Plasma Concentrations of Vinculin versus Talin-1 in Coronary Artery Disease
Abstract
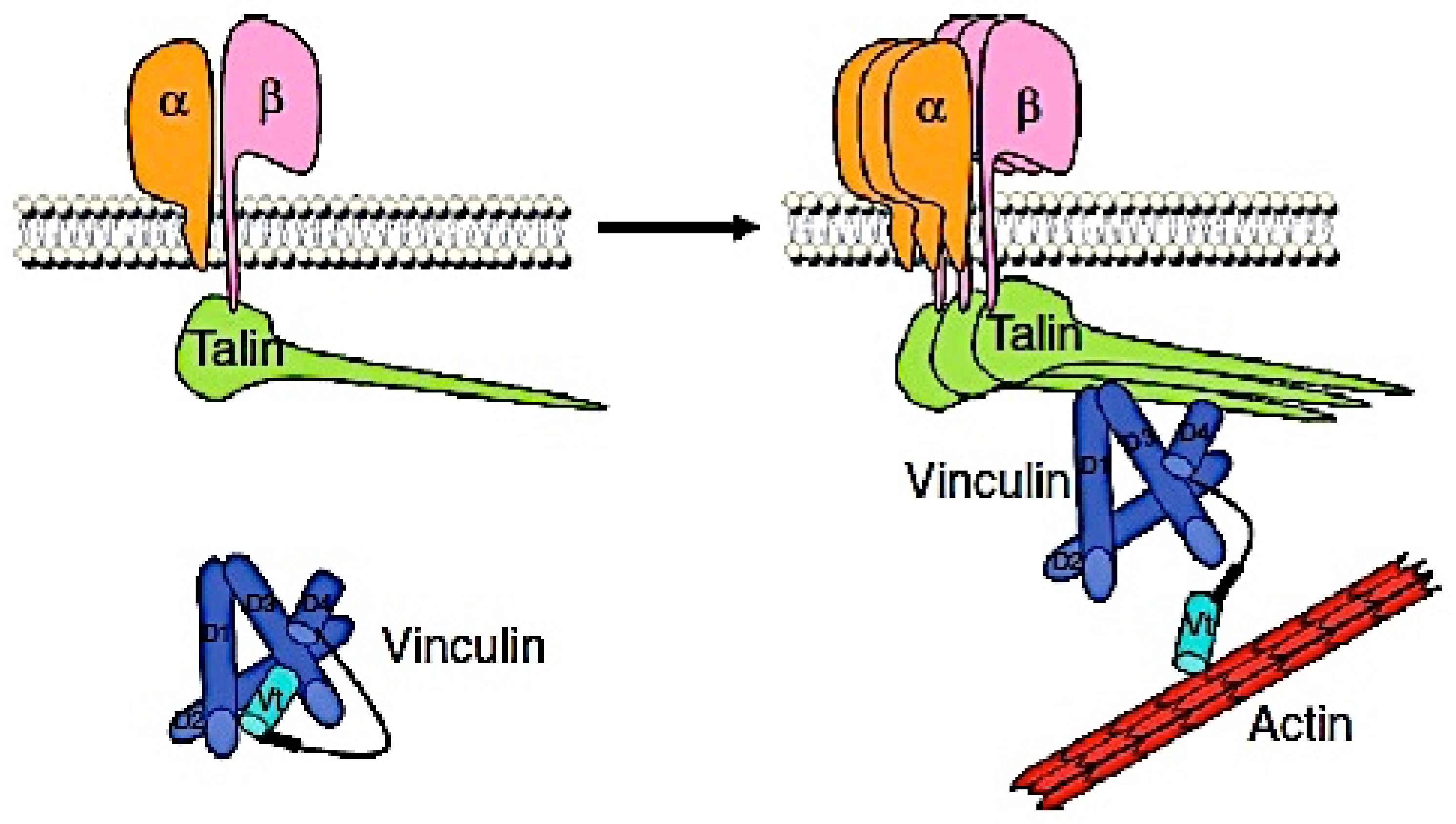
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Measurements of Vinculin, Talin-1, and C-Reactive Protein (CRP) Concentrations
2.3. Angiographic Analysis
2.4. Statistical Analysis
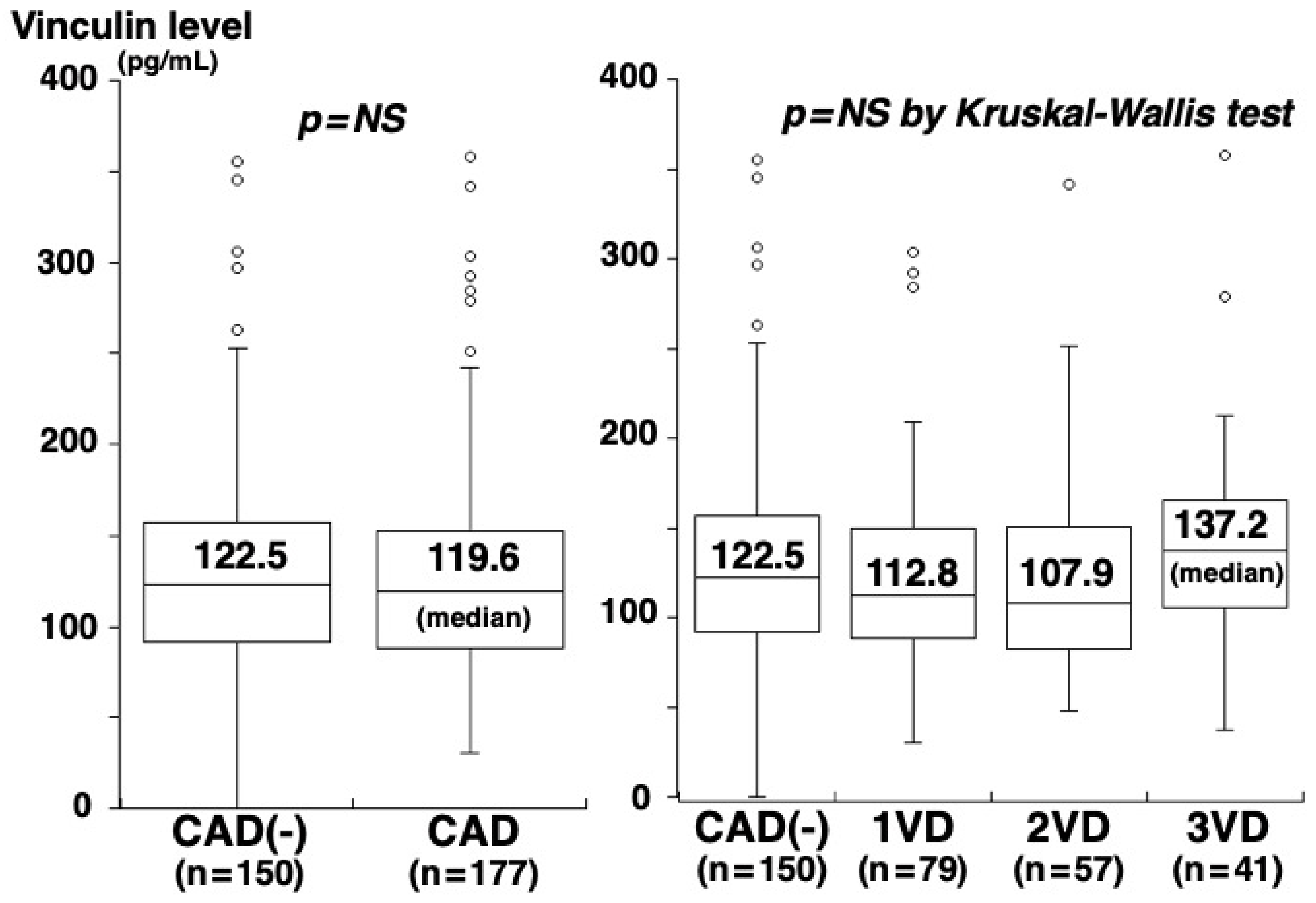
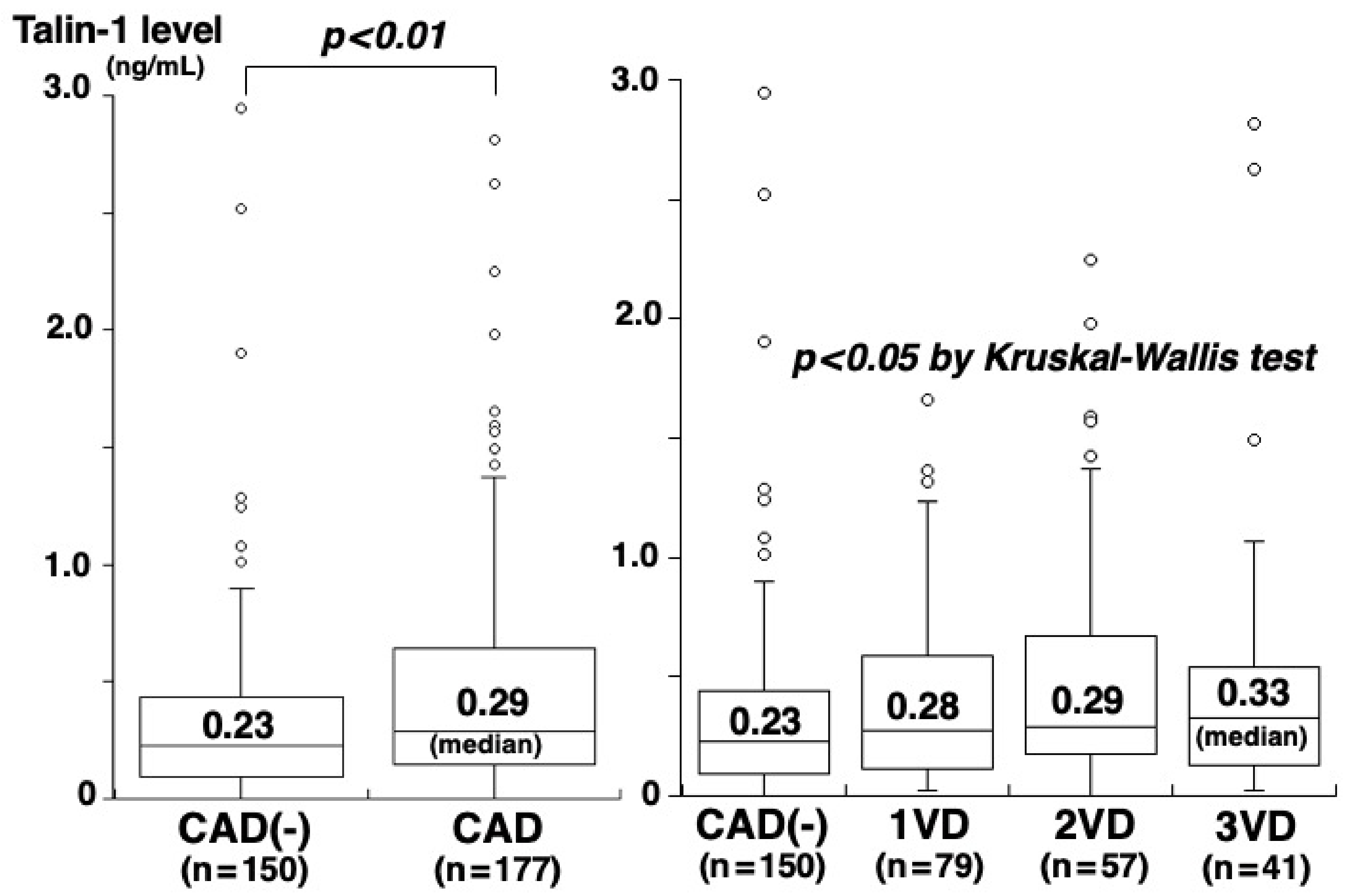
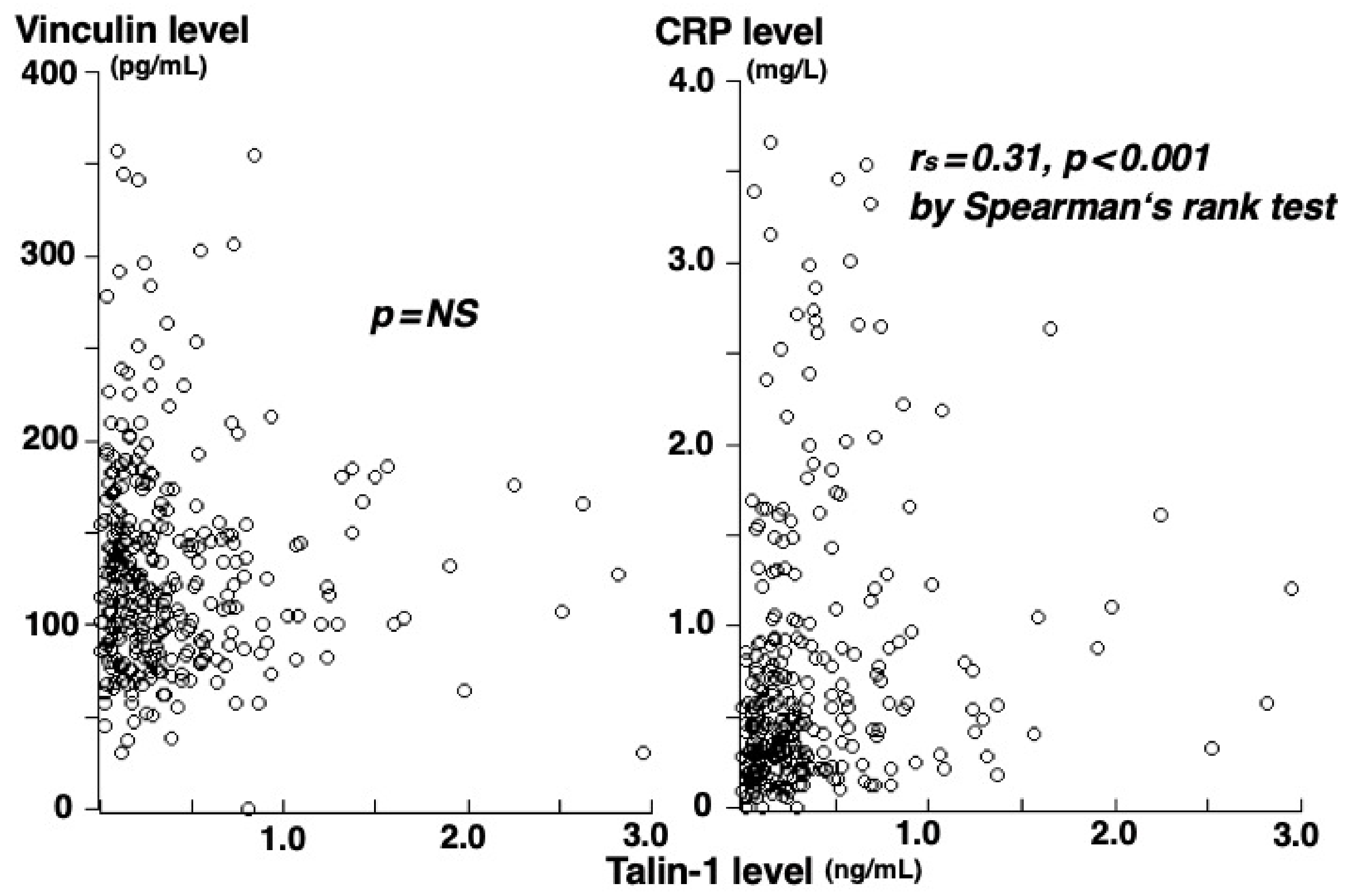
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Das, M.; Ithychanda, S.; Qin, J.; Plow, E.F. Mechanisms of talin-dependent integrin signaling and crosstalk. Biochim. Biophys. Acta 2014, 1838, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Klapholz, B.; Brown, N.H. Talin: The master of integrin adhesions. J. Cell Sci. 2017, 130, 2435–2446. [Google Scholar] [CrossRef]
- Yan, B.; Calderwood, D.A.; Yaspan, B.; Ginsberg, M.H. Calpain cleavage promotes talin binding to the beta 3 integrin cytoplasmic domain. J. Biol. Chem. 2001, 276, 28164–28170. [Google Scholar] [CrossRef] [PubMed]
- Manevich, E.; Grabovsky, V.; Feigelson, S.W.; Alon, R. Talin 1 and paxillin facilitate distinct steps in rapid VLA-4-mediated adhesion strengthening to vascular cell adhesion molecule 1. J. Biol. Chem. 2007, 282, 25338–25348. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ballestrem, C.; Chi, S. The C terminus of talin links integrins to cell cycle progression. J. Cell Biol. 2011, 195, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Carisey, A.; Ballestrem, C. Vinculin, an adapter protein in control of cell adhesion signalling. Eur. J. Cell Biol. 2011, 90, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, H.W.; Gingras, A.R.; Critchley, D.R.; Emsley, J. Integrin connections to the cytoskeleton through talin and vinculin. Biochem. Soc. Trans. 2008, 36, 235–239. [Google Scholar] [CrossRef] [PubMed]
