Prognostic Value of HIF-1α-Induced Genes in Sepsis/Septic Shock
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characteristics of the Study Population
2.2. Total RNA Extraction
2.3. Reverse Transcription and Quantitative Real-Time PCR
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. mRNA Expression of Hypoxia-Regulated Genes in ICU Patients
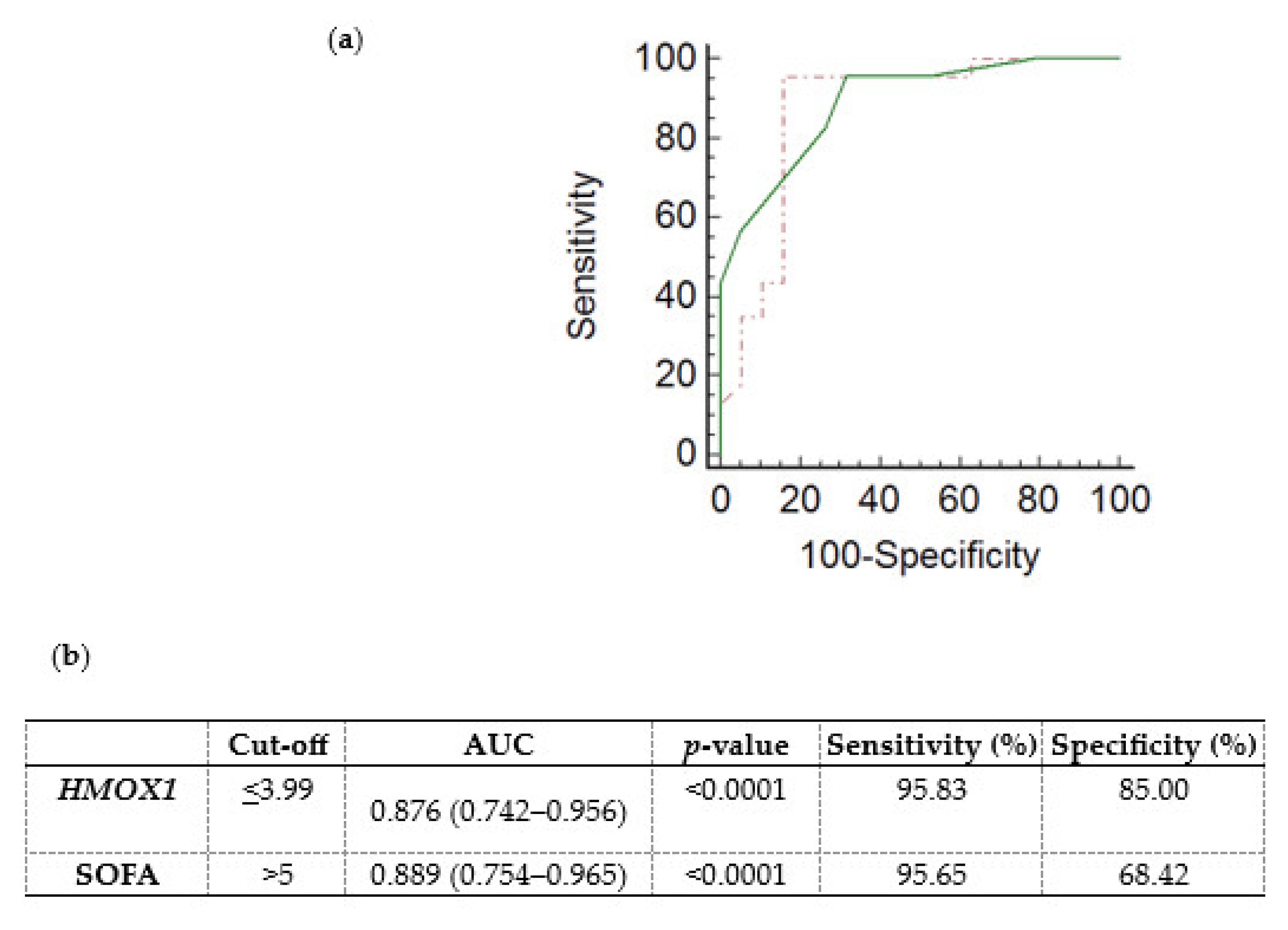
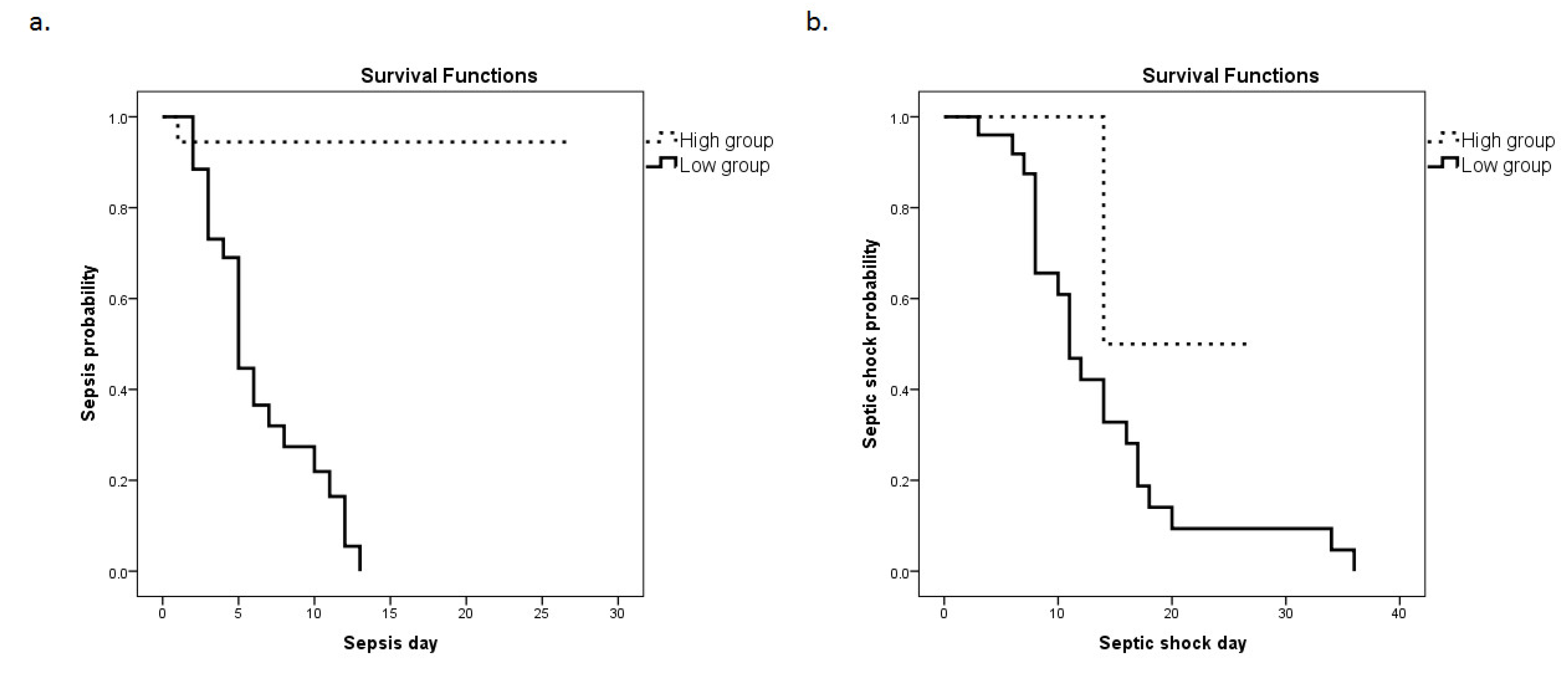
3.3. HMOX1 mRNA Expression as a Prognostic Factor for Sepsis and Septic Shock
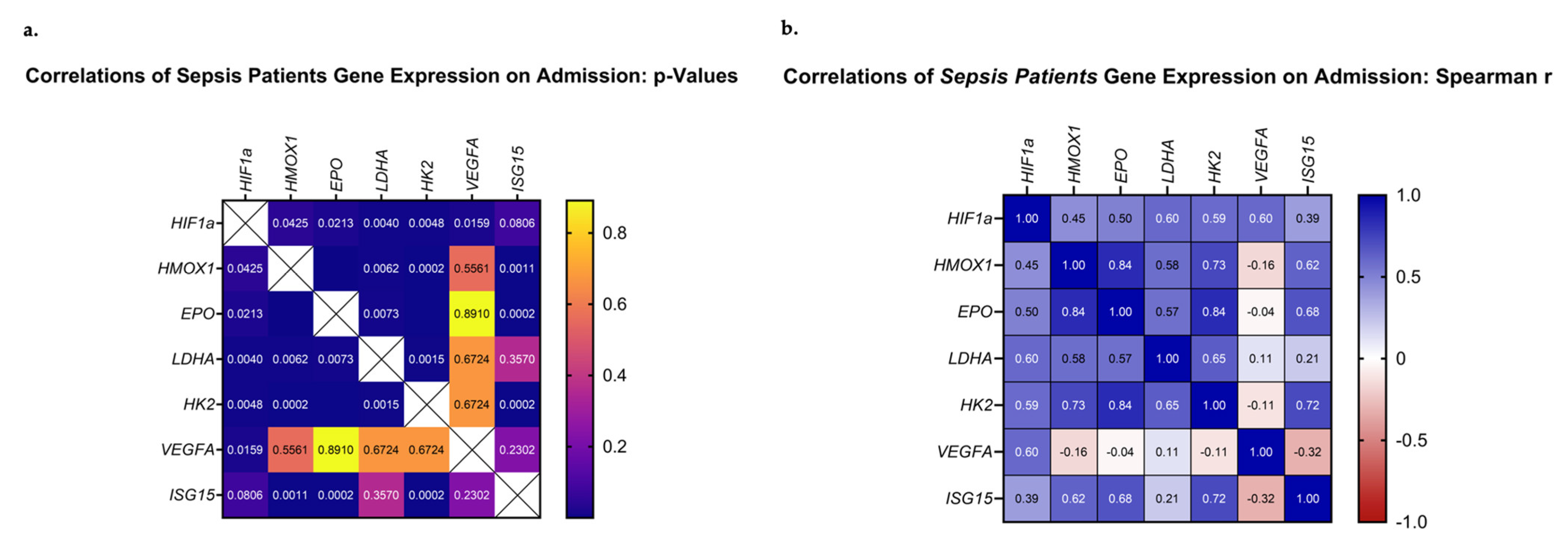
3.4. Correlations of HIF-Regulated Gene Expression on ICU Admission
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Kissoon, N.; Limmathurotsakul, D.; Bory, S.; Mutahunga, B.; Seymour, C.W.; Angus, D.C.; West, T.E. The global burden of sepsis: Barriers and potential solutions. Crit. Care 2018, 22, 232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singer, M. The role of mitochondrial dysfunction in sepsis–induced multi–organ failure. Virulence 2014, 5, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Guarino, M.; Perna, B.; Cesaro, A.E.; Maritati, M.; Spampinato, M.D.; Contini, C.; De Giorgio, R. Update on Sepsis and Septic Shock in Adult Patients: Management in the Emergency Department. J. Clin. Med. 2023, 12, 3188. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-Inducible Factor 1 (HIF-1) Pathway. Sci. STKE 2007, 2007, cm8. [Google Scholar] [CrossRef]
- Kaelin, W.G., Jr.; Ratcliffe, P.J. Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Mol. Cell 2008, 30, 393–402. [Google Scholar] [CrossRef]
- Yeh, Y.H.; Yang, Y.C.; Hsieh, M.Y.; Yeh, Y.C.; Li, T.K. A negative feedback of the HIF-1a pathway via the interferon-stimulated gene 15 and ISGylation. Clin. Cancer Res. 2013, 19, 5927–5939. [Google Scholar] [CrossRef] [Green Version]
- Perng, Y.C.; Lenschow, D.J. ISG15 in antiviral immunity and beyond. Nat. Rev. Microbiol. 2018, 16, 423–439. [Google Scholar] [CrossRef]
