Prevention and Treatment of Chemotherapy-Induced Peripheral Neuropathy (CIPN) with Non-Pharmacological Interventions: Clinical Recommendations from a Systematic Scoping Review and an Expert Consensus Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Phase 1: Scoping Review
2.1.1. Literature Search
2.1.2. Study Screening and Selection Criteria
2.1.3. Data Extraction and Reporting
2.1.4. Risk of Bias Assessment
2.2. Phase 2: Structured Expert Consensus Process
Criteria for Consensus Finding
- S = safe;
- CE = clinical experience (rated on a numerical scale 0 to 5, with 0 = no effect and 5 = maximum effect);
- ET = effort of training (education requirements in addition to a nursing grade; 0 = no additional instructions or education needed, 1 = instructions needed, 2 = application under guidance, 3 = repeated practice needed, 4 = basic training of rhythmical embrocation (200 h) recommended but partial skills can be acquired with less than 200 h, and 5 = basic training of rhythmical embrocation (200 h) needed);
- PF = practical feasibility (PFt = feasibility limited due to time requirements; PFtt = feasibility strongly limited due to time requirements; PFc = feasibility limited due to high costs (>30 EUR per month)).
3. Results
3.1. Search Results
3.2. Consensus from the Expert Panel
3.3. Preventative Options for CIPN
3.4. Complementary Treatment Options for CIPN

- −
- 1 incl. aromatherapy, topical therapy, no oral phytotherapeutics, and flaxseed bath;
- −
- 2 incl. physical therapy, sensorimotor training, exercise, closed kinematic chain exercise, resistance training, cardiovascular exercises, walking, cycling, whole-body-vibration, passive mobilization, coordination training, and tactile stimulation;
- −
- 3 incl. relaxation, PMR, yoga, meditation, hypnosis, guided imagery, cognitive therapies, and distraction therapy, as well as Qi Gong and Tai Chi;
- −
- 4 incl. vitamin and mineral supplements and dietary modification;
- −
- 5 incl. alcaline bath and cold knee and/or arm showers;
- −
- 6 incl. Tai Chi, Qi Gong, and massage acc. to TCM;
- −
- 7 incl. cryocompression, cold applications, and hypothermia;
- −
- 8 incl. hyperthermia;
- −
- 9 incl. massage, reflexology, and foot reflexology;
- −
- 10 incl. healing touch, Reiki, and therapeutic touch;
- −
- 11 incl. compression, cupping (draining procedures), hydroelectric bath, music therapy, support groups, patient education, and nurse-led follow-up.
- −
- Note. Study quality of the included studies varied—see Table S3 (Supplementary S5) for critical appraisal.
| O for p or t | Author 1 | Study Design 2 | p 3 | t 3 | Intervention | Outcome Measures | Result/Clinical Experience (CE) 4 |
|---|---|---|---|---|---|---|---|
| Phytotherapy | Arslan et al. 2020 [17] | RCT (n = 60) | √ | - | Henna application | CIPN assessment tool | Significant beneficial effect. Low cost, safe intervention, and well tolerated by patients. |
| Fallon et al. 2015 in S3 clinical guideline Supportive therapy [37] | Proof of concept study | - | √ | Application of menthol crème 1% | Brief Pain Inventory (BPI), Quantitative Sensory Testing | Significant reduction in pain symptoms. | |
| Izgu et al. 2019 [101] | Pilot RCT (n = 46) | - | √ | Aroma hand and foot massage. | Neuropathic symptoms, numeric rating scale | Significant lower severity of pain based on NRS. | |
| Li et al. 2019 [35] | Meta-analysis | √ | √ | All types of Chinese herbal medicine in TCM | CIPN grade, Levi’s grade | Improvement of sensory nerve conduction velocity and motor nerve conduction velocity. | |
| Noh et al. 2018 [36] | Syst. Review of RCTs (n = 28) | √ | √ | All types of Phy used for medicinal purposes | Clinical improvement, nerve conduction study (NCS) | Potentially preventive and/or therapeutic effects for CIPN | |
| Noh and Park 2019 [50] | RCT (n = 31) | - | √ | Aroma foot reflexology | CIPN assessment tool | Statistically significant reduction of symptoms. | |
| Rostami et al. 2019 [75] | RCT (n = 34) | - | √ | Topical c. colocynthis oil | Functional Assessment of Cancer Therapy (FACT), Neurotoxicity score | Failed to improve the symptoms of CIPN compared with placebo. | |
| Consensus process | N/A | √ | √ | Aconit oil application | Clinical improvement | CE 3 | |
| Consensus process | N/A | √ | Solum oil application | Clinical improvement | CE 1 | ||
| Consensus process | N/A | √ | √ | Flaxseed bath | Clinical improvement | CE 4 | |
| Consensus process | N/A | √ | √ | Arnica comp/Formica oil application | Clinical improvement | CE 3 | |
| Consensus process | N/A | - | √ | Arnica comp/Formica ointment (for stronger effect of Aconit) | Clinical improvement | CE 3–4 | |
| Consensus process | N/A | - | √ | Rosemary ointment | Clinical improvement | CE 3–4 | |
| Consensus process | N/A | - | √ | Peppermint oil application for heat sensations and paraesthesia | Clinical improvement | CE2 | |
| Consensus process | N/A | - | √ | Eucalyptus oil application for heat sensations and paraesthesia | Clinical improvement | CE 2 | |
| Movement therapies | Andersen et al. 2020 [38] | Single-blind ex-ploratory RCT (n = 48) | √ | √ | Physical therapy | Patient questionnaires, quantitative sensory testing | Improvement of CIPN pain for patients with breast cancer. Correlation to preservation of sensory function. |
| Brami et al. 2016 [16] | Systematic review of RCTs (n = 13) | - | √ | Physical activity | Nerve conduction velocity, (NCV), Neurological Symptom Score, Total Neuropathy Score, QoL | Evidence was reported for interventions consisting of physical activity components; for strength and endurance training; and for multimodal self-help strategies including physical activity, yoga, and mindfulness. | |
| Fernandes and Kumar 2016 [69] | Single-group pre-post prospective study (n = 25) | - | √ | Closed kinematic chain exercise | Modified Total Neuropathy Score (mTNS), Berg Balance Score (BBS) | Significant change in values before and after the exercise. | |
| Kanzawa-Lee et al. 2020 [54] | Comprehensive inte-grative review(7 RCTs, 6 quasi-experimental studies) | - | √ | Exercise with Aerobic, strength training, and balance training | CIPN, balance, and fitness | Empirical evidence is insufficient to definitively conclude that exercise interventions ameliorate CIPN. | |
| Kleckner et al. 2018 [48] | Secondary analysis of a phase III RCT (n = 355) | - | √ | EXCAP©® a standardized, individualized, moderate-intensity, home-based, six-week progressive walking and resistance exercise program | Patient-reported CIPN symptoms | Reduction of CIPN symptoms (hot/coldness in hands/feet, numbness, and tingling). | |
