Could Insulin Be a Better Regulator of Appetite/Satiety Balance and Body Weight Maintenance in Response to Glucose Exposure Compared to Sucrose Substitutes? Unraveling Current Knowledge and Searching for More Appropriate Choices
Abstract
:1. Introduction
2. Methods
3. Results
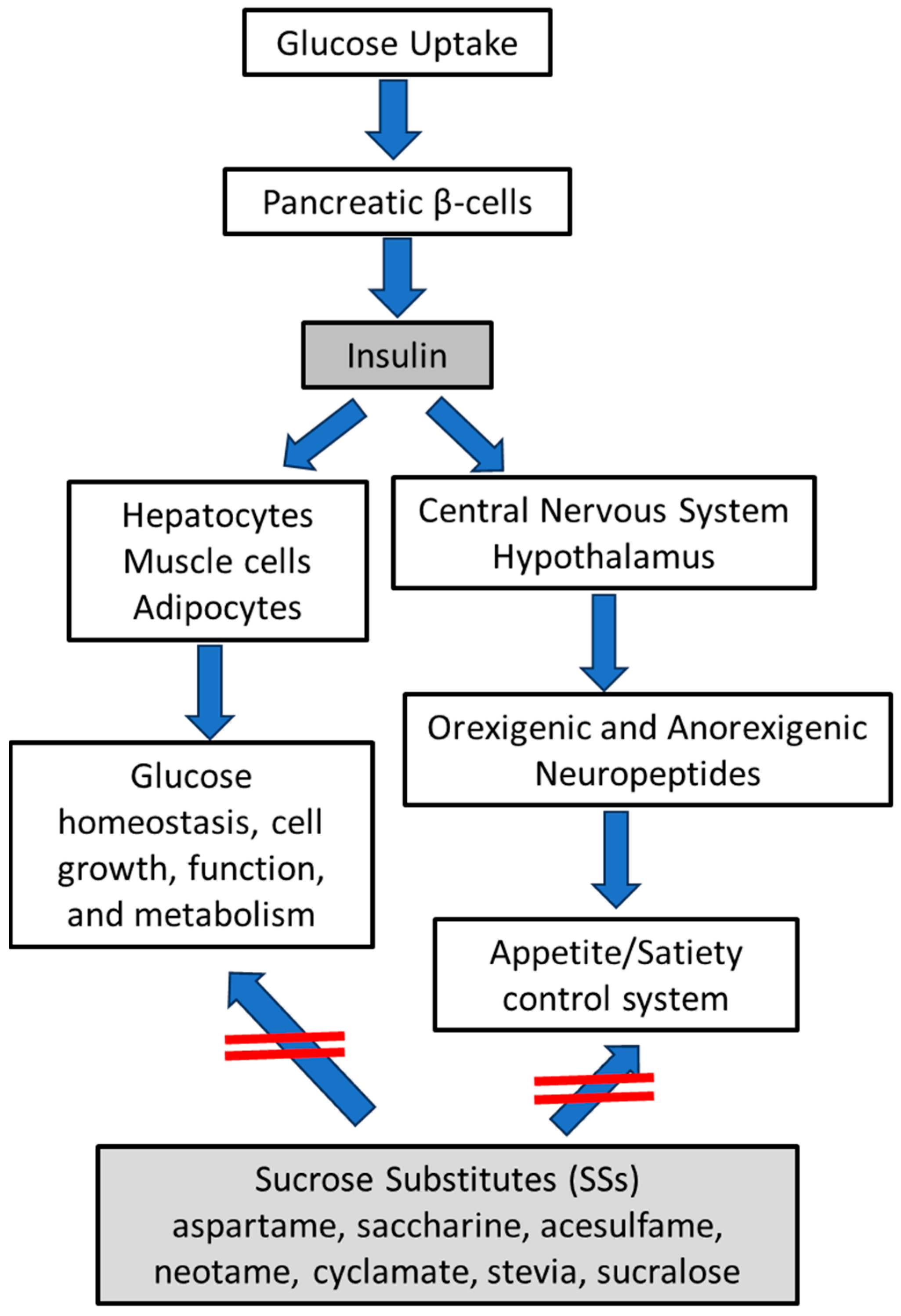
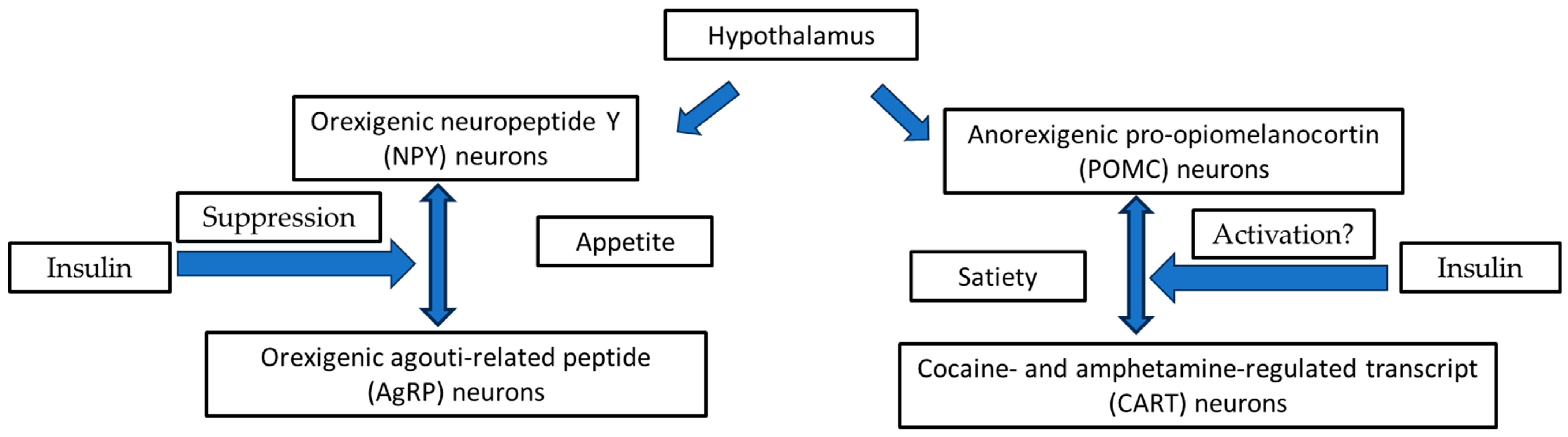
3.1. Hypothalamic Orexigenic/Anorexigenic Complex System: The Insulin Action
3.2. The Cooperation of Insulin-Leptin Hormones in Hypothalamic Orexigenic/Anorexigenic Complex System
3.3. Sucrose Substitutes (SSs) in the Control of Appetite/Hunger Complex System and Their Sweet Potency
3.4. Experimental and Clinical Evidence and Potential Mechanisms for SSs in the Control of body Weight Gain
3.5. Do SSs Stimulate Insulin Secretion or Other Molecular Signs to Control Appetite/Hunger Complex System?
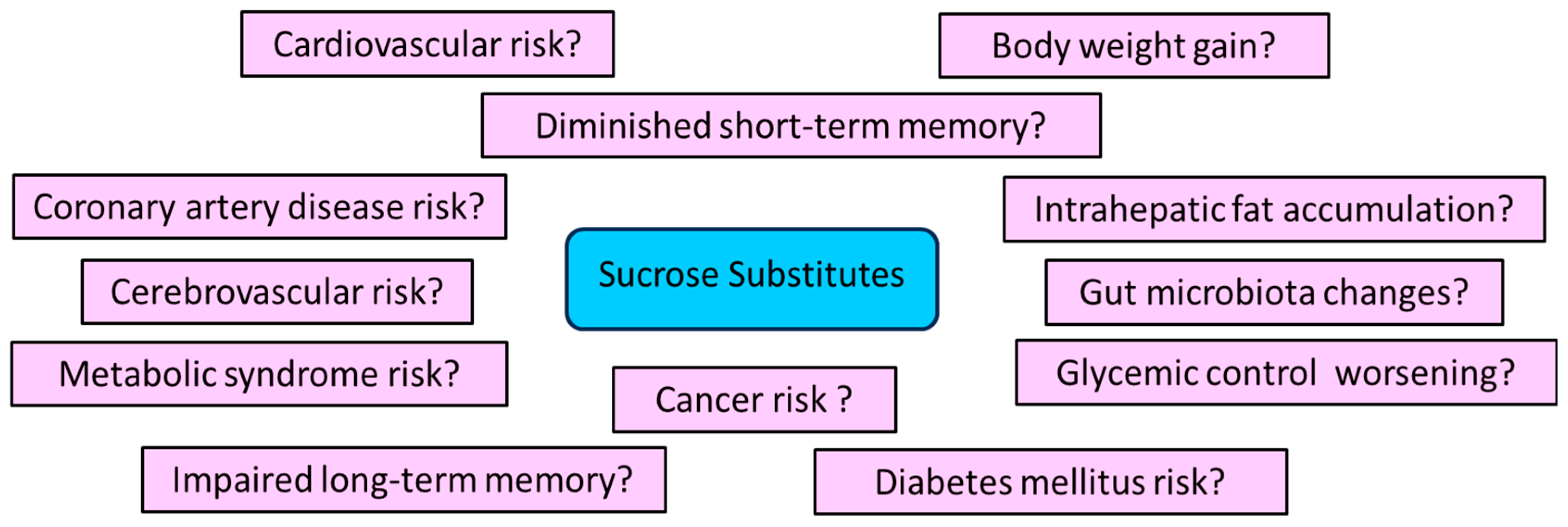
3.6. May SSs Increase the Risk of Adverse Effects?
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahman, M.S.; Hossain, K.S.; Das, S.; Kundu, S.; Adegoke, E.O.; Rahman, M.A.; Hannan, M.A.; Uddin, M.J.; Pang, M.G. Role of Insulin in Health and Disease: An Update. Int. J. Mol. Sci. 2021, 22, 6403. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Gilbert, E.R.; Liu, D. Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr. Diabetes Rev. 2013, 9, 25–53. [Google Scholar] [CrossRef] [PubMed]
- Rohli, K.E.; Boyer, C.K.; Blom, S.E.; Stephens, S.B. Nutrient Regulation of Pancreatic Islet β-Cell Secretory Capacity and Insulin Production. Biomolecules 2022, 12, 335. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [PubMed]
- Porte, D., Jr.; Baskin, D.G.; Schwartz, M.W. Insulin signaling in the central nervous system: A critical role in metabolic homeostasis and disease from C. elegans to humans. Diabetes 2005, 54, 1264–1276. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhang, L.; Hu, Z. Cerebral insulin, insulin signaling pathway, and brain angiogenesis. Neurol. Sci. 2016, 37, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Gerozissis, K. Brain insulin: Regulation, mechanisms of action and functions. Cell. Mol. Neurobiol. 2003, 23, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, A.; Nasreen, A.; Menzel, A.; Gasmi Benahmed, A.; Pivina, L.; Noor, S.; Peana, M.; Chirumbolo, S.; Bjørklund, G. Neurotransmitters Regulation and Food Intake: The Role of Dietary Sources in Neurotransmission. Molecules 2022, 28, 210. [Google Scholar] [CrossRef] [PubMed]
- Carreiro, A.L.; Dhillon, J.; Gordon, S.; Higgins, K.A.; Jacobs, A.G.; McArthur, B.M.; Redan, B.W.; Rivera, R.L.; Schmidt, L.R.; Mattes, R.D. The Macronutrients, Appetite, and Energy Intake. Annu. Rev. Nutr. 2016, 36, 73–103. [Google Scholar] [CrossRef]
- Rhea, E.M.; Banks, W.A. Role of the Blood-Brain Barrier in Central Nervous System Insulin Resistance. Front. Neurosci. 2019, 13, 521. [Google Scholar] [CrossRef]
- Roger, C.; Lasbleiz, A.; Guye, M.; Dutour, A.; Gaborit, B.; Ranjeva, J.P. The Role of the Human Hypothalamus in Food Intake Networks: An MRI Perspective. Front. Nutr. 2022, 8, 760914. [Google Scholar] [CrossRef] [PubMed]
- Timper, K.; Brüning, J.C. Hypothalamic circuits regulating appetite and energy homeostasis: Pathways to obesity. Dis. Model. Mech. 2017, 10, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.G.; Kanoski, S.E.; Sanchez-Watts, G.; Langhans, W. The physiological control of eating: Signals, neurons, and networks. Physiol. Rev. 2022, 102, 689–813. [Google Scholar] [CrossRef] [PubMed]
- Pliquett, R.U.; Führer, D.; Falk, S.; Zysset, S.; von Cramon, D.Y.; Stumvoll, M. The effects of insulin on the central nervous system–focus on appetite regulation. Horm. Metab. Res. 2006, 38, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Robbins, J.; Busquets, O.; Tong, M.; de la Monte, S.M. Dysregulation of Insulin-Linked Metabolic Pathways in Alzheimer’s Disease: Co-Factor Role of Apolipoprotein E ɛ4. J. Alzheimers Dis. Rep. 2020, 4, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, E.; Velázquez, E.; Hurtado-Carneiro, V.; Ruiz-Albusac, J.M. Insulin in the brain: Its pathophysiological implications for States related with central insulin resistance, type 2 diabetes and Alzheimer’s disease. Front. Endocrinol. 2014, 5, 161. [Google Scholar] [CrossRef] [PubMed]