- Vanderhaeghen, T.; Vandewalle, J.; Libert, C. Hypoxia-Inducible Factors in Metabolic Reprogramming during Sepsis. FEBS J. 2020, 287, 1478–1495. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, B.L.; Leite, G.G.F.; Brunialti, M.K.C.; Assuncao, M.; Azevedo, L.C.P.; Freitas, F.; Salomao, R. HIF-1α and Hypoxia Responsive Genes are Differentially Expressed in Leukocytes from Survivors and Non-Survivors Patients During Clinical Sepsis. Shock 2021, 56, 80–91. [Google Scholar] [CrossRef]
- Riddle, S.R.; Ahmad, A.; Ahmad, S.; Deeb, S.S.; Malkki, M.; Schneider, B.K.; Allen, C.B.; White, C.W. Hypoxia induces hexokinase II gene expression in human lung cell line A549. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 278, L407–L416. [Google Scholar] [CrossRef] [Green Version]
- Semenza, G.; Roth, P.; Fang, H.; Wang, G. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J. Biol. Chem. 1994, 269, 23757–23763. [Google Scholar] [CrossRef]
- Yang, C.; Jiang, L.; Zhang, H.; Shimoda, L.A.; DeBerardinis, R.J.; Semenza, G.L. Analysis of hypoxia-induced metabolic reprogramming. Methods Enzymol. 2014, 542, 425–455. [Google Scholar]
- Roberts, D.J.; Miyamoto, S. Hexokinase II integrates energy metabolism and cellular protection: Akting on mitochondria and TORCing to autophagy. Cell Death Differ. 2015, 22, 248–257. [Google Scholar] [CrossRef] [Green Version]
- Van Wyngene, L.; Vandewalle, J.; Libert, C. Reprogramming of basic metabolic pathways in microbial sepsis: Therapeutic targets at last? EMBO Mol. Med. 2018, 10, e8712. [Google Scholar] [CrossRef]
- Chillappagari, S.; Venkatesan, S.; Garapati, V.; Mahavadi, P.; Munder, A.; Seubert, A.; Sarode, G.; Guenther, A.; Schmeck, B.T.; Tümmler, B.; et al. Impaired TLR4 and HIF expression in cystic fibrosis bronchial epithelial cells downregulates hemeoxygenase-1 and alters iron homeostasis in vitro. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, 791–799. [Google Scholar] [CrossRef] [Green Version]
