.; Cortes Calabuig, A.; Swinnen, J.V.; Agostinis, P.; Baekelandt, V.; et al. ATP13A3 is a major component of the enigmatic mammalian polyamine transport system. J. Biol. Chem. 2021, 296, 100182. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, V.; Andl, T.; Phanstiel, O. ATP13A3 facilitates polyamine transport in human pancreatic cancer cells. Sci. Rep. 2022, 12, 4045. [Google Scholar] [CrossRef]
- van Veen, S.; Martin, S.; Van den Haute, C.; Benoy, V.; Lyons, J.; Vanhoutte, R.; Kahler, J.P.; Decuypere, J.P.; Gelders, G.; Lambie, E.; et al. ATP13A2 deficiency disrupts lysosomal polyamine export. Nature 2020, 578, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Kusama-Eguchi, K.; Kobayashi, H.; Igarashi, K. Estimation of polyamine binding to macromolecules and ATP in bovine lymphocytes and rat liver. J. Biol. Chem. 1991, 266, 20803–20809. [Google Scholar] [CrossRef]
- Igarashi, K.; Kashiwagi, K. The functional role of polyamines in eukaryotic cells. Int. J. Biochem. Cell Biol. 2019, 107, 104–115. [Google Scholar] [CrossRef]
- Butcher, N.J.; Broadhurst, G.M.; Minchin, R.F. Polyamine-dependent regulation of spermidine-spermine N1-acetyltransferase mRNA translation. J. Biol. Chem. 2007, 282, 28530–28539. [Google Scholar] [CrossRef]
- Xiao, L.; Rao, J.N.; Zou, T.; Liu, L.; Marasa, B.S.; Chen, J.; Turner, D.J.; Zhou, H.; Gorospe, M.; Wang, J.Y. Polyamines regulate the stability of activating transcription factor-2 mRNA through RNA-binding protein HuR in intestinal epithelial cells. Mol. Biol. Cell 2007, 18, 4579–4590. [Google Scholar] [CrossRef]
- Matsufuji, S.; Matsufuji, T.; Miyazaki, Y.; Murakami, Y.; Atkins, J.F.; Gesteland, R.F.; Hayashi, S. Autoregulatory frameshifting in decoding mammalian ornithine decarboxylase antizyme. Cell 1995, 80, 51–60. [Google Scholar] [CrossRef]
- Hasan, R.; Alam, M.K.; Ali, R. Polyamine induced Z-conformation of native calf thymus DNA. FEBS Lett. 1995, 368, 27–30. [Google Scholar] [CrossRef]
- D’Agostino, L.; di Pietro, M.; Di Luccia, A. Nuclear aggregates of polyamines are supramolecular structures that play a crucial role in genomic DNA protection and conformation. FEBS J. 2005, 272, 3777–3787. [Google Scholar] [CrossRef] [PubMed]
- Brooks, W.H. Increased polyamines alter chromatin and stabilize autoantigens in autoimmune diseases. Front. Immunol. 2013, 4, 91. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.T.; Grafham, D.V.; Coffey, A.J.; Scherer, S.; McLay, K.; Muzny, D.; Platzer, M.; Howell, G.R.; Burrows, C.; Bird, C.P.; et al. The DNA sequence of the human X chromosome. Nature 2005, 434, 325–337. [Google Scholar] [CrossRef]
- Kramerov, D.A.; Vassetzky, N.S. Short retroposons in eukaryotic genomes. Int. Rev. Cytol. 2005, 247, 165–221. [Google Scholar] [CrossRef]
- Roy-Engel, A.M.; Carroll, M.L.; Vogel, E.; Garber, R.K.; Nguyen, S.V.; Salem, A.H.; Batzer, M.A.; Deininger, P.L. Alu insertion polymorphisms for the study of human genomic diversity. Genetics 2001, 159, 279–290. [Google Scholar] [CrossRef]
- Sun, H.; Xiang, J.; Liu, Y.; Li, L.; Li, Q.; Xu, G.; Tang, Y. A stabilizing and denaturing dual-effect for natural polyamines interacting with G-quadruplexes depending on concentration. Biochimie 2011, 93, 1351–1356. [Google Scholar] [CrossRef]
- Murray Stewart, T.; Dunston, T.T.; Woster, P.M.; Casero, R.A., Jr. Polyamine catabolism and oxidative damage. J. Biol. Chem. 2018, 293, 18736–18745. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Ren, W.; Rahu, N.; Dad, R.; Kalhoro, D.H.; Yin, Y. Polyamines: Therapeutic perspectives in oxidative stress and inflammatory diseases. Amino Acids 2017, 49, 1457–1468. [Google Scholar] [CrossRef]
- Ha, H.C.; Sirisoma, N.S.; Kuppusamy, P.; Zweier, J.L.; Woster, P.M.; Casero, R.A., Jr. The natural polyamine spermine functions directly as a free radical scavenger. Proc. Natl. Acad. Sci. USA 1998, 95, 11140–11145. [Google Scholar] [CrossRef]
- Pedreno, E.; Lopez-Contreras, A.J.; Cremades, A.; Penafiel, R. Protecting or promoting effects of spermine on DNA strand breakage induced by iron or copper ions as a function of metal concentration. J. Inorg. Biochem. 2005, 99, 2074–2080. [Google Scholar] [CrossRef] [PubMed]