- Lohner, S.; Toews, I.; Meerpohl, J.J. Health outcomes of non-nutritive sweeteners: Analysis of the research landscape. Nutr. J. 2017, 16, 55. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.; Pang, M.; Castelnuovo, G.; Finlayson, G.; Blaak, E.; Gibbons, C.; Navas-Carretero, S.; Almiron-Roig, E.; Harrold, J.; Raben, A.; et al. A rational review on the effects of sweeteners and sweetness enhancers on appetite, food reward and metabolic/adiposity outcomes in adults. Food Funct. 2021, 12, 442–465. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.; Port, J.D.; Acosta, A. Integrative Hedonic and Homeostatic Food Intake Regulation by the Central Nervous System: Insights from Neuroimaging. Brain Sci. 2022, 12, 431. [Google Scholar] [CrossRef]
- Higgins, K.A.; Rawal, R.; Baer, D.; O’Connor, L.E.; Appleton, K.M. Scoping Review and Evidence Map of the Relation between Exposure to Dietary Sweetness and Body Weight-Related Outcomes in Adults. Adv. Nutr. 2022, 13, 2341–2356. [Google Scholar] [CrossRef]
- Song, J.; Choi, S.Y. Arcuate Nucleus of the Hypothalamus: Anatomy, Physiology, and Diseases. Exp. Neurobiol. 2023, 32, 371–386. [Google Scholar] [CrossRef] [PubMed]
- Sohn, J.W. Network of hypothalamic neurons that control appetite. BMB Rep. 2015, 48, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hernandez-Sanchez, D.; Herzog, H. Regulation of Feeding-Related Behaviors by Arcuate Neuropeptide Y Neurons. Endocrinology 2019, 160, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- De la Cruz-Concepción, B.; Flores-Cortez, Y.A.; Barragán-Bonilla, M.I.; Mendoza-Bello, J.M.; Espinoza-Rojo, M. Insulin: A connection between pancreatic β cells and the hypothalamus. World J. Diabetes 2023, 14, 76–91. [Google Scholar] [CrossRef] [PubMed]
- Vohra, M.S.; Benchoula, K.; Serpell, C.J.; Hwa, W.E. AgRP/NPY and POMC neurons in the arcuate nucleus and their potential role in treatment of obesity. Eur. J. Pharmacol. 2022, 915, 174611. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, T.; Nakamura, Y.; Yamanaka, D.; Shibano, T.; Chida, K.; Minami, S.; Asano, T.; Hakuno, F.; Takahashi, S. Phosphatidylinositol 3-kinase (PI3K) activity bound to insulin-like growth factor-I (IGF-I) receptor, which is continuously sustained by IGF-I stimulation, is required for IGF-I-induced cell proliferation. J. Biol. Chem. 2012, 287, 29713–29721. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Jain, S.K. Phosphatidylinositol-3,4,5-triphosphate and cellular signaling: Implications for obesity and diabetes. Cell Physiol. Biochem. 2015, 35, 1253–1275. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, R.; Elliott, B. Akt/PKB activation and insulin signaling: A novel insulin signaling pathway in the treatment of type 2 diabetes. Diabetes Metab. Syndr. Obes. 2014, 7, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Wipperman, M.F.; Montrose, D.C.; Gotto, A.M., Jr.; Hajjar, D.P. Mammalian Target of Rapamycin: A Metabolic Rheostat for Regulating Adipose Tissue Function and Cardiovascular Health. Am. J. Pathol. 2019, 189, 492–501. [Google Scholar] [CrossRef]
- Pan, C.W.; Jin, X.; Zhao, Y.; Pan, Y.; Yang, J.; Karnes, R.J.; Zhang, J.; Wang, L.; Huang, H. AKT-phosphorylated FOXO1 suppresses ERK activation and chemoresistance by disrupting IQGAP1-MAPK interaction. EMBO J. 2017, 36, 995–1010. [Google Scholar] [CrossRef]
- Cheng, Z.; White, M.F. Targeting Forkhead box O1 from the concept to metabolic diseases: Lessons from mouse models. Antioxid. Redox Signal. 2011, 14, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Kouidhi, S.; Clerget-Froidevaux, M.S. Integrating Thyroid Hormone Signaling in Hypothalamic Control of Metabolism: Crosstalk Between Nuclear Receptors. Int. J. Mol. Sci. 2018, 19, 2017. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, K.; Iwasaki, Y.; Daimon, M. Hypothalamic Regulation of Corticotropin-Releasing Factor under Stress and Stress Resilience. Int. J. Mol. Sci. 2021, 22, 12242. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Wagner, E.J.; Rønnekleiv, O.K.; Kelly, M.J. Insulin and leptin excite anorexigenic pro-opiomelanocortin neurones via activation of TRPC5 channels. J. Neuroendocrinol. 2018, 30, 10. [Google Scholar] [CrossRef]