- Haase, V.H. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev. 2013, 27, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Ebert, B.L.; Bunn, H.F. Regulation of the Erythropoietin Gene. Blood J. Am. Soc. Hematol. 1999, 94, 1864–1877. [Google Scholar]
- Semenza, G.L. HIF-1: Mediator of physiological and pathophysiological responses to hypoxia. J. Appl. Physiol. 2000, 88, 1474–1480. [Google Scholar] [CrossRef] [Green Version]
- Ryter, S.W. Heme Oxgenase-1, a Cardinal Modulator of Regulated Cell Death and Inflammation. Cells 2021, 10, 515. [Google Scholar] [CrossRef]
- Fredenburgh, L.E.; Perrella, M.A.; Mitsialis, S.A. The Role of Heme Oxygenase-1 in Pulmonary Disease. Am. J. Respir. Cell Mol. Biol. 2007, 36, 158–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Yu, J.; Gong, L.; Zhang, Y.; Dong, S.; Shi, J.; Li, C.; Li, Y.; Zhang, Y.; Li, H. Heme oxygenase-1(HO-1) regulates Golgi stress and attenuates endotoxin-induced acute lung injury through hypoxia inducible factor-1α (HIF-1α)/HO-1 signaling pathway. Free Radic. Biol. Med. 2021, 165, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Y.; Xie, X.; Chen, H.; Zhu, Q.; Ge, Z.; Wei, H.; Deng, J.; Xia, Z.; Lian, Q. Heme Oxygenase-1 Reduces Sepsis-Induced Endoplasmic Reticulum Stress and Acute Lung Injury. Mediat. Inflamm. 2018, 2018, 9413876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jelkmann, W. Erythropoietin. Front. Horm. Res. 2016, 47, 115–127. [Google Scholar]
- Broxmeyer, H.E. Erythropoietin: Multiple targets, actions, and modifying influences for biological and clinical consideration. J. Exp. Med. 2013, 210, 205–208. [Google Scholar] [CrossRef] [Green Version]
- Chancharoenthana, W.; Udompronpitak, K.; Manochantr, Y.; Kantagowit, P.; Kaewkanha, P.; Issara-Amphorn, J.; Leelahavanichkul, A. Repurposing of High-Dose Erythropoietin as a Potential Drug Attenuates Sepsis in Preconditioning Renal Injury. Cells 2021, 10, 3133. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, S. Protective Effects of Erythropoietin towards Acute Lung Injuries in Rats with Sepsis and Its Related Mechanisms. Ann. Clin. Lab. Sci. 2019, 49, 257–264. [Google Scholar]