- Das, K.C.; Misra, H.P. Hydroxyl radical scavenging and singlet oxygen quenching properties of polyamines. Mol. Cell. Biochem. 2004, 262, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, M.K.; Tabor, C.W.; Tabor, H. Spermidine but not spermine is essential for hypusine biosynthesis and growth in Saccharomyces cerevisiae: Spermine is converted to spermidine in vivo by the FMS1-amine oxidase. Proc. Natl. Acad. Sci. USA 2003, 100, 13869–13874. [Google Scholar] [CrossRef] [PubMed]
- Rider, J.E.; Hacker, A.; Mackintosh, C.A.; Pegg, A.E.; Woster, P.M.; Casero, R.A., Jr. Spermine and spermidine mediate protection against oxidative damage caused by hydrogen peroxide. Amino Acids 2007, 33, 231–240. [Google Scholar] [CrossRef]
- Antony, T.; Hoyer, W.; Cherny, D.; Heim, G.; Jovin, T.M.; Subramaniam, V. Cellular polyamines promote the aggregation of alpha-synuclein. J. Biol. Chem. 2003, 278, 3235–3240. [Google Scholar] [CrossRef]
- Namba, T.; Funahashi, Y.; Nakamuta, S.; Xu, C.; Takano, T.; Kaibuchi, K. Extracellular and Intracellular Signaling for Neuronal Polarity. Physiol. Rev. 2015, 95, 995–1024. [Google Scholar] [CrossRef]
- Yu, C.H.; Chou, C.C.; Lee, Y.J.; Khoo, K.H.; Chang, G.D. Uncovering protein polyamination by the spermine-specific antiserum and mass spectrometric analysis. Amino Acids 2015, 47, 469–481. [Google Scholar] [CrossRef]
- Cordella-Miele, E.; Miele, L.; Beninati, S.; Mukherjee, A.B. Transglutaminase-catalyzed incorporation of polyamines into phospholipase A2. J. Biochem. 1993, 113, 164–173. [Google Scholar] [CrossRef]
- Song, Y.; Kirkpatrick, L.L.; Schilling, A.B.; Helseth, D.L.; Chabot, N.; Keillor, J.W.; Johnson, G.V.; Brady, S.T. Transglutaminase and polyamination of tubulin: Posttranslational modification for stabilizing axonal microtubules. Neuron 2013, 78, 109–123. [Google Scholar] [CrossRef]
- Wilhelmus, M.M.; Grunberg, S.C.; Bol, J.G.; van Dam, A.M.; Hoozemans, J.J.; Rozemuller, A.J.; Drukarch, B. Transglutaminases and transglutaminase-catalyzed cross-links colocalize with the pathological lesions in Alzheimer’s disease brain. Brain Pathol. 2009, 19, 612–622. [Google Scholar] [CrossRef]
- Fernandez, C.O.; Hoyer, W.; Zweckstetter, M.; Jares-Erijman, E.A.; Subramaniam, V.; Griesinger, C.; Jovin, T.M. NMR of alpha-synuclein-polyamine complexes elucidates the mechanism and kinetics of induced aggregation. EMBO J. 2004, 23, 2039–2046. [Google Scholar] [CrossRef] [PubMed]
- Goers, J.; Uversky, V.N.; Fink, A.L. Polycation-induced oligomerization and accelerated fibrillation of human alpha-synuclein in vitro. Protein Sci. 2003, 12, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Krasnoslobodtsev, A.V.; Peng, J.; Asiago, J.M.; Hindupur, J.; Rochet, J.C.; Lyubchenko, Y.L. Effect of spermidine on misfolding and interactions of alpha-synuclein. PLoS ONE 2012, 7, e38099. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, K.; Brooks, M.; Barone, S.; Rahmati, N.; Murray Stewart, T.; Dunworth, M.; Destefano-Shields, C.; Dasgupta, N.; Davidson, S.; Lindquist, D.M.; et al. Ablation of polyamine catabolic enzymes provokes Purkinje cell damage, neuroinflammation, and severe ataxia. J. Neuroinflamm. 2020, 17, 301. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.J.; Crossland, J.; Shaw, G.G. The actions of spermidine and spermine on the central nervous system. Neuropharmacology 1975, 14, 571–577. [Google Scholar] [CrossRef]
- Bowie, D. Polyamine-mediated channel block of ionotropic glutamate receptors and its regulation by auxiliary proteins. J. Biol. Chem. 2018, 293, 18789–18802. [Google Scholar] [CrossRef]
- Nichols, C.G.; Lee, S.J. Polyamines and potassium channels: A 25-year romance. J. Biol. Chem. 2018, 293, 18779–18788. [Google Scholar] [CrossRef]
- Baronas, V.A.; Kurata, H.T. Inward rectifiers and their regulation by endogenous polyamines. Front. Physiol. 2014, 5, 325. [Google Scholar] [CrossRef]
- Bowie, D.; Mayer, M.L. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron 1995, 15, 453–462. [Google Scholar] [CrossRef]