| McCrary et al. 2019 [84] | Prospective pilot intervention study, single group pre-post design (n = 35) | - | √ | 8-week multimodal exercises (resistance, balance, cardiovascular training) | Total Neuropathy Score—clinical version (TNSc), EORTC CIPN-20, functional assessment tools, disability, and QoL | Reduction of CIPN symptoms and related functional and quality of life deficits. No changes in sensory or motor neurophysiologic parameters. | |
| Schönsteiner et al. 2017 [89] | Randomized exploratory phase 2 study (n = 131) | - | √ | Whole-body vibration including massage, passive mobilization, and physical exercise. | Functional Assessment of Cancer Therapy/Gynecologic Oncology Group neurotoxicity subscale (FACT/GOG-NTX), EORTC QLQ-C30 Quantitative sensory testing (QST) | Significantly and clinically relevant beneficial impact on symptom relief, physical fitness, and sensory function. | |
| Schwenk et al. 2016 [90] | Single blinded, randomized controlled pilot study (n = 22) | - | √ | Interactive motor adaptation balance training program | VPT score, numeric rating scale for pain (NRS), neuropathy-related numbness in feet (NRS score), Short-Form Health Survey (SF-12), Falls, Efficacy Scale-International (FES-I) | Significant reductions in postural sway parameters in challenging semi-tandem position. No significant changes were noted for balance with “eyes closed”, gait speed, and fear of falling. | |
| Steinmann et al. 2011 in S3 clinical guidelineS3 Guideline Supportive therapy 2020 [37] | Overview article | √ | √ | Tactile Stimulation (e.g., been bath) | Clinical improvement | 81% of patients consider tactile stimulation to be very effective or effective. | |
| Streckmann, Kneis et al. 2014 in S3 Guideline Supportive therapy 2020 [37] | RCT (n = 62) | - | √ | Exercise (sensorimotor training, endurance, strength) | QOL; coordination, endurance, strength, therapy-induced side-effects. | Due to the highly significant physiological parameters, the study was terminated prematurely. | |
| Streckmann, Zopf et al. 2014 [60] | Systematic review of RCTs (n = 10), CCT (n = 8) | - | √ | Exercise interventions | Side effects of Polyneuropathy | Number of patients with reduced deep sensitivity could be diminished. Only one RCT related to CIPN. | |
| S3 Guideline Supportive therapy 2020 [37] | S3 Guideline | - | √ | Non-drug methods | Not described | Sensorimotor training and whole-body vibration represent new options for CIPN treatment. Clear evidence of improvement of functional limitation through non-medicinal procedures such as sports therapy, occupational therapy, physiotherapy, and physical therapy including electrotherapy. | |
| Tofthagen et al. 2012 [96] | Review of RCTs (n = 10), single-arm study (n = 1), cross-over-study (n = 1), quasi-experimental study (n = 1) | - | √ | Strength training and balance training | Neuropathy symptoms, strength, balance | Recommendation of physical therapy as a treatment option, but no studies were identified that evaluate strength training and balance training for treatment of CIPN. | |
| Zimmer et al. 2018 [94] | RCT (n = 30) | - | √ | Multimodal exercise program, (endurance, resistance, balance, coordination) | Trial Outcome Index (TOI),NCI-CTC/FACT/GOG-NTX | Regarding CIPN (TOI), there were significant differences between groups in the main analysis. | |
| Consensus process | N/A | - | √ | Sugar oil peeling | Clinical improvement | CE 3 | |
| Consensus process | N/A | √ | √ | Tactile stimulation | Clinical improvement | CE 2–3 | |
| Mind-body therapies | Brami et al. 2016 [16] | Systematic review of RCTs (n = 13) | - | √ | Mind-Body modalities | NCV, Neurological Symptom Score, Total Neuropathy Score, QoL | Evidence was reported for self-management strategies including yoga and mindfulness. |
| Galantino et al. 2019 [80] | Open-label, single-arm, feasibility trial | - | √ | Yoga, Meditation | Functional Reach, Timed Up and Go, Patient Neurotoxicity Questionnaire (PNQ), (FACT-GOG-NTX) | Significant improvements were found in flexibility, balance, and fall risk. | |
| Kanzawa-Lee et al. 2020 [54] | Comprehensive inte-grative review(7 RCTs, 6 quasi-experimental studies) | - | √ | Yoga, exercises | CIPN, balance, and fitness | Empirical evidence is insufficient to definitively conclude that exercise interventions ameliorate CIPN. | |
| Nutritional therapy | Brami et al. 2016 [16] | Systematic review of RCTs (n = 13) | √ | √ | Glutamine, Goshajinkigan, vitamin E, Omega 3, Acetyl-l-carnitine, Alpha-lipoic-acid | NCV, Neurological Symptom Score, Total Neuropathy Score, QoL | Vitamin E, Glutamine, Goshajinkigan, and Omega-3 may prevent CIPN. Acetyl-l-carnitine may worsen CIPN; Alpha-lipoic-acid activity is unknown. |
| Greenlee et al. 2017 [42] | Clinical practice guideline based on a systematic literature review of RCTs. | √ | √ | Omega-3, fatty acids, vitamin E, alpha-lipoic acid, dietary modification | - | Acetyl-l carnitine is not recommended to prevent CIPN. Insufficient evidence that Omega-3, fatty acids, and vitamin E help to reduce neuropathy. | |
| Rostock et al. 2013 [88] | Four arm RCT (n = 60) | - | √ | Vitamin B complex | Detailed questionnaire, NRS | Positive effects. No statistically significant results. | |
| Hydrotherapy | Consensus process | N/A | - | √ | Alkaline bath for hand/foot, then Aconit oil application | Clinical improvement | CE 3 |
| Consensus process | N/A | - | √ | Cold knee and/or arm showers | Clinical improvement | CE 3 | |
| Acupuncture/Acupressure | Brami et al. 2016 [16] | Systematic review of RCTs (n = 13) | - | √ | Electroacupuncture | NCV, Neurological Symptom Score, Total Neuropathy Score, QoL | Not superior to placebo. |
| Deng et al. 2013 [53] | Systematic review of meta-analyses (n = 4), syst. Reviews (n = 14), RCT (n = 16) | - | √ | Acupuncture | VAS, neuropathy symptoms, QoL. | Some improvement regarding VAS and neuropathy symptoms. | |
| Donald et al. 2011 [68] | Retrospective Evaluation (n = 18) | - | √ | Acupuncture | CIPN symptoms. | 82% (n = 14) reported improvement of neuropathy symptoms. | |
| Greenlee et al. 2017 [42] | Clinical practice guideline based on a systematic literature review of RCTs. | √ | √ | Acupuncture, electroacupuncture | - | Insufficient evidence that electroacupuncture help to reduce neuropathy. | |
| Rostock et al. 2013 [88] | Four arm RCT (n = 60) | - | √ | Electroacupuncture | Detailed questionnaire, NRS Scale | Positive effects. No statistically significant results. | |
| S3 guideline complementary medicine in the treatment of oncology patients [57] | S3 guideline | - | √ | Acupuncture, electroacupuncture | BPI, Total Neuropathy Score, NCS, Functional Assessment, QoL. | Data are available from a meta-analysis and two RCTs on the efficacy of A- for CIPN. | |
| Wong et al. 2016 [93] | Prospective phase 2 study (n = 40) | - | √ | Acupuncture like TENS | Numbness score, mTNS, Edmonton Symptoms Assessment Scale (ESAS) | Statistically significant difference at 6 months from the baseline pain score. | |