- Walden, A.P.; Young, J.D.; Sharples, E. Bench to bedside: A role for erythropoietin in sepsis. Crit. Care 2010, 14, 227. [Google Scholar] [CrossRef] [Green Version]
- Kannan, K.; Choudhury, S.; Ramasamy, T.; Kesavan, M.; Parida, S.; Singh, T.U. Erythropoietin prevents sepsis-induced clinical severity and haematological changes in mice. Pharma Innov. J. 2020, 9, 81–84. [Google Scholar]
- Ghezzi, P.; Brines, M. Erythropoietin as an antiapoptotic, tissue-protective cytokine. Cell Death Differ. 2004, 11, S37–S44. [Google Scholar] [CrossRef] [Green Version]
- Bates, D.O. Vascular endothelial growth factors and vascular permeability. Cardiovasc. Res. 2010, 87, 262–271. [Google Scholar] [CrossRef] [Green Version]
- Tomita, K.; Saito, Y.; Suzuki, T.; Imbaby, S.; Hattori, K.; Matsuda, N.; Hattori, Y. Vascular endothelial growth factor contributes to lung vascular hyperpermeability in sepsis-associated acute lung injury. Naunyn Schmiedebergs Arch Pharmacol. 2020, 393, 2365–2374. [Google Scholar] [CrossRef]
- Livak, K.L.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta DeltaC(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, M.; Liu, H.; Dong, H.; Wu, N.; Wiedermann, C.J.; Andaluz-Ojeda, D.; Chen, H.; Li, N. Heme oxygenase-1 as a predictor of sepsis-induced acute kidney injury: A cross-sectional study. Ann. Transl. Med. 2022, 10, 1177. [Google Scholar] [CrossRef]
- Chen, H.Y.; Tzeng, I.S.; Tsai, K.W.; Wu, Y.K.; Cheng, C.F.; Lu, K.C.; Chung, H.W.; Chao, Y.C.; Su, W.L. Association between heme oxygenase one and sepsis development in patients with moderate-to-critical COVID-19: A single center, retrospective observational study. Eur. J. Med. Res. 2022, 27, 275. [Google Scholar] [CrossRef]