- DeMali, K.A.; Barlow, C.A.; Burridge, K. Recruitment of the Arp2/3 complex to vinculin: Coupling membrane protrusion to matrix adhesion. J. Cell Biol. 2002, 159, 881–891. [Google Scholar] [CrossRef]
- Izard, T.; Brown, D.T. Mechanisms and functions of vinculin interactions with phospholipids at cell adhesion sites. J. Biol. Chem. 2016, 291, 2548–2555. [Google Scholar] [CrossRef]
- Bays, J.L.; DeMali, K.A. Vinculin in cell-cell and cell-matrix adhesions. Cell Mol. Life Sci. 2017, 74, 2999–3009. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.; Nelson, E.S.; Maiers, J.L.; DeMali, K.A. New insights into vinculin function and regulation. Int. Rev. Cell Mol. Biol. 2011, 287, 191–231. [Google Scholar]
- Hu, X.; Jing, C.; Xu, X.; Nakazawa, N.; Cornish, V.W.; Margadant, F.M.; Sheetz, M.P. Cooperative vinculin binding to talin mapped by time-resolved super resolution microscopy. Nano Lett. 2016, 16, 4062–4068. [Google Scholar] [CrossRef]
- Humphries, J.D.; Wang, P.; Streuli, C.; Geiger, B.; Humphries, M.J.; Ballestrem, C. Vinculin controls focal adhesion formation by direct interactions with talin and actin. J. Cell Biol. 2007, 179, 1043–1057. [Google Scholar] [CrossRef]
- Banno, A.; Goult, B.T.; Lee, H.; Bate, N.; Critchley, D.R.; Ginsberg, M.H. Subcellular localization of talin is regulated by inter-domain interactions. J. Biol. Chem. 2012, 287, 13799–13812. [Google Scholar] [CrossRef]