- Haspula, D.; Cui, Z. Neurochemical Basis of Inter-Organ Crosstalk in Health and Obesity: Focus on the Hypothalamus and the Brainstem. Cells 2023, 12, 1801. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.J.; Qiu, J.; Rønnekleiv, O.K. TRPCing around the hypothalamus. Front. Neuroendocrinol. 2018, 51, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Casado, M.E.; Collado-Pérez, R.; Frago, L.M.; Barrios, V. Recent Advances in the Knowledge of the Mechanisms of Leptin Physiology and Actions in Neurological and Metabolic Pathologies. Int. J. Mol. Sci. 2023, 24, 1422. [Google Scholar] [CrossRef]
- Thon, M.; Hosoi, T.; Ozawa, K. Possible Integrative Actions of Leptin and Insulin Signaling in the Hypothalamus Targeting Energy Homeostasis. Front. Endocrinol. 2016, 7, 138. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Martínez, M. PI3K: An Attractive Candidate for the Central Integration of Metabolism and Reproduction. Front. Endocrinol. 2012, 2, 110. [Google Scholar] [CrossRef]
- Porniece Kumar, M.; Cremer, A.L.; Klemm, P.; Steuernagel, L.; Sundaram, S.; Jais, A.; Hausen, A.C.; Tao, J.; Secher, A.; Pedersen, T.Å.; et al. Insulin signalling in tanycytes gates hypothalamic insulin uptake and regulation of AgRP neuron activity. Nat. Metab. 2021, 3, 1662–1679. [Google Scholar] [CrossRef]
- Nakagawa, T.; Hosoi, T. Recent progress on action and regulation of anorexigenic adipokine leptin. Front. Endocrinol. 2023, 14, 1172060. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Raychaudhuri, U.; Chakraborty, R. Artificial sweeteners-A review. J. Food Sci. Technol. 2014, 51, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Mooradian, A.D.; Smith, M.; Tokuda, M. The role of artificial and natural sweeteners in reducing the consumption of table sugar: A narrative review. Clin. Nutr. ESPEN 2017, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Praveena, S.M.; Cheema, M.S.; Guo, H.R. Non-nutritive artificial sweeteners as an emerging contaminant in environment: A global review and risks perspectives. Ecotoxicol. Environ. Saf. 2019, 170, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, K. Is the Use of Artificial Sweeteners Beneficial for Patients with Diabetes Mellitus? The Advantages and Disadvantages of Artificial Sweeteners. Nutrients 2022, 14, 4446. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Amarnath, S.; Thulasimani, M.; Ramaswamy, S. Artificial sweeteners as a sugar substitute: Are they really safe? Indian J. Pharmacol. 2016, 48, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Lohner, S.; Kuellenberg de Gaudry, D.; Toews, I.; Ferenci, T.; Meerpohl, J.J. Non-nutritive sweeteners for diabetes mellitus. Cochrane Database Syst. Rev. 2020, 5, CD012885. [Google Scholar] [CrossRef] [PubMed]
- Chatelan, A.; Raeisi-Dehkordi, H.; Salehi-Abargouei, A. Substituting Low-Calorie Sweetened Beverages for Sugar-Sweetened Beverages to Prevent Obesity and Cardiometabolic Diseases: Still a Good Idea? Curr. Dev. Nutr. 2024, 8, 102105. [Google Scholar] [CrossRef] [PubMed]
- Malek, A.M.; Hunt, K.J.; DellaValle, D.M.; Greenberg, D.; St Peter, J.V.; Marriott, B.P. Reported Consumption of Low-Calorie Sweetener in Foods, Beverages, and Food and Beverage Additions by US Adults: NHANES 2007–2012. Curr. Dev. Nutr. 2018, 2, nzy054. [Google Scholar] [CrossRef]
- Bartoshuk, L.M.; Duffy, V.B.; Hayes, J.E.; Moskowitz, H.R.; Snyder, D.J. Psychophysics of sweet and fat perception in obesity: Problems, solutions and new perspectives. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1137–1148. [Google Scholar] [CrossRef]
- Hwang, L.D.; Cuellar-Partida, G.; Ong, J.S.; Breslin, P.A.; Reed, D.R.; MacGregor, S.; Gharahkhani, P.; Martin, N.G.; Rentería, M.E. Sweet Taste Perception is Associated with Body Mass Index at the Phenotypic and Genotypic Level. Twin Res. Hum. Genet. 2016, 19, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Owyang, C. Sugars, Sweet Taste Receptors, and Brain Responses. Nutrients 2017, 9, 653. [Google Scholar] [CrossRef] [PubMed]
- Proserpio, C.; Laureati, M.; Bertoli, S.; Battezzati, A.; Pagliarini, E. Determinants of Obesity in Italian Adults: The Role of Taste Sensitivity, Food Liking, and Food Neophobia. Chem. Sens. 2016, 41, 169. [Google Scholar] [CrossRef] [PubMed]