- Ekregbesi, P.; Shankar-Hari, M.; Bottomley, C.; Riley, E.M.; Mooney, J.P. Relationship between Anaemia, Haemolysis, Inflammation and Haem Oxygenase-1 at Admission with Sepsis: A pilot study. Sci. Rep. 2018, 8, 11198. [Google Scholar] [CrossRef] [Green Version]
- Tamion, F.; Le Cam-Duchez, V.; Menard, J.F.; Girault, C.; Coquerel, A.; Bonmarchand, G. Erythropoietin and renin as biological markers in critically ill patients. Crit. Care 2004, 8, R328–R335. [Google Scholar] [CrossRef] [Green Version]
- Maragno, A.L.; Pironin, M.; Alcalde, H.; Cong, X.; Knobeloch, K.P.; Tangy., F.; Zhang, D.E.; Ghysdael, J.; Quang, C.T. ISG15 modulates development of the erythroid lineage. PLoS ONE 2011, 6, e26068. [Google Scholar] [CrossRef]
- Tang, A.L.; Peng, Y.; Shen, M.J.; Liu, X.Y.; Li, S.; Xiong, M.C.; Gao, N.; Hu, T.P.; Zhang, G.Q. Prognostic role of elevated VEGF in sepsis: A systematic review and meta-analysis. Front. Physiol. 2022, 13, 941257. [Google Scholar] [CrossRef]
- Ryoo, S.M.; Lee, J.; Lee, Y.S.; Lee, J.H.; Lim, K.S.; Huh, J.W.; Hong, S.B.; Lim, C.M.; Koh, Y.; Kim, W.Y. Lactate level versus lactate clearance for predicting mortality in patients with septic shock defined by sepsis-3. Crit. Care Med. 2018, 46, e489–e495. [Google Scholar] [CrossRef]
- Lee, S.G.; Song, J.; Park, D.W.; Moon, S.; Cho, H.J.; Kim, J.Y.; Park, J.; Cha, J.H. Prognostic value of lactate levels and lactate clearance in sepsis and septic shock with initial hyperlactatemia: A retrospective cohort study according to the Sepsis-3 definitions. Medicine 2021, 100, e24835. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, K.; Shi, L.; Xiang, F.; Tao, K.; Wang, G. Prognostic Significance of the Metabolic Marker Hexokinase-2 in Various Solid Tumors: A Meta-Analysis. PLoS ONE 2016, 11, e0166230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katagiri, M.; Karasawa, H.; Takagi, K.; Nakayama, S.; Yabuuchi, S.; Fujishima, F.; Naitoh, T.; Watanabe, M.; Suzuki, T.; Unno, M.; et al. Hexokinase 2 in colorectal cancer: A potent prognostic factor associated with glycolysis, proliferation and migration. Histol. Histopathol. 2017, 32, 351–360. [Google Scholar] [PubMed]