- Rozov, A.; Zakharova, Y.; Vazetdinova, A.; Valiullina-Rakhmatullina, F. The Role of Polyamine-Dependent Facilitation of Calcium Permeable AMPARs in Short-Term Synaptic Enhancement. Front. Cell. Neurosci. 2018, 12, 345. [Google Scholar] [CrossRef]
- Mony, L.; Zhu, S.; Carvalho, S.; Paoletti, P. Molecular basis of positive allosteric modulation of GluN2B NMDA receptors by polyamines. EMBO J. 2011, 30, 3134–3146. [Google Scholar] [CrossRef] [PubMed]
- Caballero, R.; Dolz-Gaiton, P.; Gomez, R.; Amoros, I.; Barana, A.; Gonzalez de la Fuente, M.; Osuna, L.; Duarte, J.; Lopez-Izquierdo, A.; Moraleda, I.; et al. Flecainide increases Kir2.1 currents by interacting with cysteine 311, decreasing the polyamine-induced rectification. Proc. Natl. Acad. Sci. USA 2010, 107, 15631–15636. [Google Scholar] [CrossRef] [PubMed]
- Kucheryavykh, Y.V.; Pearson, W.L.; Kurata, H.T.; Eaton, M.J.; Skatchkov, S.N.; Nichols, C.G. Polyamine permeation and rectification of Kir4.1 channels. Channels 2007, 1, 172–178. [Google Scholar] [CrossRef]
- Park, M.H.; Cooper, H.L.; Folk, J.E. Identification of hypusine, an unusual amino acid, in a protein from human lymphocytes and of spermidine as its biosynthetic precursor. Proc. Natl. Acad. Sci. USA 1981, 78, 2869–2873. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Wolff, E.C. Hypusine, a polyamine-derived amino acid critical for eukaryotic translation. J. Biol. Chem. 2018, 293, 18710–18718. [Google Scholar] [CrossRef] [PubMed]
- Tauc, M.; Cougnon, M.; Carcy, R.; Melis, N.; Hauet, T.; Pellerin, L.; Blondeau, N.; Pisani, D.F. The eukaryotic initiation factor 5A (eIF5A1), the molecule, mechanisms and recent insights into the pathophysiological roles. Cell Biosci. 2021, 11, 219. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H. The post-translational synthesis of a polyamine-derived amino acid, hypusine, in the eukaryotic translation initiation factor 5A (eIF5A). J. Biochem. 2006, 139, 161–169. [Google Scholar] [CrossRef]
- Gutierrez, E.; Shin, B.S.; Woolstenhulme, C.J.; Kim, J.R.; Saini, P.; Buskirk, A.R.; Dever, T.E. eIF5A promotes translation of polyproline motifs. Mol. Cell 2013, 51, 35–45. [Google Scholar] [CrossRef]
- Landau, G.; Bercovich, Z.; Park, M.H.; Kahana, C. The role of polyamines in supporting growth of mammalian cells is mediated through their requirement for translation initiation and elongation. J. Biol. Chem. 2010, 285, 12474–12481. [Google Scholar] [CrossRef]
- Mandal, A.; Mandal, S.; Park, M.H. Global quantitative proteomics reveal up-regulation of endoplasmic reticulum stress response proteins upon depletion of eIF5A in HeLa cells. Sci. Rep. 2016, 6, 25795. [Google Scholar] [CrossRef]
- Li, C.H.; Ohn, T.; Ivanov, P.; Tisdale, S.; Anderson, P. eIF5A promotes translation elongation, polysome disassembly and stress granule assembly. PLoS ONE 2010, 5, e9942. [Google Scholar] [CrossRef] [PubMed]
- Maier, B.; Ogihara, T.; Trace, A.P.; Tersey, S.A.; Robbins, R.D.; Chakrabarti, S.K.; Nunemaker, C.S.; Stull, N.D.; Taylor, C.A.; Thompson, J.E.; et al. The unique hypusine modification of eIF5A promotes islet beta cell inflammation and dysfunction in mice. J. Clin. Investig. 2010, 120, 2156–2170. [Google Scholar] [CrossRef] [PubMed]
- Melis, N.; Rubera, I.; Cougnon, M.; Giraud, S.; Mograbi, B.; Belaid, A.; Pisani, D.F.; Huber, S.M.; Lacas-Gervais, S.; Fragaki, K.; et al. Targeting eIF5A Hypusination Prevents Anoxic Cell Death through Mitochondrial Silencing and Improves Kidney Transplant Outcome. J. Am. Soc. Nephrol. 2017, 28, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.C.; Martin, E.N.; Lee, G.; Taylor, C.; Dondero, R.; Reznikov, L.L.; Dinarello, C.; Thompson, J.; Scheld, W.M. Eukaryotic translation initiation factor 5A small interference RNA-liposome complexes reduce inflammation and increase survival in murine models of severe sepsis and acute lung injury. J. Infect. Dis. 2008, 198, 1407–1414. [Google Scholar] [CrossRef]
- Park, M.H.; Kar, R.K.; Banka, S.; Ziegler, A.; Chung, W.K. Post-translational formation of hypusine in eIF5A: Implications in human neurodevelopment. Amino Acids 2022, 54, 485–499. [Google Scholar] [CrossRef]