- Kamil, A.; Wilson, A.R. Sweet taste perceptions and preferences may not be associated with food intakes or obesity. Nutr. Today 2021, 56, 62–69. [Google Scholar] [CrossRef]
- Hill, C.; Wardle, J.; Cooke, L. Adiposity is not associated with children’s reported liking for selected foods. Appetite 2009, 52, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Warner, J.O. Artificial food additives: Hazardous to long-term health. Arch. Dis. Child. 2024, in press. [CrossRef] [PubMed]
- Daher, M.I.; Matta, J.M.; Abdel Nour, A.M. Non-nutritive sweeteners and type 2 diabetes: Should we ring the bell? Diabetes Res. Clin. Pract. 2019, 155, 107786. [Google Scholar] [CrossRef] [PubMed]
- Fernstrom, J.D. Non-nutritive sweeteners and obesity. Annu. Rev. Food Sci. Technol. 2015, 6, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Mattes, R.D.; Popkin, B.M. Nonnutritive sweetener consumption in humans: Effects on appetite and food intake and their putative mechanisms. Am. J. Clin. Nutr. 2009, 89, 1–14. [Google Scholar] [CrossRef]
- Swithers, S.E. Artificial sweeteners produce the counterintuitive effect of inducing metabolic derangements. Trends Endocrinol. Metab. 2013, 24, 431–441. [Google Scholar] [CrossRef]
- Fowler, S.P.; Williams, K.; Resendez, R.G.; Hunt, K.J.; Hazuda, H.P.; Stern, M.P. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity 2008, 16, 1894–1900. [Google Scholar] [CrossRef] [PubMed]
- Renwick, A.G.; Molinary, S.V. Sweet-taste receptors, low-energy sweeteners, glucose absorption and insulin release. Br. J. Nutr. 2010, 104, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Benton, D. Can artificial sweeteners help control body weight and prevent obesity? Nutr. Res. Rev. 2005, 18, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Ragi, M.; El-Helou, N.; El-Mallah, C.; Eid, A.; Obeid, O. Effect of temperature and/or sweetness of beverages on body composition in rats. Br. J. Nutr. 2021, 125, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Chi, L.; Gao, B.; Tu, P.; Ru, H.; Lu, K. The artificial sweetener acesulfame potassium affects the gut microbiome and body weight gain in CD-1 mice. PLoS ONE 2017, 12, e0178426. [Google Scholar] [CrossRef] [PubMed]
- Tsan, L.; Chometton, S.; Hayes, A.M.; Klug, M.E.; Zuo, Y.; Sun, S.; Bridi, L.; Lan, R.; Fodor, A.A.; Noble, E.E.; et al. Early life low-calorie sweetener consumption disrupts glucose regulation, sugar-motivated behavior, and memory function in rats. JCI Insight 2022, 7, e157714. [Google Scholar] [CrossRef] [PubMed]
- Ragi, M.; El-Haber, R.; El-Masri, F.; Obeid, O. The effect of aspartame and sucralose intake on body weight measures and blood metabolites: Role of their form (solid and/or liquid) of ingestion. Br. J. Nutr. 2022, 128, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Feijó, F.M.; Ballard, C.R.; Foletto, K.C.; Batista, B.A.M.; Neves, A.M.; Ribeiro, M.F.M.; Bertoluci, M.C. Saccharin and aspartame, compared with sucrose, induce greater weight gain in adult Wistar rats, at similar total caloric intake levels. Appetite 2013, 60, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Foletto, K.C.; Melo Batista, B.A.; Neves, A.M.; de Matos Feijó, F.; Ballard, C.R.; Marques Ribeiro, M.F.; Bertoluci, M.C. Sweet taste of saccharin induces weight gain without increasing caloric intake, not related to insulin-resistance in Wistar rats. Appetite 2016, 96, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Guthrie, N.; Pezzullo, J.; Sanli, T.; Fielding, R.A.; Bellamine, A. Efficacy of a novel formulation of L-Carnitine, creatine, and leucine on lean body mass and functional muscle strength in healthy older adults: A randomized, double-blind placebo-controlled study. Nutr. Metab. 2017, 14, 7. [Google Scholar] [CrossRef]
- Zhao, X.; Yan, J.; Chen, K.; Song, L.; Sun, B.; Wei, X. Effects of saccharin supplementation on body weight, sweet receptor mRNA expression and appetite signals regulation in post-weanling rats. Peptides 2018, 107, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.T.; Lin, C.H.; Pai, H.L.; Chen, Y.C.; Cheng, K.P.; Kuo, H.Y.; Li, C.H.; Ou, H.Y. Sucralose, a Non-nutritive Artificial Sweetener Exacerbates High Fat Diet-Induced Hepatic Steatosis Through Taste Receptor Type 1 Member 3. Front. Nutr. 2022, 9, 823723. [Google Scholar] [CrossRef] [PubMed]