- Martin-Lorenzo, M.; Gonzalez-Calero, L.; Maroto, A.S.; Martinez, P.J.; Zubiri, I.; de la Cuesta, F.; Mourino-Alvarez, L.; Barderas, M.G.; Heredero, A.; Aldamiz-Echevarría, G.; et al. Cytoskeleton deregulation and impairment in amino acids and energy metabolism in early atherosclerosis at aortic tissue with reflection in plasma. Biochim. Biophys. Acta 2016, 1862, 725–732. [Google Scholar] [CrossRef]
- De la Cuesta, F.; Zubiri, I.; Maroto, A.S.; Posada, M.; Padial, L.R.; Vivanco, F.; Alvarez-Llamas, G.; Barderas, M.G. Deregulation of smooth muscle cell cytoskeleton within the human atherosclerotic coronary media layer. J. Proteom. 2013, 82, 155–165. [Google Scholar] [CrossRef]
- Von Essen, M.; Rahikainen, R.; Oksala, N.; Raitoharju, E.; Seppälä, I.; Mennander, A.; Sioris, T.; Kholová, I.; Klopp, N.; Illig, T.; et al. Talin and vinculin are downregulated in atherosclerotic plaque; Tampere Vascular Study. Atherosclerosis 2016, 255, 43–53. [Google Scholar] [CrossRef]
- Aoyama, M.; Kishimoto, Y.; Saita, E.; Ikegami, Y.; Ohmori, R.; Nakamura, M.; Kondo, K.; Momiyama, Y. High plasma levels of soluble talin-1 in patients with coronary artery disease. Dis. Markers 2020, 2020, 2479830. [Google Scholar] [CrossRef]
- Bostanci, O.; Kemik, O.; Kemik, A.; Battal, M.; Demir, U.; Purisa, S.; Mihmanli, M. A novel screening test for colon cancer: Talin-1. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2533–2537. [Google Scholar]
- Yano, M.; Miura, S.; Shiga, Y.; Miyase, Y.; Suematsu, Y.; Norimatsu, K.; Nakamura, A.; Adachi, S.; Nishikawa, H.; Saku, K. Association between smoking habits and severity of coronary stenosis as assessed by coronary computed tomography angiography. Heart Vessel. 2016, 31, 1061–1068. [Google Scholar] [CrossRef]