- Ganapathi, M.; Padgett, L.R.; Yamada, K.; Devinsky, O.; Willaert, R.; Person, R.; Au, P.B.; Tagoe, J.; McDonald, M.; Karlowicz, D.; et al. Recessive Rare Variants in Deoxyhypusine Synthase, an Enzyme Involved in the Synthesis of Hypusine, Are Associated with a Neurodevelopmental Disorder. Am. J. Hum. 2019, 104, 287–298. [Google Scholar] [CrossRef]
- Templin, A.T.; Maier, B.; Nishiki, Y.; Tersey, S.A.; Mirmira, R.G. Deoxyhypusine synthase haploinsufficiency attenuates acute cytokine signaling. Cell Cycle 2011, 10, 1043–1049. [Google Scholar] [CrossRef]
- Clarkson, A.N.; Liu, H.; Pearson, L.; Kapoor, M.; Harrison, J.C.; Sammut, I.A.; Jackson, D.M.; Appleton, I. Neuroprotective effects of spermine following hypoxic-ischemic-induced brain damage: A mechanistic study. FASEB J. 2004, 18, 1114–1116. [Google Scholar] [CrossRef]
- Duan, Q.; Yang, W.; Jiang, D.; Tao, K.; Dong, A.; Cheng, H. Spermine ameliorates ischemia/reperfusion injury in cardiomyocytes via regulation of autophagy. Am. J. Transl. Res. 2016, 8, 3976–3985. [Google Scholar]
- Gaugas, J.M.; Dewey, D.L. Evidence for serum binding of oxidized spermine and its potent G1-phase inhibition of cell proliferation. Br. J. Cancer 1979, 39, 548–557. [Google Scholar] [CrossRef][Green Version]
- Sharmin, S.; Sakata, K.; Kashiwagi, K.; Ueda, S.; Iwasaki, S.; Shirahata, A.; Igarashi, K. Polyamine cytotoxicity in the presence of bovine serum amine oxidase. Biochem. Biophys. Res. Commun. 2001, 282, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Tabor, C.W.; Tabor, H.; Bachrach, U. Identification of the Aminoaldehydes Produced by the Oxidation of Spermine and Spermidine with Purified Plasma Amine Oxidase. J. Biol. Chem. 1964, 239, 2194–2203. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Qi, C.; Shen, L.; Wang, J.; Liu, X.; Zhang, N.; Bing, T.; Shangguan, D. Oxidative degradation of polyamines by serum supplement causes cytotoxicity on cultured cells. Sci. Rep. 2018, 8, 10384. [Google Scholar] [CrossRef]
- Igarashi, K.; Ueda, S.; Yoshida, K.; Kashiwagi, K. Polyamines in renal failure. Amino Acids 2006, 31, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Noda, K.; Kawasaki, A.; Yoshida, S.; Dong, Y.; Saito, M.; Dong, Z.; Ando, R.; Mori, S.; Saito, W.; et al. Soluble Vascular Adhesion Protein-1 Mediates Spermine Oxidation as Semicarbazide-Sensitive Amine Oxidase: Possible Role in Proliferative Diabetic Retinopathy. Curr. Eye Res. 2017, 42, 1674–1683. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, R.; Venza, I.; Ceci, G.; Visalli, M.; Teti, D.; Reibaldi, A. Vitreous polyamines spermidine, putrescine, and spermine in human proliferative disorders of the retina. Br. J. Ophthalmol. 2003, 87, 1038–1042. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sakata, K.; Kashiwagi, K.; Sharmin, S.; Ueda, S.; Irie, Y.; Murotani, N.; Igarashi, K. Increase in putrescine, amine oxidase, and acrolein in plasma of renal failure patients. Biochem. Biophys. Res. Commun. 2003, 305, 143–149. [Google Scholar] [CrossRef]
- Tomitori, H.; Usui, T.; Saeki, N.; Ueda, S.; Kase, H.; Nishimura, K.; Kashiwagi, K.; Igarashi, K. Polyamine oxidase and acrolein as novel biochemical markers for diagnosis of cerebral stroke. Stroke 2005, 36, 2609–2613. [Google Scholar] [CrossRef]
- Ivanova, S.; Botchkina, G.I.; Al-Abed, Y.; Meistrell, M., 3rd; Batliwalla, F.; Dubinsky, J.M.; Iadecola, C.; Wang, H.; Gregersen, P.K.; Eaton, J.W.; et al. Cerebral ischemia enhances polyamine oxidation: Identification of enzymatically formed 3-aminopropanal as an endogenous mediator of neuronal and glial cell death. J. Exp. Med. 1998, 188, 327–340. [Google Scholar] [CrossRef]
- Ivanova, S.; Batliwalla, F.; Mocco, J.; Kiss, S.; Huang, J.; Mack, W.; Coon, A.; Eaton, J.W.; Al-Abed, Y.; Gregersen, P.K.; et al. Neuroprotection in cerebral ischemia by neutralization of 3-aminopropanal. Proc. Natl. Acad. Sci. USA 2002, 99, 5579–5584. [Google Scholar] [CrossRef]