| Cryotherapy | Bandla et al. 2020 [18] | Proof-of-concept study (n = 26) | √ | - | Cryocompression | Total neuropathy score (TNS), NCS | Potentially improve efficacy of preventing CIPN. Safe and tolerable. |
| Beijers et al. 2020 [39] | RCT (n = 180) | √ | - | Frozen glove and sock | CIPN20 | Significant reduction of CIPN symptoms. Dropout of one-third of patients. | |
| Griffiths et al. 2018 [19] | RCT (n = 29) | √ | - | Frozen glove and sock | Neuropathic Pain Symptom Inventory, BPI. | No significant differences in neuropathy or pain. Drop-out rate, more than 50 %. | |
| Sundar et al. 2017 [40] | Prospective pilot study (n = 20) | √ | - | Continuous-flow limb hypothermia. | Visual analog scale (VAS), subjective tolerance scale, NCS, | No significant difference in NCS. Well tolerated by all patients. | |
| Consensus process | N/A | √ | - | Frozen gloves and socks | Clinical improvement | Cannot be assessed. | |
| Manipulative therapies | Brami et al. 2016 [16] | Systematic review of RCTs (n = 13)) | - | √ | Massage, touch therapy | MD Anderson Symptom Inventory | Greatly reduced CIPN symptoms from grade 2 to 1 and significantly improved quality of life. |
| Cunningham et al. 2011 [74] | Case report | - | √ | Massage | MD Anderson Symptom Inventory | Greatly reduced CIPN symptoms from grade 2 to 1 and significantly improved quality of life. | |
| Izgu et al. 2019 [41] | RCT (n = 40) | √ | Massage | Self-Leeds Assessment of Neuropathic Symptoms and Sign (S-LANSS), EORCT QLQ CIPN20, NCS. | Massage successfully prevented CIPN, improved the QoL, and showed beneficial effects on the NCS findings. | ||
| Sarisoy, et al. 2020 [76] | RCT (n = 40) | - | √ | Foot-massage | VAS, Doleur Neuropatique/Neuropatic pain (DN4), Pittsburg Sleep Quality Index (PSQI) | Positive effect on CIPN pain. | |
| Schönsteiner et al. 2017 [89] | Randomized exploratory phase 2 study (n = 131) | - | √ | Whole-body vibration including massage, passive mobilization, and physical exercise. | (FACT/GOG-NTX), EORTC QLQ-C30 Quantitative sensory testing (QST) | Significantly and clinically relevant beneficial impact on symptoms relieve, physical fitness, and sensory function. | |
| Rhytmical embrocations | Consensus process | N/A | - | √ | Aconit oil—rhythmical embrocation | Clinical improvement | CE 4 |
| Consensus process | N/A | - | √ | Arnica comp/Formica oil—rhythmical embrocation | Clinical improvement | CE 4 | |
| TENS/Scrambler therapy | Coyne et al. 2013 [67] | Expanded trial, single arm trial (n = 39) | - | √ | Scrambler therapy | NRS, BPI, European Organization for Treatment and Cancer CIPN20 (EORTCCIPN20) | Clinically important and statistically significant improvements were seen in average, least, and worst pain. |
| Gewandter et al. 2019 [65] | Single-arm study (n = 29) | - | √ | TENS | EORTC-CIPN20, Utah Early Neuropathy Score | Significant improvements were observed with the EORTC-CIPN20. | |
| Loprinzi et al. 2020 [71] | RCT, two arm phase II pilot trial (n = 50). | - | √ | Scrambler therapy, TENS | EORTC CIPN20, NAS questionnaire regarding CIPN-associated pain | Scrambler therapy improves CIPN symptoms more than TENS. | |
| Other supportive interventions | Kotani et al. 2021 [43] | Double-blind phase 2 trial (n = 56) | √ | - | Compression | Incidence of Grade ≥ 2 CIPN. | No significant reduction of CIPN incidence. |
| Rostock et al. 2013 [88] | Four arm RCT (n = 60) | - | √ | Hydroelectric bath | Detailed questionnaire, NRS | Positive effects. No statistically significant results. | |
| Consensus process | N/A | √ | - | Compression | Clinical improvement | Cannot be assessed. | |
| Consensus process | N/A | - | √ | Copper ointment (0.4%) | Clinical improvement | E 2 |
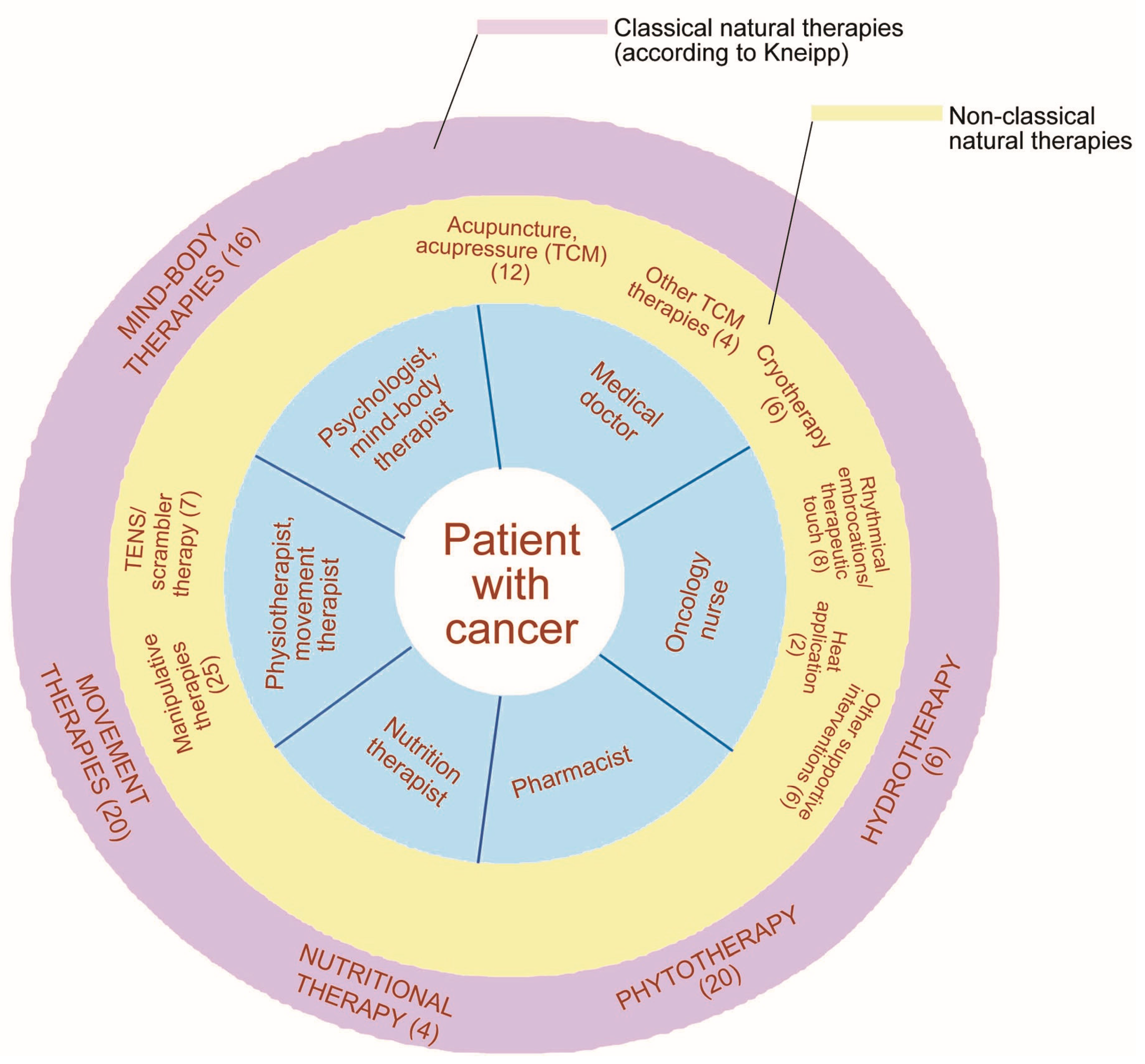
3.5. Manipulative Therapies
Massage, Reflexology, and Foot Reflexology
3.6. Rhythmical Embrocations (Including Healing Touch, Therapeutic Touch, Reiki)
3.7. Phytotherapy (including Herbal Medicines)
Aromatherapy, Aromatherapy Massage, and Aromatherapy Reflexology
3.8. Movement Therapies
3.9. Mind–Body Therapies
3.9.1. Yoga
3.9.2. Distraction Therapies and Relaxation
3.9.3. Additional MBMs
Progressive Muscle Relaxation and Relaxation
Problem-Solving Therapies
3.10. Acupuncture/Acupressure (TCM)
3.11. TENS/Scrambler Therapy
3.11.1. TENS
3.11.2. Scrambler Therapy
3.12. Conceptual Therapeutic Approach
3.13. Side Effects and/or Interactions
4. Discussion
4.1. Interprofessional Teamwork
4.2. Challenges of Categorizing Non-Pharmacological Therapies
4.3. Further Integration of Non-Pharmacological Therapies in the Healthcare System
4.4. Directions for Future Research
4.5. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hou, S.; Huh, B.; Kim, H.K.; Kim, K.-H.; Abdi, S. Treatment of Chemotherapy-Induced Peripheral Neuropathy: Systematic Review and Recommendations. Pain Physician 2018, 21, 571–592. [Google Scholar] [PubMed]
- Colvin, L.A. Chemotherapy-induced peripheral neuropathy: Where are we now? Pain 2019, 160 (Suppl. 1), S1–S10. [Google Scholar] [CrossRef] [PubMed]
- Areti, A.; Yerra, V.G.; Komirishetty, P.; Kumar, A. Potential Therapeutic Benefits of Maintaining Mitochondrial Health in Peripheral Neuropathies. Curr. Neuropharmacol. 2016, 14, 593–609. [Google Scholar] [CrossRef] [Green Version]
- Kolb, N.A.; Smith, A.G.; Singleton, J.R.; Beck, S.L.; Stoddard, G.J.; Brown, S.; Mooney, K. The Association of Chemotherapy-Induced Peripheral Neuropathy Symptoms and the Risk of Falling. JAMA Neurol. 2016, 73, 860–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molassiotis, A.; Cheng, H.L.; Lopez, V.; Au, J.S.K.; Chan, A.; Bandla, A.; Leung, K.T.; Li, Y.C.; Wong, K.H.; Suen, L.K.P.; et al. Are we mis-estimating chemotherapy-induced peripheral neuropathy? Analysis of assessment methodologies from a prospective, multinational, longitudinal cohort study of patients receiving neurotoxic chemotherapy. BMC Cancer 2019, 19, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staff, N.P.; Grisold, A.; Grisold, W.; Windebank, A.J. Chemotherapy-induced peripheral neuropathy: A current review. Ann. Neurol. 2017, 81, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Bandos, H.; Melnikow, J.; Rivera, D.R.; Swain, S.M.; Sturtz, K.; Fehrenbacher, L.; Wade, J.L.; Brufsky, A.M.; Julian, T.B.; Margolese, R.G.; et al. Long-term Peripheral Neuropathy in Breast Cancer Patients Treated with Adjuvant Chemotherapy: NRG Oncology/NSABP B-30. J. Natl. Cancer Inst. 2018, 110, djx162. [Google Scholar] [CrossRef] [Green Version]
- Zajączkowska, R.; Kocot-Kępska, M.; Leppert, W.; Wrzosek, A.; Mika, J.; Wordliczek, J. Mechanisms of Chemotherapy-Induced Peripheral Neuropathy. Int. J. Mol. Sci. 2019, 20, 1451. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.M.L.; Pang, H.; Cirrincione, C.; Fleishman, S.; Paskett, E.D.; Ahles, T.; Bressler, L.R.; Fadul, C.E.; Knox, C.; Le-Lindqwister, N.; et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: A randomized clinical trial. JAMA 2013, 309, 1359–1367. [Google Scholar] [CrossRef]