- Steensels, S.; Cools, L.; Avau, B.; Vancleef, L.; Farré, R.; Verbeke, K.; Depoortere, I. Supplementation of oligofructose, but not sucralose, decreases high-fat diet induced body weight gain in mice independent of gustducin-mediated gut hormone release. Mol. Nutr. Food Res. 2017, 61, 1600716. [Google Scholar] [CrossRef] [PubMed]
- van Opstal, A.M.; Kaal, I.; van den Berg-Huysmans, A.A.; Hoeksma, M.; Blonk, C.; Pijl, H.; Rombouts, S.; van der Grond, J. Dietary sugars and non-caloric sweeteners elicit different homeostatic and hedonic responses in the brain. Nutrition 2019, 60, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Appleton, K.M.; Tuorila, H.; Bertenshaw, E.J.; de Graaf, C.; Mela, D.J. Sweet taste exposure and the subsequent acceptance and preference for sweet taste in the diet: Systematic review of the published literature. Am. J. Clin. Nutr. 2018, 107, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Steinert, R.; Frey, F.; Töpfer, A.; Drewe, J.; Beglinger, C. Effects of carbohydrate sugars and artificial sweeteners on appetite and the secretion of gastrointestinal satiety peptides. Br. J. Nutr. 2011, 105, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Maersk, M.; Belza, A.; Holst, J.J.; Fenger-Grøn, M.; Pedersen, S.B.; Astrup, A.; Richelsen, B. Satiety scores and satiety hormone response after sucrose-sweetened soft drink compared with isocaloric semi-skimmed milk and with non-caloric soft drink: A controlled trial. Eur. J. Clin. Nutr. 2012, 66, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Ford, H.E.; Peters, V.; Martin, N.M.; Sleeth, M.L.; Ghatei, M.A.; Frost, G.S.; Bloom, S.R. Effects of oral ingestion of sucralose on gut hormone response and appetite in healthy normal-weight subjects. Eur. J. Clin. Nutr. 2011, 65, 508–613. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Bellon, M.; Wishart, J.M.; Young, R.; Blackshaw, L.A.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Effect of the artificial sweetener, sucralose, on gastric emptying and incretin hormone release in healthy subjects. Am. J. Physiol.-Gastrointest. Liver Physiol. 2009, 296, G735–G739. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chang, J.; Checklin, H.L.; Young, R.L.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Effect of the artificial sweetener, sucralose, on small intestinal glucose absorption in healthy human subjects. Br. J. Nutr. 2010, 104, 803–806. [Google Scholar] [CrossRef]
- Fujita, Y.; Wideman, R.D.; Speck, M.; Asadi, A.; King, D.S.; Webber, T.D.; Haneda, M.; Kieffer, T.J. Incretin release from gut is acutely enhanced by sugar but not by sweeteners in vivo. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E473–E479. [Google Scholar] [CrossRef] [PubMed]
- Swithers, S.E.; Laboy, A.F.; Clark, K.; Cooper, S.; Davidson, T.L. Experience with the high-intensity sweetener saccharin impairs glucose homeostasis and GLP-1 release in rats. Behav. Brain Res. 2012, 233, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Temizkan, S.; Deyneli, O.; Yasar, M.; Arpa, M.; Gunes, M.; Yazici, D.; Sirikci, O.; Haklar, G.; Imeryuz, N.; Yavuz, D.G. Sucralose enhances GLP-1 release and lowers blood glucose in the presence of carbohydrate in healthy subjects but not in patients with type 2 diabetes. Eur. J. Clin. Nutr. 2015, 69, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Grotz, V.L.; Henry, R.R.; McGill, J.B.; Prince, M.J.; Shamoon, H.; Trout, J.R.; Pi-Sunyer, F.X. Lack of effect of sucralose on glucose homeostasis in subjects with type 2 diabetes. J. Am. Diet. Assoc. 2003, 103, 1607–1612. [Google Scholar] [CrossRef] [PubMed]