- Minois, N.; Carmona-Gutierrez, D.; Madeo, F. Polyamines in aging and disease. Aging 2011, 3, 716–732. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, B.; Raj, V.; Nandakumar, S.; Kishore, A.; Thekkuveettil, A. Spermine protects alpha-synuclein expressing dopaminergic neurons from manganese-induced degeneration. Cell Biol. Toxicol. 2019, 35, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Dogan, A.; Rao, A.M.; Hatcher, J.; Rao, V.L.; Baskaya, M.K.; Dempsey, R.J. Effects of MDL 72527, a specific inhibitor of polyamine oxidase, on brain edema, ischemic injury volume, and tissue polyamine levels in rats after temporary middle cerebral artery occlusion. J. Neurochem. 1999, 72, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Pech, U.; Bhukel, A.; Fulterer, A.; Ender, A.; Mauermann, S.F.; Andlauer, T.F.; Antwi-Adjei, E.; Beuschel, C.; Thriene, K.; et al. Spermidine Suppresses Age-Associated Memory Impairment by Preventing Adverse Increase of Presynaptic Active Zone Size and Release. PLoS Biol. 2016, 14, e1002563. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, T.; Knauer, H.; Schauer, A.; Buttner, S.; Ruckenstuhl, C.; Carmona-Gutierrez, D.; Ring, J.; Schroeder, S.; Magnes, C.; Antonacci, L.; et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 2009, 11, 1305–1314. [Google Scholar] [CrossRef]
- Buttner, S.; Broeskamp, F.; Sommer, C.; Markaki, M.; Habernig, L.; Alavian-Ghavanini, A.; Carmona-Gutierrez, D.; Eisenberg, T.; Michael, E.; Kroemer, G.; et al. Spermidine protects against alpha-synuclein neurotoxicity. Cell Cycle 2014, 13, 3903–3908. [Google Scholar] [CrossRef]
- Minois, N.; Carmona-Gutierrez, D.; Bauer, M.A.; Rockenfeller, P.; Eisenberg, T.; Brandhorst, S.; Sigrist, S.J.; Kroemer, G.; Madeo, F. Spermidine promotes stress resistance in Drosophila melanogaster through autophagy-dependent and -independent pathways. Cell Death Dis. 2012, 3, e401. [Google Scholar] [CrossRef]
- Minois, N.; Rockenfeller, P.; Smith, T.K.; Carmona-Gutierrez, D. Spermidine feeding decreases age-related locomotor activity loss and induces changes in lipid composition. PLoS ONE 2014, 9, e102435. [Google Scholar] [CrossRef]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef]
- Kim, J. Spermidine is protective against kidney ischemia and reperfusion injury through inhibiting DNA nitration and PARP1 activation. Anat. Cell Biol. 2017, 50, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, S.; Zhang, Y.; Lin, X.; Song, Y.; Xue, Z.; Qian, H.; Wang, S.; Wan, G.; Zheng, X.; et al. Induction of autophagy by spermidine is neuroprotective via inhibition of caspase 3-mediated Beclin 1 cleavage. Cell Death Dis. 2017, 8, e2738. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zheng, C.; Cao, J.; Cao, G.; Shou, P.; Lin, L.; Velletri, T.; Jiang, M.; Chen, Q.; Han, Y.; et al. Spermidine alleviates experimental autoimmune encephalomyelitis through inducing inhibitory macrophages. Cell Death Differ. 2016, 23, 1850–1861. [Google Scholar] [CrossRef] [PubMed]
- Hyvonen, M.T.; Sinervirta, R.; Grigorenko, N.; Khomutov, A.R.; Vepsalainen, J.; Keinanen, T.A.; Alhonen, L. alpha-Methylspermidine protects against carbon tetrachloride-induced hepatic and pancreatic damage. Amino Acids 2010, 38, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Bowlin, T.L.; McKown, B.J.; Sunkara, P.S. Ornithine decarboxylase induction and polyamine biosynthesis are required for the growth of interleukin-2- and interleukin-3-dependent cell lines. Cell Immunol. 1986, 98, 341–350. [Google Scholar] [CrossRef]
- Ueda, A.; Araie, M.; Kubota, S. Polyamine depletion induces G1 and S phase arrest in human retinoblastoma Y79 cells. Cancer Cell Int. 2008, 8, 2. [Google Scholar] [CrossRef]
- Meehan, T.F.; Conte, N.; West, D.B.; Jacobsen, J.O.; Mason, J.; Warren, J.; Chen, C.K.; Tudose, I.; Relac, M.; Matthews, P.; et al. Disease model discovery from 3,328 gene knockouts by The International Mouse Phenotyping Consortium. Nat. Genet. 2017, 49, 1231–1238. [Google Scholar] [CrossRef]
- Prokop, J.W.; Bupp, C.P.; Frisch, A.; Bilinovich, S.M.; Campbell, D.B.; Vogt, D.; Schultz, C.R.; Uhl, K.L.; VanSickle, E.; Rajasekaran, S.; et al. Emerging Role of ODC1 in Neurodevelopmental Disorders and Brain Development. Genes 2021, 12, 470. [Google Scholar] [CrossRef]