- Hershman, D.L.; Lacchetti, C.; Dworkin, R.H.; Smith, E.M.L.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Gavin, P.; Lavino, A.; Lustberg, M.B.; et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 2014, 32, 1941–1967. [Google Scholar] [CrossRef]
- Boon, H.S.; Olatunde, F.; Zick, S.M. Trends in complementary/alternative medicine use by breast cancer survivors: Comparing survey data from 1998 and 2005. BMC Womens Health 2007, 7, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keene, M.R.; Heslop, I.M.; Sabesan, S.S.; Glass, B.D. Complementary and alternative medicine use in cancer: A systematic review. Complement. Ther. Clin. Pract. 2019, 35, 33–47. [Google Scholar] [CrossRef]
- Buckner, C.A.; Lafrenie, R.; Dénommée, J.A.; Caswell, J.M.; Want, D.A. Complementary and alternative medicine use in patients before and after a cancer diagnosis. Curr. Oncol. 2018, 25, e275–e281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teoh, D.; Smith, T.J.; Song, M.; Spirtos, N.M. Care After Chemotherapy: Peripheral Neuropathy, Cannabis for Symptom Control, and Mindfulness. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 469–479. [Google Scholar] [CrossRef]
- Bardia, A.; Barton, D.L.; Prokop, L.J.; Bauer, B.A.; Moynihan, T.J. Efficacy of complementary and alternative medicine therapies in relieving cancer pain: A systematic review. J. Clin. Oncol. 2006, 24, 5457–5464. [Google Scholar] [CrossRef] [PubMed]
- Brami, C.; Bao, T.; Deng, G. Natural products and complementary therapies for chemotherapy-induced peripheral neuropathy: A systematic review. Crit. Rev. Oncol. Hematol. 2016, 98, 325–334. [Google Scholar] [CrossRef] [Green Version]
- Arslan, S.; Bahceli, P.Z.; Ilik, Y.; Artaç, M. The preliminary effects of henna on chemotherapy-induced peripheral neuropathy in women receiving oxaliplatin-based treatment: A parallel-group, randomized, controlled pilot trial. Eur. J. Oncol. Nurs. 2020, 48, 101827. [Google Scholar] [CrossRef]
- Bandla, A.; Tan, S.; Kumarakulasinghe, N.B.; Huang, Y.; Ang, S.; Magarajah, G.; Hairom, Z.; Lim, J.S.J.; Wong, A.; Chan, G.; et al. Safety and tolerability of cryocompression as a method of enhanced limb hypothermia to reduce taxane-induced peripheral neuropathy. Support. Care Cancer 2020, 28, 3691–3699. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, C.; Kwon, N.; Beaumont, J.L.; Paice, J.A. Cold therapy to prevent paclitaxel-induced peripheral neuropathy. Support. Care Cancer 2018, 26, 3461–3469. [Google Scholar] [CrossRef]
- Stolz, R.; Klafke, N.; Kröger, B.; Boltenhagen, U.; Kaltenbach, A.; Heine, R.; Idler, C.; Layer, M.; Kohler, S.; Winkler, M. Creating evidence for naturopathic nursing interventions in oncology—A systematic approach. Z Evid. Fortbild. Qual. Gesundhwes. 2021, 166, 1–7. [Google Scholar] [CrossRef]
- Steinmann, D.; Babadağ Savaş, B.; Felber, S.; Joy, S.; Mertens, I.; Cramer, H.; Paul, A.; Layer, M.; Klafke, N.; Stolz, R. Nursing Procedures for the Prevention and Treatment of Mucositis Induced by Cancer Therapies: Clinical Practice Guideline Based on an Interdisciplinary Consensus Process and a Systematic Literature Search. Integr. Cancer Ther. 2021, 20, 1534735420940412. [Google Scholar] [CrossRef] [PubMed]
- Sackett, D.L.; Rosenberg, W.; Gray, J.A.M.; Haynes, R.B.; Richardson, W.S. Evidence based medicine: What it is and what it isn’t. BMJ 1996, 312, 71–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CEBM. Oxford Centre for Evidence-Based Medicine: Levels of Evidence (March 2009). Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009 (accessed on 18 July 2022).
- Assaf, C.; Booken, N.; Dippel, E.; Guenova, E.; Jonak, C.; Klemke, C.D.; Nicolay, J.P.; Schlaak, M.; Wobser, M.; Trautinger, F. Chlormethin-Gel zur Behandlung der Mycosis fungoides: Ein Expertenkonsens aus Deutschland, Österreich und der Schweiz (DACH-Region) zum Therapiemanagement. J. Dtsch. Dermatol. Ges. 2022, 20, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef] [Green Version]
- Peters, M.; Godfrey, C.; McInerney, P.; Munn, Z.; Tricco, A.C.; Khalil, H. Chapter 11: Scoping Reviews (2020 Version). In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; JBI: Adelaide, Australia, 2020; Available online: https://synthesismanual.jbi.global (accessed on 18 July 2022).
- National Center for Complementary and Integrative Health. Complementary, Alternative, or Integrative Health: What’s in a Name? 2021. Available online: https://www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a-name (accessed on 4 November 2022).
- Witt, C.M.; Balneaves, L.G.; Cardoso, M.J.; Cohen, L.; Greenlee, H.; Johnstone, P.; Kücük, .; Mailman, J.; Mao, J.J. A Comprehensive Definition for Integrative Oncology. J. Natl. Cancer Inst. Monogr. 2017, 2017, lgx012. [Google Scholar] [CrossRef] [Green Version]
- Stolz, R.; Klafke, N. Complementary Nursing Procedures to Treat Polyneuropathy in Cancer Patients Undergoing Chemotherapy. 2020. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020165851 (accessed on 2 February 2020).
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [Green Version]
- Critical Appraisal Skills Programme. CASP (Randomised Controlled Trials Checklist, Systematic Review Checklist, Qualitative Studies Checklist, Cohort Study Checklist). 2019. Available online: https://casp-uk.net/casp-tools-checklists/ (accessed on 18 July 2022).
- AWMF. AWMF-Regelwerk Leitlinien: Strukturierte Konsensfindung. 2022. Available online: https://www.awmf.org/leitlinien/awmf-regelwerk/leitlinien-entwicklung/awmf-regelwerk-03-leitlinienentwicklung/ll-entwicklung-strukturierte-konsensfindung.html (accessed on 18 July 2022).
- Li, Z.; Jin, H.; Yan, Q.; Sun, L.; Wasan, H.S.; Shen, M.; Ruan, S. The Method of Activating Blood and Dredging Collaterals for Reducing Chemotherapy-Induced Peripheral Neuropathy: A Systematic Review and Meta-Analysis. Evid. Based Complement. Alternat. Med. 2019, 2019, 1029626. [Google Scholar] [CrossRef] [Green Version]
- Noh, H.; Yoon, S.; Park, B. A Systematic Review of Herbal Medicine for Chemotherapy Induced Peripheral Neuropathy. Evid. Based Complement. Alternat. Med. 2018, 2018, 6194184. [Google Scholar] [CrossRef]
- AWMF. Leitlinienprogramm Onkologie der Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften e.V. (AWMF), Deutschen Krebsgesellschaft e.V. (DKG) und Deutschen Krebshilfe (DKH). Supportive Therapie bei onkologischen PatientInnen—Langversion 1.3. 2020. Available online: https://register.awmf.org/assets/guidelines/032-054OLl_S3_Supportiv_2020-07-abgelaufen.pdf (accessed on 22 December 2022).