- Romo-Romo, A.; Aguilar-Salinas, C.A.; Brito-Córdova, G.X.; Gómez-Díaz, R.A.; Almeda-Valdes, P. Sucralose decreases insulin sensitivity in healthy subjects: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.Y.; Friel, J.K.; MacKay, D.S. The effect of the artificial sweeteners on glucose metabolism in healthy adults: A randomized, double-blinded, crossover clinical trial. Appl. Physiol. Nutr. Metab. 2020, 45, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Dalenberg, J.R.; Patel, B.P.; Denis, R.; Veldhuizen, M.G.; Nakamura, Y.; Vinke, P.C.; Luquet, S.; Small, D.M. Short-Term Consumption of Sucralose with, but Not without, Carbohydrate Impairs Neural and Metabolic Sensitivity to Sugar in Humans. Cell Metab. 2020, 31, 493–502.e7. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.E.; Perez, V. Low-calorie sweeteners and body weight and composition: A meta-analysis of randomized controlled trials and prospective cohort studies. Am. J. Clin. Nutr. 2014, 100, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Abou-Setta, A.M.; Chauhan, B.F.; Rabbani, R.; Lys, J.; Copstein, L.; Mann, A.; Jeyaraman, M.M.; Reid, A.E.; Fiander, M.; et al. Nonnutritive sweeteners and cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ 2017, 189, E929–E939. [Google Scholar] [CrossRef]
- Toews, I.; Küllenberg de Gaudry, D.; Lohner, S.; Sommer, H.; Meerpohl, J.J. Association between intake of non-sugar sweeteners and health outcomes: Systematic review and meta-analyses of randomised and non-randomised controlled trials and observational studies. BMJ 2019, 364, k4718. [Google Scholar] [CrossRef]
- Walbolt, J.; Koh, Y. Non-nutritive Sweeteners and Their Associations with Obesity and Type 2 Diabetes. J. Obes. Metab. Syndr. 2020, 29, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Mbambo, N.P.; Dlamini, S.N.; Chukwuma, C.I.; Islam, M.S. Comparative effects of commonly used commercially available non-nutritive sweeteners on diabetes-related parameters in non-diabetic rats. J. Food Biochem. 2020, 44, e13453. [Google Scholar] [CrossRef]
- Faraoni, D.; Schaefer, S.T. Randomized controlled trials vs. observational studies: Why not just live together? BMC Anesthesiol. 2016, 16, 102. [Google Scholar] [CrossRef] [PubMed]
- Fernainy, P.; Cohen, A.A.; Murray, E.; Losina, E.; Lamontagne, F.; Sourial, N. Rethinking the pros and cons of randomized controlled trials and observational studies in the era of big data and advanced methods: A panel discussion. BMC Proc. 2024, 18 (Suppl. S2), 1. [Google Scholar] [CrossRef]
- Cason, A.M.; Aston-Jones, G. Attenuation of saccharin-seeking in rats by orexin/hypocretin receptor 1 antagonist. Psychopharmacology 2013, 228, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Stuber, G.D.; Wise, R.A. Lateral hypothalamic circuits for feeding and reward. Nat. Neurosci. 2016, 19, 198–205. [Google Scholar] [CrossRef]
- De Araujo, I.E.; Schatzker, M.; Small, D.M. Rethinking Food Reward. Annu. Rev. Psychol. 2020, 71, 139–164. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q. Gain weight by “going diet”? Artificial sweeteners and the neurobiology of sugar cravings: Neuroscience 2010. Yale J. Biol. Med. 2010, 83, 101–108. [Google Scholar]
- Han, P.; Bagenna, B.; Fu, M. The sweet taste signalling pathways in the oral cavity and the gastrointestinal tract affect human appetite and food intake: A review. Int. J. Food Sci. Nutr. 2019, 70, 125–135. [Google Scholar] [CrossRef]
- Smeets, P.A.; de Graaf, C.; Stafleu, A.; van Osch, M.J.; van der Grond, J. Functional magnetic resonance imaging of human hypothalamic responses to sweet taste and calories. Am. J. Clin. Nutr. 2005, 82, 1011–1016. [Google Scholar] [CrossRef]
- Suzuki, K.; Jayasena, C.N.; Bloom, S.R. Obesity and appetite control. Exp. Diabetes Res. 2012, 2012, 824305. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.D.; Goossens, G.H.; Blaak, E.E. The Impact of Artificial Sweeteners on Body Weight Control and Glucose Homeostasis. Front. Nutr. 2021, 7, 598340. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.-J.; Kokrashvili, Z.; Theodorakis, M.J.; Carlson, O.D.; Kim, B.-J.; Zhou, J.; Kim, H.H.; Xu, X.; Chan, S.L.; Juhaszova, M.; et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc. Natl. Acad. Sci. USA 2007, 104, 15069–15074. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, J.; Lee, J.Y.; Mattes, R.D. The cephalic phase insulin response to nutritive and low-calorie sweeteners in solid and beverage form. Physiol. Behav. 2017, 181, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Pullicin, A.J.; Glendinning, J.I.; Lim, J. Cephalic phase insulin release: A review of its mechanistic basis and variability in humans. Physiol. Behav. 2021, 239, 113514. [Google Scholar] [CrossRef] [PubMed]