| Gene | Sequence (5′-3′) | nt | |
|---|---|---|---|
| HIF1A | F | 5′-GGCGCGAACGACAAGAAAAAG-3′ | 21 |
| R | 5′-CCTTATCAAGATGCGAACTCACA-3′ | 23 | |
| HMOX-1 | F | 5′-GGCCTAAACTTCAGAGGGGG-3′ | 20 |
| R | 5′-AGACAGCTGCCACATTAGGG-3′ | 20 | |
| EPO | F | 5′-GCCCCACCACGCCTCATCTGT-3′ | 21 |
| R | 5′-CTTCCAGGCATAGAAATTAAC-3′ | 21 | |
| ISG15 | F | 5′-CCACCTGAAGCAAGCAAGTGA-3′ | 21 |
| R | 5′-CGCAGGCGCAGATTCATGAA-3′ | 20 | |
| LDHA | F | 5′-TTCACCCATTAAGCTGTCATGG-3′ | 22 |
| R | 5′-GACACCAGCAACATTCATTCCA-3′ | 22 | |
| HK2 | F | 5′-GAGCCACCACTCACCCTACT-3′ | 20 |
| R | 5′-ACCCAAAGCACACGGAAGTT-3′ | 20 | |
| VEGFA | F | 5′-CTTGCCTTGCTGCTCTAC-3′ | 18 |
| R | 5′-TGGCTTGAAGATGTACTCG-3′ | 19 | |
| GAPDH | F | 5′-ATGGGGAAGGTGAAGGTCG-3′ | 19 |
| R | 5′-TACATGAGGGCACGGAAGATG-3′ | 23 |
| Characteristics | Septic Patients | Non-Septic Patients | p-Value |
|---|---|---|---|
| Number of patients, n | 25 | 21 | |
| Age (years), (mean ± SD) | 51 ± 28 | 53 ± 18 | 0.76 |
| Sex, n (%) | 0.35 | ||
| Male | 19 (59.4%) | 13 (40.6%) | |
| Female | 6 (42.9%) | 8 (57.1%) | |
| Diagnosis, n (%) | 0.15 | ||
| Surgical | 3 (12.0%) | 7 (33.3%) | |
| Medical | 5 (20.0%) | 5 (23.8%) | |
| Trauma | 17 (68.0%) | 9 (42.9%) | |
| APACHE II score (median, IQR) | 15 (12–20) | 14 (11–16) | 0.16 |
| SOFA score (median, IQR) | 8 (7–10) | 5 (3–7) | <0.0001 **** |
| PaO2/FiO2 (mmHg), (median, IQR) | 360 (215–413) | 381 (256–430) | 0.32 |
| Lactate (mmol/L) (median, IQR) | 1.7 (1.0–2.9) | 1.5 (0.8–1.7) | 0.21 |
| CRP (mg/dL), (median, IQR) | 4.2 (1.6–8.9) | 3 (1.4–8.5) | 0.41 |
| PCT (ng/mL), (median, IQR) | 0.4 (0.1–1.7) | 0.3 (0.1–0.8) | 0.78 |
| IL-6 (pg/mL), (median, IQR) | 21.8 (8.6–64.4) | 31.8 (8.4–75.6) | 0.96 |
| IL-8 (pg/mL), (median, IQR) | 64.1 (23.4–102.3) | 40.2 (20.0–137.3) | 0.70 |
| TNF-α (pg/mL), (median, IQR) | 40.5 (29.3–73.6) | 83.1 (50.6–173.1) | 0.004 ** |