- Muszynski, C.A.; Robertson, C.S.; Goodman, J.C.; Henley, C.M. DFMO reduces cortical infarct volume after middle cerebral artery occlusion in the rat. J. Cereb. Blood Flow Metab. 1993, 13, 1033–1037. [Google Scholar] [CrossRef]
- Zahedi, K.; Lentsch, A.B.; Okaya, T.; Barone, S.; Sakai, N.; Witte, D.P.; Arend, L.J.; Alhonen, L.; Jell, J.; Janne, J.; et al. Spermidine/spermine-N1-acetyltransferase ablation protects against liver and kidney ischemia-reperfusion injury in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G899–G909. [Google Scholar] [CrossRef]
- Hogarty, M.D.; Norris, M.D.; Davis, K.; Liu, X.; Evageliou, N.F.; Hayes, C.S.; Pawel, B.; Guo, R.; Zhao, H.; Sekyere, E.; et al. ODC1 is a critical determinant of MYCN oncogenesis and a therapeutic target in neuroblastoma. Cancer Res. 2008, 68, 9735–9745. [Google Scholar] [CrossRef]
- Rounbehler, R.J.; Li, W.; Hall, M.A.; Yang, C.; Fallahi, M.; Cleveland, J.L. Targeting ornithine decarboxylase impairs development of MYCN-amplified neuroblastoma. Cancer Res. 2009, 69, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Clifford, A.; Morgan, D.; Yuspa, S.H.; Soler, A.P.; Gilmour, S. Role of ornithine decarboxylase in epidermal tumorigenesis. Cancer Res. 1995, 55, 1680–1686. [Google Scholar] [PubMed]
- Hayes, C.S.; DeFeo-Mattox, K.; Woster, P.M.; Gilmour, S.K. Elevated ornithine decarboxylase activity promotes skin tumorigenesis by stimulating the recruitment of bulge stem cells but not via toxic polyamine catabolic metabolites. Amino Acids 2014, 46, 543–552. [Google Scholar] [CrossRef]
- Brown, I.; Halliday, S.; Greig, H.; Heys, S.D.; Wallace, H.M.; Schofield, A.C. Genetic polymorphism in ornithine decarboxylase and risk of breast cancer. Fam. Cancer 2009, 8, 307–311. [Google Scholar] [CrossRef]
- Gamble, L.D.; Purgato, S.; Henderson, M.J.; Di Giacomo, S.; Russell, A.J.; Pigini, P.; Murray, J.; Valli, E.; Milazzo, G.; Giorgi, F.M.; et al. A G316A Polymorphism in the Ornithine Decarboxylase Gene Promoter Modulates MYCN-Driven Childhood Neuroblastoma. Cancers 2021, 13, 1807. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.E.; O’Brien, T.G.; Fultz, K.E.; Babbar, N.; Yerushalmi, H.; Qu, N.; Guo, Y.; Boorman, D.; Einspahr, J.; Alberts, D.S.; et al. Pronounced reduction in adenoma recurrence associated with aspirin use and a polymorphism in the ornithine decarboxylase gene. Proc. Natl. Acad. Sci. USA 2003, 100, 7859–7864. [Google Scholar] [CrossRef]
- Visvanathan, K.; Helzlsouer, K.J.; Boorman, D.W.; Strickland, P.T.; Hoffman, S.C.; Comstock, G.W.; O’Brien, T.G.; Guo, Y. Association among an ornithine decarboxylase polymorphism, androgen receptor gene (CAG) repeat length and prostate cancer risk. J. Urol. 2004, 171, 652–655. [Google Scholar] [CrossRef]
- Zell, J.A.; Ziogas, A.; Ignatenko, N.; Honda, J.; Qu, N.; Bobbs, A.S.; Neuhausen, S.L.; Gerner, E.W.; Anton-Culver, H. Associations of a polymorphism in the ornithine decarboxylase gene with colorectal cancer survival. Clin. Cancer Res. 2009, 15, 6208–6216. [Google Scholar] [CrossRef]
- Liu, L.; Li, L.; Rao, J.N.; Zou, T.; Zhang, H.M.; Boneva, D.; Bernard, M.S.; Wang, J.Y. Polyamine-modulated expression of c-myc plays a critical role in stimulation of normal intestinal epithelial cell proliferation. Am. J. Physiol. Cell Physiol. 2005, 288, C89–C99. [Google Scholar] [CrossRef]
- Rao, J.N.; Li, L.; Bass, B.L.; Wang, J.Y. Expression of the TGF-beta receptor gene and sensitivity to growth inhibition following polyamine depletion. Am. J. Physiol. Cell Physiol. 2000, 279, C1034–C1044. [Google Scholar] [CrossRef]
- Wang, J.Y. Polyamines and mRNA stability in regulation of intestinal mucosal growth. Amino Acids 2007, 33, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Lukkarinen, J.A.; Grohn, O.H.; Alhonen, L.I.; Janne, J.; Kauppinen, R.A. Enhanced ornithine decarboxylase activity is associated with attenuated rate of damage evolution and reduction of infarct volume in transient middle cerebral artery occlusion in the rat. Brain Res. 1999, 826, 325–329. [Google Scholar] [CrossRef]