- Saita, E.; Miura, K.; Suzuki-Sugihara, N.; Miyata, K.; Ikemura, N.; Ohmori, R.; Ikegami, Y.; Kishimoto, Y.; Kondo, K.; Momiyama, Y. Plasma soluble endoglin levels are inversely associated with the severity of coronary atherosclerosis: Brief report. Arter. Thromb. Vasc. Biol. 2017, 37, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Khassawneh, B.Y.; Samrah, S.M.; Jarrah, M.I.; Ibdah, R.K.; Ibnian, A.M.; Al-Mistarehi, A.W.; Zghayer, A.A.; Abuqudairi, S.I.; Khader, Y.S. Prevalence of undiagnosed COPD in male patients with coronary artery disease: A cross-sectional study in Jordan. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 2759–2766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishimoto, Y.; Niki, H.; Saita, E.; Ibe, S.; Umei, T.; Miura, K.; Ikegami, Y.; Ohmori, R.; Kondo, K.; Momiyama, Y. Blood levels of heme oxygenase-1 versus bilirubin in patients with coronary artery disease. Clin. Chim. Acta 2020, 504, 30–35. [Google Scholar] [CrossRef] [PubMed]
- De la Cuesta, F.; Barderas, M.G.; Calvo, E.; Zubiri, I.; Maroto, A.S.; Darde, V.M.; Martin-Rojas, T.; Gil-Dones, F.; Posada-Ayala, M.; Tejerina, T.; et al. Secretome analysis of atherosclerotic and non-atherosclerotic arteries reveals dynamic extracellular remodeling during pathogenesis. J. Proteom. 2012, 75, 2960–2971. [Google Scholar] [CrossRef]
- Zhong, T.; Li, Y.; He, X.; Liu, Y.; Dong, Y.; Ma, H.; Zheng, Z.; Zhang, Y. Adaptation of endothelial cells to shear stress under atheroprone conditions by modulating internalization of vascular endothelial cadherin and vinculin. Ann. Transl. Med. 2020, 8, 1423. [Google Scholar] [CrossRef]