- Andersen Hammond, E.; Pitz, M.; Steinfeld, K.; Lambert, P.; Shay, B. An Exploratory Randomized Trial of Physical Therapy for the Treatment of Chemotherapy-Induced Peripheral Neuropathy. Neurorehabilit. Neural Repair 2020, 34, 235–246. [Google Scholar] [CrossRef]
- Beijers, A.; Bonhof, C.; Mols, F.; Ophorst, J.; de Vos-Geelen, J.; Jacobs, E.; van de Poll-Franse, L.; Vreugdenhil, G. Multicenter randomized controlled trial to evaluate the efficacy and tolerability of frozen gloves for the prevention of chemotherapy-induced peripheral neuropathy. Ann. Oncol. 2020, 31, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Sundar, R.; Bandla, A.; Tan, S.S.H.; Liao, L.-D.; Kumarakulasinghe, N.B.; Jeyasekharan, A.D.; Ow, S.G.W.; Ho, J.; Tan, D.S.P.; Lim, J.S.J.; et al. Limb hypothermia for preventing paclitaxel-induced peripheral neuropathy in breast cancer patients: A pilot study. Front. Oncol. 2017, 6, 274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izgu, N.; Metin, Z.G.; Karadas, C.; Ozdemir, L.; Çetin, N.; Demirci, U. Prevention of chemotherapy-induced peripheral neuropathy with classical massage in breast cancer patients receiving paclitaxel: An assessor-blinded randomized controlled trial. Eur. J. Oncol. Nurs. 2019, 40, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, H.; DuPont-Reyes, M.J.; Rn, L.G.B.; Carlson, L.E.; Cohen, M.R.; Deng, G.; Johnson, J.A.; Mumber, M.; Seely, D.; Zick, S.M.; et al. Clinical practice guidelines on the evidence-based use of integrative therapies during and after breast cancer treatment. CA A Cancer J. Clin. 2017, 67, 194–232. [Google Scholar] [CrossRef] [PubMed]
- Kotani, H.; Terada, M.; Mori, M.; Horisawa, N.; Sugino, K.; Kataoka, A.; Adachi, Y.; Gondou, N.; Yoshimura, A.; Hattori, M.; et al. Compression therapy using surgical gloves does not prevent paclitaxel-induced peripheral neuropathy: Results from a double-blind phase 2 trial. BMC Cancer 2021, 21, 548. [Google Scholar] [CrossRef] [PubMed]
- Volger, E.; Brinkhaus, B. Einteilung der Naturheilverfahren. In Kursbuch Naturheilverfahren für die Ärztliche Weiterbildung; Volger, E., Brinkhaus, B., Eds.; Urban & Fischer: Munich, Germany, 2017; pp. 5–6. [Google Scholar]
- Dikmen, H.A.; Terzioglu, F. Effects of reflexology and progressive muscle relaxation on pain, fatigue, and quality of life during chemotherapy in gynecologic cancer patients. Pain Manag. Nurs. 2019, 20, 47–53. [Google Scholar] [CrossRef]
- Dyer, J.; Thomas, K.; Sandsund, C.; Shaw, C. Is reflexology as effective as aromatherapy massage for symptom relief in an adult outpatient oncology population? Complement. Ther. Clin. Pract. 2013, 19, 139–146. [Google Scholar] [CrossRef]
- Gok Metin, Z.; Arikan Donmez, A.; Izgu, N.; Ozdemir, L.; Arslan, I.E. Aromatherapy massage for neuropathic pain and quality of life in diabetic patients. J. Nurs. Scholarsh. 2017, 49, 379–388. [Google Scholar] [CrossRef]
- Kleckner, I.R.; Kamen, C.; Gewandter, J.S.; Mohile, N.A.; Heckler, C.E.; Culakova, E.; Fung, C.; Janelsins, M.C.; Asare, M.; Lin, P.-J. Effects of exercise during chemotherapy on chemotherapy-induced peripheral neuropathy: A multicenter, randomized controlled trial. Support. Care Cancer 2018, 26, 1019–1028. [Google Scholar] [CrossRef]
- Kutner, J.S.; Smith, M.C.; Corbin, L.; Hemphill, L.; Benton, K.; Mellis, B.K.; Beaty, B.; Felton, S.; Yamashita, T.E.; Bryant, L.L. Massage therapy versus simple touch to improve pain and mood in patients with advanced cancer [with consumer summary]. Ann. Intern. Med. 2008, 149, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Noh, G.O.; Park, K.S. Effects of aroma self-foot reflexology on peripheral neuropathy, peripheral skin temperature, anxiety, and depression in gynaecologic cancer patients undergoing chemotherapy: A randomised controlled trial. Eur. J. Oncol. Nurs. 2019, 42, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Boyd, C.; Crawford, C.; Paat, C.F.; Price, A.; Xenakis, L.; Zhang, W.; Group, E.f.M.T.W. The impact of massage therapy on function in pain populations—A systematic review and meta-analysis of randomized controlled trials: Part II, cancer pain populations. Pain Med. 2016, 17, 1553–1568. [Google Scholar] [CrossRef]
- Buffart, L.M.; Van Uffelen, J.G.Z.; I Riphagen, I.; Brug, J.; Van Mechelen, W.; Brown, W.J.; Chinapaw, M.J.M. Physical and psychosocial benefits of yoga in cancer patients and survivors, a systematic review and meta-analysis of randomized controlled trials. BMC Cancer 2012, 12, 559. [Google Scholar] [CrossRef] [Green Version]
- Deng, G.E.; Rausch, S.M.; Jones, L.W.; Gulati, A.; Kumar, N.B.; Greenlee, H.; Pietanza, M.C.; Cassileth, B.R. Complementary therapies and integrative medicine in lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143 (Suppl. 5), e420S–e436S. [Google Scholar] [CrossRef] [PubMed]
- Kanzawa-Lee, G.A.; Larson, J.L.; Resnicow, K.; Smith, E.M.L. Exercise Effects on Chemotherapy-Induced Peripheral Neuropathy: A Comprehensive Integrative Review. Cancer Nurs. 2020, 43, E172–E185. [Google Scholar] [CrossRef]
- Kim, J.-I.; Lee, M.S.; Lee, D.-H.; Boddy, K.; Ernst, E. Cupping for treating pain: A systematic review. Evid. Based Complement. Altern. Med. 2011, 2011, 467014. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-H.; Kim, J.-Y.; Jong-Yeop, K.; Kim, S.; Lim, S. Meta-analysis of massage therapy on cancer pain. Integr. Cancer Ther. 2015, 14, 297–304. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.I.; Scherer, R.W.; Snyder, C.; Geigle, P.M.; Berlanstein, D.; Topaloglu, O. Exercise interventions on health-related quality of life for cancer survivors (Cochrane review) [with consumer summary]. Cochrane Database Syst. Rev. 2012, 8, CD007566. [Google Scholar]
- Pan, Y.Q.; Yang, K.H.; Wang, Y.L.; Zhang, L.P.; Liang, H.Q. Massage interventions and treatment-related side effects of breast cancer: A systematic review and meta-analysis. Int. J. Clin. Oncol. 2013, 19, 829–841. [Google Scholar] [CrossRef]
- Stier-Jarmer, M.; Throner, V.; Kirschneck, M.; Frisch, D.; Schuh, A. Effekte der Kneipp-Therapie: Ein systematischer Review der aktuellen wissenschaftlichen Erkenntnisse (2000–2019). Complement. Med. Res. 2021, 28, 146–159. [Google Scholar] [CrossRef]
- Streckmann, F.; Zopf, E.M.; Lehmann, H.C.; May, K.; Rizza, J.; Zimmer, P.; Gollhofer, A.; Bloch, W.; Baumann, F.T. Exercise intervention studies in patients with peripheral neuropathy: A systematic review. Sports Med. 2014, 44, 1289–1304. [Google Scholar] [CrossRef] [PubMed]
- Van Vu, D.; Molassiotis, A.; Ching, S.S.Y.; Le, T.T. Effects of Qigong on symptom management in cancer patients: A systematic review. Complement. Ther. Clin. Pract. 2017, 29, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Wayne, P.M.; Lee, M.; Novakowski, J.; Osypiuk, K.; Ligibel, J.; Carlson, L.; Song, R. Tai Chi and Qigong for cancer-related symptoms and quality of life: A systematic review and meta-analysis. J. Cancer Surviv. 2018, 12, 256–267. [Google Scholar] [CrossRef] [PubMed]
- AWMF. Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): Komplementärmedizin in der Behandlung von onkologischen PatientInnen, Langversion 1.1. Available online: https://www.leitlinienprogramm-onkologie.de/fileadmin/user_upload/Downloads/Leitlinien/Komplement%C3%A4r/Version_1/LL_Komplement%C3%A4r_Langversion_1.0.pdf (accessed on 22 December 2022).