- Lukkarinen, J.A.; Kauppinen, R.A.; Grohn, O.H.; Oja, J.M.; Sinervirta, R.; Jarvinen, A.; Alhonen, L.I.; Janne, J. Neuroprotective role of ornithine decarboxylase activation in transient focal cerebral ischaemia: A study using ornithine decarboxylase-overexpressing transgenic rats. Eur. J. Neurosci. 1998, 10, 2046–2055. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra Rao, V.L.; Dogan, A.; Bowen, K.K.; Dempsey, R.J. Ornithine decarboxylase knockdown exacerbates transient focal cerebral ischemia-induced neuronal damage in rat brain. J. Cereb. Blood Flow Metab. 2001, 21, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Temiz, C.; Dogan, A.; Baskaya, M.K.; Dempsey, R.J. Effect of difluoromethylornithine on reperfusion injury after temporary middle cerebral artery occlusion. J. Clin. Neurosci. 2005, 12, 449–452. [Google Scholar] [CrossRef]
- Bupp, C.P.; Schultz, C.R.; Uhl, K.L.; Rajasekaran, S.; Bachmann, A.S. Novel de novo pathogenic variant in the ODC1 gene in a girl with developmental delay, alopecia, and dysmorphic features. Am. J. Med. Genet. Part A 2018, 176, 2548–2553. [Google Scholar] [CrossRef]
- Rodan, L.H.; Anyane-Yeboa, K.; Chong, K.; Klein Wassink-Ruiter, J.S.; Wilson, A.; Smith, L.; Kothare, S.V.; Rajabi, F.; Blaser, S.; Ni, M.; et al. Gain-of-function variants in the ODC1 gene cause a syndromic neurodevelopmental disorder associated with macrocephaly, alopecia, dysmorphic features, and neuroimaging abnormalities. Am. J. Med. Genet. Part A 2018, 176, 2554–2560. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Bupp, C.P.; Leimanis-Laurens, M.; Shukla, A.; Russell, C.; Junewick, J.; Gleason, E.; VanSickle, E.A.; Edgerly, Y.; Wittmann, B.M.; et al. Repurposing eflornithine to treat a patient with a rare ODC1 gain-of-function variant disease. eLife 2021, 10, e67097. [Google Scholar] [CrossRef]
- Pegg, A.E.; Wang, X. Mouse models to investigate the function of spermine. Commun. Integr. Biol. 2009, 2, 271–274. [Google Scholar] [CrossRef]
- Becerra-Solano, L.E.; Butler, J.; Castaneda-Cisneros, G.; McCloskey, D.E.; Wang, X.; Pegg, A.E.; Schwartz, C.E.; Sanchez-Corona, J.; Garcia-Ortiz, J.E. A missense mutation, p.V132G, in the X-linked spermine synthase gene (SMS) causes Snyder-Robinson syndrome. Am. J. Med. Genet. Part A 2009, 149A, 328–335. [Google Scholar] [CrossRef]
- Cason, A.L.; Ikeguchi, Y.; Skinner, C.; Wood, T.C.; Holden, K.R.; Lubs, H.A.; Martinez, F.; Simensen, R.J.; Stevenson, R.E.; Pegg, A.E.; et al. X-linked spermine synthase gene (SMS) defect: The first polyamine deficiency syndrome. Eur. J. Hum. Genet. 2003, 11, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Murray-Stewart, T.; Dunworth, M.; Foley, J.R.; Schwartz, C.E.; Casero, R.A., Jr. Polyamine Homeostasis in Snyder-Robinson Syndrome. Med. Sci. 2018, 6, 112. [Google Scholar] [CrossRef] [PubMed]
- Hato, T.; Zollman, A.; Plotkin, Z.; El-Achkar, T.M.; Maier, B.F.; Pay, S.L.; Dube, S.; Cabral, P.; Yoshimoto, M.; McClintick, J.; et al. Endotoxin Preconditioning Reprograms S1 Tubules and Macrophages to Protect the Kidney. J. Am. Soc. Nephrol. 2018, 29, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, K.; Barone, S.; Kramer, D.L.; Amlal, H.; Alhonen, L.; Janne, J.; Porter, C.W.; Soleimani, M. The role of spermidine/spermine N1-acetyltransferase in endotoxin-induced acute kidney injury. Am. J. Physiol. Cell Physiol. 2010, 299, C164–C174. [Google Scholar] [CrossRef]
- Zahedi, K.; Barone, S.L.; Xu, J.; Steinbergs, N.; Schuster, R.; Lentsch, A.B.; Amlal, H.; Wang, J.; Casero, R.A., Jr.; Soleimani, M. Hepatocyte-specific ablation of spermine/spermidine-N1-acetyltransferase gene reduces the severity of CCl4-induced acute liver injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G546–G560. [Google Scholar] [CrossRef]
- Zahedi, K.; Wang, Z.; Barone, S.; Prada, A.E.; Kelly, C.N.; Casero, R.A.; Yokota, N.; Porter, C.W.; Rabb, H.; Soleimani, M. Expression of SSAT, a novel biomarker of tubular cell damage, increases in kidney ischemia-reperfusion injury. Am. J. Physiol. Ren. Physiol. 2003, 284, F1046–F1055. [Google Scholar] [CrossRef]