- Pepino, M.Y.; Tiemann, C.D.; Patterson, B.W.; Wice, B.M.; Klein, S. Sucralose Affects Glycemic and Hormonal Responses to an Oral Glucose Load. Diabetes Care 2013, 36, 2530–2535. [Google Scholar] [CrossRef] [PubMed]
- Sylvetsky, A.C.; Brown, R.J.; Blau, J.E.; Walter, M.; Rother, K.I. Hormonal responses to non-nutritive sweeteners in water and diet soda. Nutr. Metab. 2016, 13, 71. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zhao, B.R.; Bound, M.J.; Checklin, H.L.; Bellon, M.; Little, T.J.; Young, R.; Jones, K.; Horowitz, M.; Rayner, C.K. Effects of different sweet preloads on incretin hormone secretion, gastric emptying, and postprandial glycemia in healthy humans. Am. J. Clin. Nutr. 2012, 95, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Bound, M.J.; Standfield, S.D.; Bellon, M.; Young, R.L.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Artificial Sweeteners Have No Effect on Gastric Emptying, Glucagon-Like Peptide-1, or Glycemia after Oral Glucose in Healthy Humans. Diabetes Care 2013, 36, e202–e203. [Google Scholar] [CrossRef]
- Horwitz, D.L.; McLane, M.; Kobe, P. Response to Single Dose of Aspartame or Saccharin by NIDDM Patients. Diabetes Care 1988, 11, 230–234. [Google Scholar] [CrossRef]
- Hall, W.L.; Millward, D.J.; Rogers, P.J.; Morgan, L.M. Physiological mechanisms mediating aspartame-induced satiety. Physiol. Behav. 2003, 78, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Woodward, O.R.M.; Gribble, F.M.; Reimann, F.; Lewis, J.E. Gut peptide regulation of food intake—Evidence for the modulation of hedonic feeding. J. Physiol. 2022, 600, 1053–1078. [Google Scholar] [CrossRef] [PubMed]
- Bowers, M.E.; Choi, D.C.; Ressler, K.J. Neuropeptide regulation of fear and anxiety: Implications of cholecystokinin, endogenous opioids, and neuropeptide Y. Physiol. Behav. 2012, 107, 699–710. [Google Scholar] [CrossRef] [PubMed]
- NamKoong, C.; Kim, M.S.; Jang, B.T.; Lee, Y.H.; Cho, Y.M.; Choi, H.J. Central administration of GLP-1 and GIP decreases feeding in mice. Biochem. Biophys. Res. Commun. 2017, 490, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Ten Kulve, J.S.; Veltman, D.J.; van Bloemendaal, L.; Barkhof, F.; Drent, M.L.; Diamant, M.; IJzerman, R.G. Liraglutide Reduces CNS Activation in Response to Visual Food Cues Only After Short-term Treatment in Patients With Type 2 Diabetes. Diabetes Care 2016, 39, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Schnabl, K.; Gabler, S.M.; Willershäuser, M.; Reber, J.; Karlas, A.; Laurila, S.; Lahesmaa, M.; U Din, M.; Bast-Habersbrunner, A.; et al. Secretin-Activated Brown Fat Mediates Prandial Thermogenesis to Induce Satiation. Cell 2018, 175, 1561–1574.e12. [Google Scholar] [CrossRef] [PubMed]
- Batterham, R.L.; Cohen, M.A.; Ellis, S.M.; Le Roux, C.W.; Withers, D.J.; Frost, G.S.; Ghatei, M.A.; Bloom, S.R. Inhibition of food intake in obese subjects by peptide YY3-36. N. Engl. J. Med. 2003, 349, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Torruella-Suárez, M.L.; McElligott, Z.A. Neurotensin in reward processes. Neuropharmacology 2020, 167, 108005. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, K.; Yabe, D. Dietary and Nutritional Guidelines for People with Diabetes. Nutrients 2023, 15, 4314. [Google Scholar] [CrossRef]
- Green, E.; Murphy, C. Altered processing of sweet taste in the brain of diet soda drinkers. Physiol. Behav. 2012, 107, 560–567. [Google Scholar] [CrossRef]
- Rudenga, K.J.; Small, D.M. Amygdala response to sucrose consumption is inversely related to artificial sweetener use. Appetite 2012, 58, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Mossavar-Rahmani, Y.; Kamensky, V.; Manson, J.E.; Silver, B.; Rapp, S.R.; Haring, B.; Beresford, S.A.A.; Snetselaar, L.; Wassertheil-Smoller, S. Artificially Sweetened Beverages and Stroke, Coronary Heart Disease, and All-Cause Mortality in the Women’s Health Initiative. Stroke 2019, 50, 555–562. [Google Scholar] [CrossRef]
- Debras, C.; Chazelas, E.; Sellem, L.; Porcher, R.; Druesne-Pecollo, N.; Esseddik, Y.; de Edelenyi, F.S.; Agaësse, C.; De Sa, A.; Lutchia, R.; et al. Artificial sweeteners and risk of cardiovascular diseases: Results from the prospective NutriNet-Santé cohort. BMJ 2022, 378, e071204. [Google Scholar] [CrossRef] [PubMed]