- Möller, U.O.; Beck, I.; Rydén, L.; Malmström, M. A comprehensive approach to rehabilitation interventions following breast cancer treatment—A systematic review of systematic reviews. BMC Cancer 2019, 19, 472. [Google Scholar]
- Gewandter, J.S.; Chaudari, J.; Ibegbu, C.; Kitt, R.; Serventi, J.; Burke, J.; Culakova, E.; Kolb, N.; Sluka, K.A.; Tejani, M.A.; et al. Wireless transcutaneous electrical nerve stimulation device for chemotherapy-induced peripheral neuropathy: An open-label feasibility study. Support. Care Cancer 2019, 27, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Deutsches Netzwerk für Qualitätsentwicklung in der Pflege (DNQP). Expertenstandard Schmerzmanagement in der Pflege. In Schriftenreihe des Deutschen Netzwerks für Qualitätsentwicklung in der Pflege; DNQP: Osnabrück, Germany, 2020. [Google Scholar]
- Coyne, P.J.; Wan, W.; Dodson, P.; Swainey, C.; Smith, T.J. A Trial of Scrambler Therapy in the Treatment of Cancer Pain Syndromes and Chronic Chemotherapy-Induced Peripheral Neuropathy. J. Pain Palliat. Care Pharmacother. 2013, 27, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Donald, G.K.; Tobin, I.; Stringer, J. Evaluation of acupuncture in the management of chemotherapy-induced peripheral neuropathy. Acupunct. Med. 2011, 29, 230–233. [Google Scholar] [CrossRef]
- Fernandes, J.; Kumar, S. Effect of lower limb closed kinematic chain exercises on balance in patients with chemotherapy-induced peripheral neuropathy: A pilot study. Int. J. Rehabil. Res. 2016, 39, 368–371. [Google Scholar] [CrossRef]
- Kwekkeboom, K.L. Pain management strategies used by patients with breast and gynecologic cancer with postoperative pain. Cancer Nurs. 2001, 24, 378–386. [Google Scholar] [CrossRef]
- Loprinzi, C.; Le-Rademacher, J.G.; Majithia, N.; McMurray, R.P.; O’Neill, C.R.; Bendel, M.A.; Beutler, A.; Lachance, D.H.; Cheville, A.; Strick, D.M.; et al. Scrambler therapy for chemotherapy neuropathy: A randomized phase II pilot trial. Support. Care Cancer 2020, 28, 1183–1197. [Google Scholar] [CrossRef] [PubMed]
- Ernst, E. Is reflexology an effective intervention? A systematic review of randomised controlled trials. Med. J. Aust. 2009, 191, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Telles, S.; Sayal, N.; Nacht, C.; Chopra, A.; Patel, K.; Wnuk, A.; Dalvi, P.; Bhatia, K.; Miranpuri, G.; Anand, A. Yoga: Can it be integrated with treatment of neuropathic pain? Ann. Neurosci. 2019, 26, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.E.; Kelechi, T.; Sterba, K.; Barthelemy, N.; Falkowski, P.; Chin, S.H. Case report of a patient with chemotherapy-induced peripheral neuropathy treated with manual therapy (massage). Support. Care Cancer 2011, 19, 1473–1476. [Google Scholar] [CrossRef] [PubMed]
- Rostami, N.; Mosavat, S.H.; Heydarirad, G.; Arbab Tafti, R.; Heydari, M. Efficacy of topical Citrullus colocynthis (bitter apple) extract oil in chemotherapy-induced peripheral neuropathy: A pilot double-blind randomized placebo-controlled clinical trial. Phytother. Res. 2019, 33, 2685–2691. [Google Scholar] [CrossRef] [PubMed]
- Sarısoy, P.; Ovayolu, O. The Effect of Foot Massage on Peripheral Neuropathy-Related Pain and Sleep Quality in Patients with Non-Hodgkin’s Lymphoma. Holist. Nurs. Pract. 2020, 34, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, N.; Dalton, J.A.; Carlson, J. The effect of foot reflexology on pain in patients with metastatic cancer. Appl. Nurs. Res. 2003, 16, 284–286. [Google Scholar] [CrossRef]
- Wyatt, G.; Sikorskii, A.; Tesnjak, I.; Frambes, D.; Holmstrom, A.; Luo, Z.; Victorson, D.; Tamkus, D. A randomized clinical trial of caregiver-delivered reflexology for symptom management during breast cancer treatment. J. Pain Symptom Manag. 2017, 54, 670–679. [Google Scholar] [CrossRef] [Green Version]
- Doorenbos, A.; Given, B.; Given, C.; Verbitsky, N.; Cimprich, B.; McCorkle, R. Reducing symptom limitations: A cognitive behavioral intervention randomized trial. Psychooncology 2005, 14, 574–584. [Google Scholar] [CrossRef]
- Galantino, M.L.; Tiger, R.; Brooks, J.; Jang, S.; Wilson, K. Impact of somatic yoga and meditation on fall risk, function, and quality of life for chemotherapy-induced peripheral neuropathy syndrome in cancer survivors. Integr. Cancer Ther. 2019, 18, 1534735419850627. [Google Scholar] [CrossRef]
- Giasson, M.; Bouchard, L. Effect of therapeutic touch on the well-being of persons with terminal cancer. J. Holist. Nurs. 1998, 16, 383–398. [Google Scholar] [CrossRef]
- Koch, B. Evaluation of Efficacy of Home-Based Kneipp Hydrotherapy in Patients with Polyneuropathic Compaints of the Lower Extremities; Charité—Universitätsmedizin Berlin: Berlin, Germany, 2015. [Google Scholar]
- Listing, M.; Reißhauer, A.; Krohn, M.; Voigt, B.; Tjahono, G.; Becker, J.; Klapp, B.F.; Rauchfuß, M. Massage therapy reduces physical discomfort and improves mood disturbances in women with breast cancer. Psychooncology 2009, 18, 1290–1299. [Google Scholar] [CrossRef]
- McCrary, J.M.; Goldstein, D.; Sandler, C.X.; Barry, B.K.; Marthick, M.; Timmins, H.C.; Li, T.; Horvath, L.; Grimison, P.; Park, S.B. Exercise-based rehabilitation for cancer survivors with chemotherapy-induced peripheral neuropathy. Support. Care Cancer 2019, 27, 3849–3857. [Google Scholar] [CrossRef]
- Moore, S.; Corner, J.; Haviland, J.; Wells, M.; Salmon, E.; Normand, C.; Brada, M.; Smith, I. Nurse led follow up and conventional medical follow up in management of patients with lung cancer: Randomised trial. BMJ 2002, 325, 1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostermann, T.; Blaser, G.; Bertram, M.; Michalsen, A.; Matthiessen, P.F.; Kraft, K. Effects of rhythmic embrocation therapy with solum oil in chronic pain patients: A prospective observational study. Clin. J. Pain 2008, 24, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Post-White, J.; Kinney, M.E.; Savik, K.; Gau, J.B.; Wilcox, C.; Lerner, I. Therapeutic massage and healing touch improve symptoms in cancer. Integr. Cancer Ther. 2003, 2, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Rostock, M.; Jaroslawski, K.; Guethlin, C.; Ludtke, R.; Schröder, S.; Bartsch, H.H. Chemotherapy-Induced Peripheral Neuropathy in Cancer Patients: A Four-Arm Randomized Trial on the Effectiveness of Electroacupuncture. Evid. Based Complement. Altern. Med. 2013, 2013, 349653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schönsteiner, S.S.; Bauder Mißbach, H.; Benner, A.; Mack, S.; Hamel, T.; Orth, M.; Landwehrmeyer, B.; Süßmuth, S.D.; Geitner, C.; Mayer-Steinacker, R.; et al. A randomized exploratory phase 2 study in patients with chemotherapy-related peripheral neuropathy evaluating whole-body vibration training as adjunct to an integrated program including massage, passive mobilization and physical exercises. Exp. Hematol. Oncol. 2017, 6, 5. [Google Scholar] [CrossRef] [Green Version]