- Kim, H.J.; Woo, S.J.; Suh, E.J.; Ahn, J.; Park, J.H.; Hong, H.K.; Lee, J.E.; Ahn, S.J.; Hwang, D.D.-J.; Kim, K.W.; et al. Identification of vinculin as a potential plasma marker for age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7166–7176. [Google Scholar] [CrossRef]
- Orwoll, E.S.; Wiedrick, J.; Jacobs, J.; Baker, E.S.; Piehowski, P.; Petyuk, V.; Gao, Y.; Shi, T.; Smith, R.D.; Bauer, D.C.; et al. High-throughput serum proteomics for the identification of protein biomarkers of mortality in older men. Aging Cell 2018, 17, e12717. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, L.; Xie, Y.; Tan, Y.; Chang, C.; Qiu, X.; Li, X. Characterization of differentially expressed plasma proteins in patients with acute myocardial infarction. J. Proteom. 2020, 227, 103923. [Google Scholar] [CrossRef]
- Wang, H.Q.; Yang, H.; Tang, Q.; Gong, Y.C.; Fu, Y.H.; Wan, F.; Yang, B.; Guo, R.; Zhong, Y.-L.; Zhu, J.-M.; et al. Identification of vinculin as a potential diagnostic biomarker for acute aortic dissection using label-free proteomics. BioMed Res. Int. 2020, 2020, 7806409. [Google Scholar] [CrossRef]
- Chen, H.; Cohen, D.M.; Choudhury, D.M.; Kioka, N.; Craig, S.W. Spatial distribution and functional significance of activated vinculin in living cells. J. Cell Biol. 2005, 169, 459–470. [Google Scholar] [CrossRef]
- Kristensen, L.P.; Larsen, M.R.; Mickley, H.; Saaby, L.; Diederichsen, A.C.; Lambrechtsen, J.; Rasmussen, L.M.; Overgaard, M. Plasma proteome profiling of atherosclerotic disease manifestations reveals elevated levels of cytoskeletal protein vinculin. J. Proteom. 2014, 101, 141–153. [Google Scholar] [CrossRef]