| EPO-R (ng/mL) (median, IQR) | 3.5 (1.1–10.3) | 3.3 (1.5–6.9) | 0.88 |
| Day of Sepsis (median, IQR) | 5 (3–8) | N/A | |
| Day of septic shock (median, IQR) | 12 (8–17) | N/A | |
| Site of infection, n | N/A | ||
| Pneumonia | 23 | ||
| CNS | 1 | ||
| Blood | 1 | ||
| Type of infection, n | N/A | ||
| Gram-negative bacteria | 22 | ||
| Gram-positive bacteria | 3 | ||
| Mechanical ventilation duration (median, IQR) | 27 (19–38) | 2 (1–4) | <0.0001 **** |
| Length of stay (median, IQR) | 36 (24–47) | 6 (3–10) | <0.0001 **** |
| ICU mortality, n (%) | 8 (32%) | 0 (0%) | 0.005 ** |
| Variable | Univariate Model | Multivariate Model | ||||
|---|---|---|---|---|---|---|
| O.R. | 95% C.I. | p-Value | O.R. | 95% C.I. | p-Value | |
| SOFA score | 2.635 | 1.456–4.770 | 0.001 * | 2.919 | 1.257–6.775 | 0.013 * |
| HMOX1 | 0.259 | 0.125–0.534 | <0.0001 * | 0.258 | 0.097–0.686 | 0.007 * |
| Age | 0.995 | 0.760–0.964 | 0.760 | 0.993 | 0.937–1.052 | 0.815 |
| Sex | 0.500 | 0.138–1.809 | 0.291 | 2.855 | 0.207–39.362 | 0.433 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lotsios, N.S.; Keskinidou, C.; Jahaj, E.; Mastora, Z.; Dimopoulou, I.; Orfanos, S.E.; Vassilaki, N.; Vassiliou, A.G.; Kotanidou, A. Prognostic Value of HIF-1α-Induced Genes in Sepsis/Septic Shock. Med. Sci. 2023, 11, 41. https://doi.org/10.3390/medsci11020041
Lotsios NS, Keskinidou C, Jahaj E, Mastora Z, Dimopoulou I, Orfanos SE, Vassilaki N, Vassiliou AG, Kotanidou A. Prognostic Value of HIF-1α-Induced Genes in Sepsis/Septic Shock. Medical Sciences. 2023; 11(2):41. https://doi.org/10.3390/medsci11020041
Chicago/Turabian StyleLotsios, Nikolaos S., Chrysi Keskinidou, Edison Jahaj, Zafeiria Mastora, Ioanna Dimopoulou, Stylianos E. Orfanos, Niki Vassilaki, Alice G. Vassiliou, and Anastasia Kotanidou. 2023. "Prognostic Value of HIF-1α-Induced Genes in Sepsis/Septic Shock" Medical Sciences 11, no. 2: 41. https://doi.org/10.3390/medsci11020041