- Schwenk, M.; Grewal, G.S.; Holloway, D.; Muchna, A.; Garland, L.; Najafi, B. Interactive Sensor-Based Balance Training in Older Cancer Patients with Chemotherapy-Induced Peripheral Neuropathy: A Randomized Controlled Trial. Gerontology 2016, 62, 553–563. [Google Scholar] [CrossRef]
- Uehleke, B.; Wöhling, H.; Stange, R. A prospective “Study by Correspondance” on the effects of Kneipp hydrotherapy in patients with complaints due to peripheral neuropathy. Schweiz. Z. Ganzheitsmed. 2008, 20, 287–291. [Google Scholar] [CrossRef]
- Nagarathna, R.; Rekha, M.; Vanitha, N.; Kodaganuru, G.; Srinath, B.; Vishweshwara, M.; Madhavi, Y.; Basavalingaiah, S.A.; Bilimagga, R.S.; Rao, N.; et al. Effects of yoga on symptom management in breast cancer patients: A randomized controlled trial. Int. J. Yoga 2009, 2, 73–79. [Google Scholar] [CrossRef]
- Wong, R.; Major, P.; Sagar, S. Phase 2 Study of Acupuncture-Like Transcutaneous Nerve Stimulation for Chemotherapy-Induced Peripheral Neuropathy. Integr. Cancer Ther. 2016, 15, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, P.; Trebing, S.; Timmers-Trebing, U.; Schenk, A.; Paust, R.; Bloch, W.; Rudolph, R.; Streckmann, F.; Baumann, F.T. Eight-week, multimodal exercise counteracts a progress of chemotherapy-induced peripheral neuropathy and improves balance and strength in metastasized colorectal cancer patients: A randomized controlled trial. Support. Care Cancer 2018, 26, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Dy, S.M. Evidence-based approaches to pain in advanced cancer. Cancer J. 2010, 16, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Tofthagen, C.; Visovsky, C.; Berry, D.L. Strength and Balance Training for Adults with Peripheral Neuropathy and High Risk of Fall: Current Evidence and Implications for Future Research. Oncol. Nurs. Forum 2012, 39, E416–E424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verhulst, A.L.; Savelberg, H.H.; Vreugdenhil, G.; Mischi, M.; Schep, G. Whole-Body Vibration as a Modality for the Rehabilitation of Peripheral Neuropathies: Implications for Cancer Survivors Suffering from Chemotherapy-Induced Peripheral Neuropathy. Oncol. Rev. 2015, 9, 263. [Google Scholar] [CrossRef] [Green Version]
- Sanft, T.; Denlinger, C.S.; Armenian, S.; Baker, K.S.; Broderick, G.; Demark-Wahnefried, W.; Friedman, D.L.; Goldman, M.; Hudson, M.; Khakpour, N.; et al. NCCN Guidelines Insights: Survivorship, Version 2.2019: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2019, 17, 784–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.-I.; Lee, M.S.; Kang, J.W.; Choi, D.Y.; Ernst, E. Reflexology for the symptomatic treatment of breast cancer: A systematic review. Integr. Cancer Ther. 2010, 9, 326–330. [Google Scholar] [CrossRef]
- Hökkä, M.; Kaakinen, P.; Pölkki, T. A systematic review: Non-pharmacological interventions in treating pain in patients with advanced cancer. J. Adv. Nurs. 2014, 70, 1954–1969. [Google Scholar] [CrossRef]
- Izgu, N.; Ozdemir, L.; Bugdayci, F. Effect of Aromatherapy Massage on Chemotherapy-Induced Peripheral Neuropathic Pain and Fatigue in Patients Receiving Oxaliplatin: An Open Label Quasi-Randomized Controlled Pilot Study. Cancer Nurs. 2019, 42, 139–147. [Google Scholar] [CrossRef]
- Armstrong, T.; Almadrones, L.; Gilbert, M.R. Chemotherapy-induced peripheral neuropathy. Oncol. Nurs. Forum 2005, 32, 305–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jane, S.W.; Wilkie, D.J.; Gallucci, B.B.; Beaton, R.D. Systematic review of massage intervention for adult patients with cancer: A methodological perspective. Cancer Nurs. 2008, 31, E24–E35. [Google Scholar] [CrossRef]
- Aghabati, N.; Mohammadi, E.; Pour Esmaiel, Z. The effect of therapeutic touch on pain and fatigue of cancer patients undergoing chemotherapy. Evid. Based Complement. Altern. Med. 2010, 7, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Grealish, L.; Lomasney, A.; Whiteman, B.J.C.N. Foot massage: A nursing intervention to modify the distressing symptoms of pain and nausea in patients hospitalized with cancer. Cancer Nurs. 2000, 23, 237–243. [Google Scholar] [CrossRef]
- Long, H.A.; French, D.P.; Brooks, J.M. Optimising the value of the critical appraisal skills programme (CASP) tool for quality appraisal in qualitative evidence synthesis. Res. Methods Med. Health Sci. 2020, 1, 31–42. [Google Scholar] [CrossRef]
- Holey, E.A.; Cook, E.M. Evidence-Based Therapeutic Massage E-Book: A Practical Guide for Therapists; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Layer, M. (Ed.) Praxishandbuch Rhythmische Einreibungen nach Wegman/Hauschka; Verlag Hans Huber: Bern, Switzerland, 2014. [Google Scholar]
- Frank, L.S.; Frank, J.L.; March, D.; Makari-Judson, G.; Barham, R.B.; Mertens, W.C. Does therapeutic touch ease the discomfort or distress of patients undergoing stereotactic core breast biopsy? A randomized clinical trial. Pain Med. 2007, 8, 419–424. [Google Scholar] [CrossRef]
- Samarel, N.; Fawcett, J.; Davis, M.M.; Ryan, F.M. Effects of dialogue and therapeutic touch on preoperative and postoperative experiences of breast cancer surgery: An exploratory study. Oncol. Nurs. Forum 1998, 25, 1369–1376. [Google Scholar] [PubMed]
- Tabatabaee, A.; Tafreshi, M.; Rassouli, M.; Aledavood, S.; Majd, H.; Farahmand, A. Effect of therapeutic touch in patients with cancer: A literature review. Med. Arch. 2016, 70, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Büntzel, J.; Micke, O.; Büntzel, J. How to transfer traditional knowledge about medicinal herbs? or TCM plants: A black box for modern oncologists. J Cancer Res. Clin. Oncol. 2021, 147, 351–359. [Google Scholar] [CrossRef]
- Steflitsch, W.; Wolz, D.; Buchbauer, G.; Stadelmann, I. (Eds.) Aromatherapie in Wissenschaft und Praxis; Stadelmann Natur: Wiggensbach, Germany, 2021. [Google Scholar]
- Maßberg, D.; Hatt, H. Human Olfactory Receptors: Novel Cellular Functions Outside of the Nose. Physiol. Rev. 2018, 98, 1739–1763. [Google Scholar] [CrossRef]
- Kontaris, I.; East, B.S.; Wilson, D.A. Behavioral and Neurobiological Convergence of Odor, Mood and Emotion: A Review. Front. Behav. Neurosci. 2020, 14, 35. [Google Scholar] [CrossRef]
- National Center for Complementary and Integrative Health. 2017. Available online: https://www.nccih.nih.gov/health/mind-and-body-practices (accessed on 19 April 2022).