- López-Farré, A.J.; Zamorano-Leon, J.J.; Azcona, L.; Modrego, J.; Mateos-Cáceres, P.J.; González-Armengol, J.; Villarroel, P.; Moreno-Herrero, R.; Rodríguez-Sierra, P.; Segura, A.; et al. Proteomic changes related to “bewildered” circulating platelets in the acute coronary syndrome. Proteomics 2011, 11, 3335–3348. [Google Scholar] [CrossRef]
- Mascia, G.; Pescetelli, F.; Baldari, A.; Gatto, P.; Seitun, S.; Sartori, P.; Pieroni, M.; Calò, L.; Della Bona, R.; Porto, I. Interpretation of elevated high-sensitivity cardiac troponin I in elite soccer players previously infected by severe acute respiratory syndrome coronavirus 2. Int. J. Cardiol. 2021, 326, 248–251. [Google Scholar] [CrossRef]
- Cirer-Sastre, R.; Legaz-Arrese, A.; Francisco Corbi, F.; Keith George, K.; Nie, J.; Carranza-García, L.E.; Reverter-Masià, J. Cardiac biomarker release after exercise in healthy children and adolescents: A systematic review and meta-analysis. Pediatr. Exerc. Sci. 2019, 31, 28–36. [Google Scholar] [CrossRef]
- Welsh, P.; Preiss, D.; Hayward, C.; Shah, A.S.V.; McAllister, D.; Briggs, A.; Boachie, C.; McConnachie, A.; Padmanabhan, S.; Welsh, C.; et al. Cardiac troponin T and troponin I in the general population. Circulation 2019, 139, 2754–2764. [Google Scholar] [CrossRef]
- Zemljic-Harpf, A.; Manso, A.M.; Ross, R.S. Vinculin and talin: Focus on the myocardium. J. Investig. Med. 2009, 57, 849–855. [Google Scholar] [CrossRef]