- Golab, F.; Kadkhodaee, M.; Zahmatkesh, M.; Hedayati, M.; Arab, H.; Schuster, R.; Zahedi, K.; Lentsch, A.B.; Soleimani, M. Ischemic and non-ischemic acute kidney injury cause hepatic damage. Kidney Int. 2009, 75, 783–792. [Google Scholar] [CrossRef]
- Zahedi, K.; Barone, S.; Wang, Y.; Murray-Stewart, T.; Roy-Chaudhury, P.; Smith, R.D.; Casero, R.A., Jr.; Soleimani, M. Proximal tubule epithelial cell specific ablation of the spermidine/spermine N1-acetyltransferase gene reduces the severity of renal ischemia/reperfusion injury. PLoS ONE 2014, 9, e110161. [Google Scholar] [CrossRef]
- Cervelli, M.; Bellavia, G.; D’Amelio, M.; Cavallucci, V.; Moreno, S.; Berger, J.; Nardacci, R.; Marcoli, M.; Maura, G.; Piacentini, M.; et al. A New Transgenic Mouse Model for Studying the Neurotoxicity of Spermine Oxidase Dosage in the Response to Excitotoxic Injury. PLoS ONE 2013, 8, e64810. [Google Scholar] [CrossRef]
- Cervetto, C.; Averna, M.; Vergani, L.; Pedrazzi, M.; Amato, S.; Pelassa, S.; Giuliani, S.; Baldini, F.; Maura, G.; Mariottini, P.; et al. Reactive Astrocytosis in a Mouse Model of Chronic Polyamine Catabolism Activation. Biomolecules 2021, 11, 1274. [Google Scholar] [CrossRef]
- Leonetti, A.; Baroli, G.; Fratini, E.; Pietropaoli, S.; Marcoli, M.; Mariottini, P.; Cervelli, M. Epileptic seizures and oxidative stress in a mouse model over-expressing spermine oxidase. Amino Acids 2020, 52, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Pietropaoli, S.; Leonetti, A.; Cervetto, C.; Venturini, A.; Mastrantonio, R.; Baroli, G.; Persichini, T.; Colasanti, M.; Maura, G.; Marcoli, M.; et al. Glutamate Excitotoxicity Linked to Spermine Oxidase Overexpression. Mol. Neurobiol. 2018, 55, 7259–7270. [Google Scholar] [CrossRef] [PubMed]
- Sierra, J.C.; Piazuelo, M.B.; Luis, P.B.; Barry, D.P.; Allaman, M.M.; Asim, M.; Sebrell, T.A.; Finley, J.L.; Rose, K.L.; Hill, S.; et al. Spermine oxidase mediates Helicobacter pylori-induced gastric inflammation, DNA damage, and carcinogenic signaling. Oncogene 2020, 39, 4465–4474. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, R.; de Sablet, T.; Asim, M.; Piazuelo, M.B.; Barry, D.P.; Verriere, T.G.; Sierra, J.C.; Hardbower, D.M.; Delgado, A.G.; Schneider, B.G.; et al. Increased Helicobacter pylori-associated gastric cancer risk in the Andean region of Colombia is mediated by spermine oxidase. Oncogene 2015, 34, 3429–3440. [Google Scholar] [CrossRef] [PubMed]
- Casero, R.A., Jr.; Celano, P.; Ervin, S.J.; Wiest, L.; Pegg, A.E. High specific induction of spermidine/spermine N1-acetyltransferase in a human large cell lung carcinoma. Biochem. J. 1990, 270, 615–620. [Google Scholar] [CrossRef][Green Version]
- Casero, R.A., Jr.; Murray Stewart, T.; Pegg, A.E. Polyamine metabolism and cancer: Treatments, challenges and opportunities. Nat. Rev. Cancer 2018, 18, 681–695. [Google Scholar] [CrossRef]
- Zahedi, K.; Bissler, J.J.; Wang, Z.; Josyula, A.; Lu, L.; Diegelman, P.; Kisiel, N.; Porter, C.W.; Soleimani, M. Spermidine/spermine N1-acetyltransferase overexpression in kidney epithelial cells disrupts polyamine homeostasis, leads to DNA damage, and causes G2 arrest. Am. J. Physiol. Cell Physiol. 2007, 292, C1204–C1215. [Google Scholar] [CrossRef]
- Jell, J.; Merali, S.; Hensen, M.L.; Mazurchuk, R.; Spernyak, J.A.; Diegelman, P.; Kisiel, N.D.; Barrero, C.; Deeb, K.K.; Alhonen, L.; et al. Genetically altered expression of spermidine/spermine N1-acetyltransferase affects fat metabolism in mice via acetyl-CoA. J. Biol. Chem. 2007, 282, 8404–8413. [Google Scholar] [CrossRef]
- Liu, C.; Perez-Leal, O.; Barrero, C.; Zahedi, K.; Soleimani, M.; Porter, C.; Merali, S. Modulation of polyamine metabolic flux in adipose tissue alters the accumulation of body fat by affecting glucose homeostasis. Amino Acids 2014, 46, 701–715. [Google Scholar] [CrossRef]
- Jain, V.; Raina, S.; Gheware, A.P.; Singh, R.; Rehman, R.; Negi, V.; Murray Stewart, T.; Mabalirajan, U.; Mishra, A.K.; Casero, R.A., Jr.; et al. Reduction in polyamine catabolism leads to spermine-mediated airway epithelial injury and induces asthma features. Allergy 2018, 73, 2033–2045. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).