- Danon, N.; Al-Gobari, M.; Burnand, B.; Rodondi, P. Are mind-body therapies effective for relieving cancer-related pain in adults? A systematic review and meta-analysis. Psychooncology 2022, 31, 345–371. [Google Scholar] [CrossRef] [PubMed]
- Carlson, L.E.; Zelinski, E.; Toivonen, K.; Flynn, M.; Qureshi, M.; Piedalue, K.-A.; Grant, R. Mind-body therapies in cancer: What is the latest evidence? Curr. Oncol. Rep. 2017, 19, 67. [Google Scholar] [CrossRef] [PubMed]
- Siemens, W.; Boehlke, C.; Bennett, M.I.; Offner, K.; Becker, G.; Gaertner, J. Transcutaneous electrical nerve stimulation for advanced cancer pain inpatients in specialist palliative care-a blinded, randomized, sham-controlled pilot cross-over trial. Support Care Cancer 2020, 28, 5323–5333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lesenskyj, A.M.C.; Cruciani, R. Optimizing Neuropathic Pain Relief with Scrambler Therapy. Pract. Pain Manag. 2017, 17, 1. [Google Scholar]
- Tomasello, C.; Pinto, R.M.; Mennini, C.; Conicella, E.; Stoppa, F.; Raucci, U. Scrambler therapy efficacy and safety for neuropathic pain correlated with chemotherapy-induced peripheral neuropathy in adolescents: A preliminary study. Pediatr. Blood Cancer 2018, 65, e27064. [Google Scholar] [CrossRef]
- Karri, J.; Marathe, A.; Smith, T.J.; Wang, E.J. The Use of Scrambler Therapy in Treating Chronic Pain Syndromes: A Systematic Review. Neuromodulation 2022, 6, S1094–7159(22)00681-X. [Google Scholar] [CrossRef]
- Höxtermann, M.D.; Haller, H.; Aboudamaah, S.; Bachemir, A.; Dobos, G.; Cramer, H.; Voiss, P. Safety of acupuncture in oncology: A systematic review and meta-analysis of randomized controlled trials. Cancer 2022, 128, 2159–2173. [Google Scholar] [CrossRef]
- Cramer, H.; Lauche, R.; Klose, P.; Lange, S.; Langhorst, J.; Dobos, G.J. Yoga for improving health-related quality of life, mental health and cancer-related symptoms in women diagnosed with breast cancer. Cochrane Database Syst. Rev. 2017, 1, Cd010802. [Google Scholar] [CrossRef] [Green Version]
- Loprinzi, C.L.; Lacchetti, C.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Hertz, D.L.; Kelley, M.R.; Lavino, A.; Lustberg, M.B.; Paice, J.A.; et al. Prevention and Management of Chemotherapy-Induced Peripheral Neuropathy in Survivors of Adult Cancers: ASCO Guideline Update. J. Clin. Oncol. 2020, 38, 3325–3348. [Google Scholar] [CrossRef]
- Klafke, N.; Homberg, A.; Glassen, K.; Mahler, C. Addressing holistic healthcare needs of oncology patients: Implementation and evaluation of a complementary and alternative medicine (CAM) course within an elective module designed for healthcare professionals. BMC Complement. Ther. Med. 2016, 29, 190–195. [Google Scholar] [CrossRef]
- Homberg, A.; Klafke, N.; Loukanova, S.; Glassen, K. Findings from a three-round Delphi study: Essential topics for interprofessional training on complementary and integrative medicine. BMC Complement. Med. Ther. 2020, 20, 348. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Mamgain, R.K.; Mamgain, P.; Verma, S.K.; Pruthi, D.S.; Kandwal, A.; Saini, S. The efficacy of an ayurvedic preparation of yashtimadhu (Glycyrrhiza glabra) on radiation-induced mucositis in head-and-neck cancer patients: A pilot study. J. Cancer Res. Ther. 2020, 16, 458–462. [Google Scholar] [CrossRef]
- Rastogi, S.; Tiwari, V.; Jatav, S.P.; Singh, N.; Verma, S.; Verma, S.; Sharma, K.G.; Pandey, P.; Singh, G. A survey of patients visiting an Ayurvedic teaching hospital for factors influencing the decision to choose ayurveda as a health care provider. J. Ayurveda Integr. Med. 2022, 100539. [Google Scholar] [CrossRef] [PubMed]
- Wortmann, J.K.; Bremer, A.; Eich, H.; Wortmann, H.K.; Schuster, A.; Fühner, J.; Büntzel, J.; Muecke, R.; Prott, F.; Huebner, J. Use of complementary and alternative medicine by patients with cancer: A cross-sectional study at different points of cancer care. Med. Oncol. 2016, 33, 78. [Google Scholar] [CrossRef] [PubMed]
- Shorofi, S.A.; Arbon, P. Complementary and alternative medicine (CAM) among Australian hospital-based nurses: Knowledge, attitude, personal and professional use, reasons for use, CAM referrals, and socio-demographic predictors of CAM users. Complement. Ther. Clin. Pract. 2017, 27, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Cırık, V.; Efe, E. Pediatric Nurses’ Usage and Experience Toward Complementary Health Approaches. J. Altern. Complement. Med. 2018, 24, 1120–1127. [Google Scholar] [CrossRef]
- Bhoo-Pathy, N.; Subramaniam, S.; Khalil, S.; Kimman, M.; Kong, Y.-C.; Ng, C.-W.; Bustamam, R.S.; Yip, C.-H. Out-of-Pocket Costs of Complementary Medicine Following Cancer and the Financial Impact in a Setting with Universal Health Coverage: Findings from a Prospective Cohort Study. JCO Oncol. Pract. 2021, 17, e1592–e1602. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.Y.; Chang, H.L. A review of nurses’ knowledge, attitudes, and ability to communicate the risks and benefits of complementary and alternative medicine. J. Clin. Nurs. 2015, 24, 1466–1478. [Google Scholar] [CrossRef]
- Bossert, J.; Mahler, C.; Boltenhagen, U.; Kaltenbach, A.; Froehlich, D.; Szecsenyi, J.; Wensing, M.; Joos, S.; Klafke, N. Protocol for the process evaluation of a counselling intervention designed to educate cancer patients on complementary and integrative health care and promote interprofessional collaboration in this area (the CCC-Integrativ study). PLoS ONE 2022, 17, e0268091. [Google Scholar] [CrossRef]
- Peters, M.D.J.; Godfrey, C.M.; Khalil, H.; McInerney, P.; Parker, D.; Soares, C.B. Guidance for conducting systematic scoping reviews. Int. J. Evid. Based Healthc. 2015, 13, 141–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Steen, J.T.; ter Riet, G.; van den Bogert, C.A.; Bouter, L.M. Causes of reporting bias: A theoretical framework. F1000Research 2019, 8, 280. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klafke, N.; Bossert, J.; Kröger, B.; Neuberger, P.; Heyder, U.; Layer, M.; Winkler, M.; Idler, C.; Kaschdailewitsch, E.; Heine, R.; et al. Prevention and Treatment of Chemotherapy-Induced Peripheral Neuropathy (CIPN) with Non-Pharmacological Interventions: Clinical Recommendations from a Systematic Scoping Review and an Expert Consensus Process. Med. Sci. 2023, 11, 15. https://doi.org/10.3390/medsci11010015
Klafke N, Bossert J, Kröger B, Neuberger P, Heyder U, Layer M, Winkler M, Idler C, Kaschdailewitsch E, Heine R, et al. Prevention and Treatment of Chemotherapy-Induced Peripheral Neuropathy (CIPN) with Non-Pharmacological Interventions: Clinical Recommendations from a Systematic Scoping Review and an Expert Consensus Process. Medical Sciences. 2023; 11(1):15. https://doi.org/10.3390/medsci11010015
Chicago/Turabian StyleKlafke, Nadja, Jasmin Bossert, Birgit Kröger, Petra Neuberger, Ute Heyder, Monika Layer, Marcela Winkler, Christel Idler, Elke Kaschdailewitsch, Rolf Heine, and et al. 2023. "Prevention and Treatment of Chemotherapy-Induced Peripheral Neuropathy (CIPN) with Non-Pharmacological Interventions: Clinical Recommendations from a Systematic Scoping Review and an Expert Consensus Process" Medical Sciences 11, no. 1: 15. https://doi.org/10.3390/medsci11010015
APA StyleKlafke, N., Bossert, J., Kröger, B., Neuberger, P., Heyder, U., Layer, M., Winkler, M., Idler, C., Kaschdailewitsch, E., Heine, R., John, H., Zielke, T., Schmeling, B., Joy, S., Mertens, I., Babadag-Savas, B., Kohler, S., Mahler, C., Witt, C. M., ... Stolz, R. (2023). Prevention and Treatment of Chemotherapy-Induced Peripheral Neuropathy (CIPN) with Non-Pharmacological Interventions: Clinical Recommendations from a Systematic Scoping Review and an Expert Consensus Process. Medical Sciences, 11(1), 15. https://doi.org/10.3390/medsci11010015