- Kaushik, G.; Spenlehauer, A.; Sessions, A.O.; Trujillo, A.S.; Fuhrmann, A.; Fu, Z.; Venkatraman, V.; Pohl, D.; Tuler, J.; Wang, M.; et al. Vinculin network-mediated cytoskeletal remodeling regulates contractile function in the aging heart. Sci. Transl. Med. 2015, 7, 292ra99. [Google Scholar] [CrossRef]
- Datta, A.; Chen, C.P.; Sze, S.K. Discovery of prognostic biomarker candidates of lacunar infarction by quantitative proteomics of microvesicles enriched plasma. PLoS ONE 2014, 9, e94663. [Google Scholar] [CrossRef]
- Välimäki, E.; Cypryk, W.; Virkanen, J.; Nurmi, K.; Turunen, P.M.; Eklund, K.K.; Åkerman, K.E.; Nyman, T.A.; Matikainen, S. Calpain activity is essential for ATP-driven unconventional vesicle-mediated protein secretion and inflammasome activation in human macrophages. J. Immunol. 2016, 197, 3315–3325. [Google Scholar] [CrossRef]
- Ross, R. Atherosclerosis: An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Reumer, A.; Maes, E.; Mertens, I.; Cho, W.C.S.; Landuyt, B.; Valkenborg, D.; Schoofs, L.; Baggerman, G. Colorectal cancer biomarker discovery and validation using LC-MS/MS-based proteomics in blood: Truth or dare? Expert Rev. Proteom. 2014, 11, 449–463. [Google Scholar] [CrossRef]
| CAD(-) (n = 150) | p-Value CAD(-) vs CAD | CAD (n = 177) | 1-VD (n = 79) | 2-VD (n = 57) | 3-VD (n = 41) | Among 4 Groups | |
|---|---|---|---|---|---|---|---|
| Age (years) Sex (men) Hypertension Systolic BP (mmHg) Diabetes mellitus HbA1c (%) Smoking Hypercholesterolemia Statin use LDL-cholesterol (mg/dL) HDL-cholesterol (mg/dL) CRP (mg/L) | 65 ± 12 91 (61%) 92 (61%) 129 ± 21 23 (15%) 6.0 ± 0.7 44 (29%) 65 (43%) 41 (27%) 114 ± 28 59 ± 14 0.43 [0.21, 0.90] | <0.001 0.005 0.002 0.117 <0.001 0.002 0.033 0.013 0.001 0.641 <0.001 0.001 | 70 ± 9 134 (76%) 137 (77%) 133 ± 18 59 (33%) 6.3 ± 0.9 72 (41%) 101 (57%) 80 (45%) 112 ± 29 52 ± 14 0.58 [0.32, 1.43] | 69 ± 10 58 (73%) 59 (75%) 131 ± 16 22 (28%) 6.2 ± 0.8 35 (44%) 43 (54%) 34 (43%) 108 ± 28 56 ± 15 0.58 [0.32, 1.38] | 69 ± 10 42 (74%) 43 (75%) 137 ± 20 22 (39%) 6.4 ± 1.1 25 (44%) 33 (58%) 25 (44%) 115 ± 31 51 ± 12 0.56 [0.30, 1.11] | 72 ± 8 34 (83%) 35 (85%) 130 ± 18 15 (37%) 6.2 ± 0.9 12 (29%) 25 (61%) 21 (51%) 117 ± 26 48 ± 13 0.81 [0.42, 2.21] | <0.001 0.020 0.009 0.073 0.001 0.003 0.055 0.085 0.008 0.301 <0.001 0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aoyama, M.; Kishimoto, Y.; Saita, E.; Ohmori, R.; Tanimoto, K.; Nakamura, M.; Kondo, K.; Momiyama, Y. Plasma Concentrations of Vinculin versus Talin-1 in Coronary Artery Disease. Med. Sci. 2022, 10, 46. https://doi.org/10.3390/medsci10030046
Aoyama M, Kishimoto Y, Saita E, Ohmori R, Tanimoto K, Nakamura M, Kondo K, Momiyama Y. Plasma Concentrations of Vinculin versus Talin-1 in Coronary Artery Disease. Medical Sciences. 2022; 10(3):46. https://doi.org/10.3390/medsci10030046
Chicago/Turabian StyleAoyama, Masayuki, Yoshimi Kishimoto, Emi Saita, Reiko Ohmori, Kojiro Tanimoto, Masato Nakamura, Kazuo Kondo, and Yukihiko Momiyama. 2022. "Plasma Concentrations of Vinculin versus Talin-1 in Coronary Artery Disease" Medical Sciences 10, no. 3: 46. https://doi.org/10.3390/medsci10030046
APA StyleAoyama, M., Kishimoto, Y., Saita, E., Ohmori, R., Tanimoto, K., Nakamura, M., Kondo, K., & Momiyama, Y. (2022). Plasma Concentrations of Vinculin versus Talin-1 in Coronary Artery Disease. Medical Sciences, 10(3), 46. https://doi.org/10.3390/medsci10030046