- Morales-Ríos, E.I.; García-Machorro, J.; Briones-Aranda, A.; Gómez-Pliego, R.; Espinosa-Raya, J. Effect of Long-Term Intake of Nutritive and Non-Nutritive Sweeteners on Metabolic Health and Cognition in Adult Male Rats. J. Med. Food 2022, 25, 1059–1065. [Google Scholar] [CrossRef]
- Yan, S.; Yan, F.; Liu, L.; Li, B.; Liu, S.; Cui, W. Can Artificial Sweeteners Increase the Risk of Cancer Incidence and Mortality: Evidence from Prospective Studies. Nutrients 2022, 14, 3742. [Google Scholar] [CrossRef]
- Hodge, A.M.; Karim, M.N.; Hébert, J.R.; Shivappa, N.; de Courten, B. Association between Diet Quality Indices and Incidence of Type 2 Diabetes in the Melbourne Collaborative Cohort Study. Nutrients 2021, 13, 4162. [Google Scholar] [CrossRef]
- McCullough, M.L.; Teras, L.R.; Shah, R.; Diver, W.R.; Gaudet, M.M.; Gapstur, S.M. Artificially and sugar-sweetened carbonated beverage consumption is not associated with risk of lymphoid neoplasms in older men and women. J. Nutr. 2014, 144, 2041–2049. [Google Scholar] [CrossRef]
- Rycerz, K.; Jaworska-Adamu, J.E. Effects of aspartame metabolites on astrocytes and neurons. Folia Neuropathol. 2013, 51, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Ylmaz, S.; Uar, A. A review of the genotoxic and carcinogenic effects of aspartame: Does it safe or not? Cytotechnology 2014, 66, 875–881. [Google Scholar] [CrossRef]
- Soffritti, M.; Belpoggi, F.; Manservigi, M.; Tibaldi, E.; Lauriola, M.; Falcioni, L.; Bua, L. Aspartame administered in feed, beginning prenatally through life span, induces cancers of the liver and lung in male Swiss mice. Am. J. Ind. Med. 2010, 53, 1197–1206. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the re-evaluation of aspartame (E 951) as a food additive. Efsa J. 2013, 11, 3496. [Google Scholar]
- Soffritti, M.; Belpoggi, F.; Degli Esposti, D.; Lambertini, L.; Tibaldi, E.; Rigano, A. First experimental demonstration of the multipotential carcinogenic effects of aspartame administered in the feed to Sprague-Dawley rats. Environ. Health Perspect. 2006, 114, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Debras, C.; Chazelas, E.; Srour, B.; Druesne-Pecollo, N.; Esseddik, Y.; Szabo de Edelenyi, F.; Agaësse, C.; De Sa, A.; Lutchia, R.; Gigandet, S.; et al. Artificial sweeteners and cancer risk: Results from the NutriNet-Sante population-based cohort study. PLoS Med. 2022, 19, e1003950. [Google Scholar] [CrossRef]
- Dhingra, R.; Sullivan, L.; Jacques, P.F.; Wang, T.J.; Fox, C.S.; Meigs, J.B.; D’Agostino, R.B.; Gaziano, J.M.; Vasan, R.S. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation 2007, 116, 480–488. [Google Scholar] [CrossRef]
- Lutsey, P.L.; Steffen, L.M.; Stevens, J. Dietary intake and the development of the metabolic syndrome: The atherosclerosis risk in communities study. Circulation 2008, 117, 754–761. [Google Scholar] [CrossRef]
- Nettleton, J.A.; Lutsey, P.L.; Wang, Y.; Lima, J.A.; Michos, E.D.; Jacobs, D.R., Jr. Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2009, 32, 688–694. [Google Scholar] [CrossRef]
- Wilk, K.; Korytek, W.; Pelczyńska, M.; Moszak, M.; Bogdański, P. The Effect of Artificial Sweeteners Use on Sweet Taste Perception and Weight Loss Efficacy: A Review. Nutrients 2022, 14, 1261. [Google Scholar] [CrossRef]
- Sylvetsky, A.C.; Welsh, J.A.; Brown, R.J.; Vos, M.B. Low-calorie sweetener consumption is increasing in the United States. Am. J. Clin. Nutr. 2012, 96, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.K.; Pretorius, E. Revisiting the safety of aspartame. Nutr. Rev. 2017, 75, 718–730. [Google Scholar] [CrossRef]
- Peters, J.C.; Beck, J.; Cardel, M.; Wyatt, H.R.; Foster, G.D.; Pan, Z.; Wojtanowski, A.C.; Vander Veur, S.S.; Herring, S.J.; Brill, C.; et al. The effects of water and non-nutritive sweetened beverages on weight loss and weight maintenance: A randomized clinical trial. Obesity 2016, 24, 297–304. [Google Scholar] [CrossRef]
- Redman, L.M.; Ravussin, E. Caloric restriction in humans: Impact on physiological, psychological, and behavioral outcomes. Antioxid. Redox Signal. 2011, 14, 275–287. [Google Scholar] [CrossRef]
- Beauchamp, G.K. Why do we like sweet taste: A bitter tale? Physiol. Behav. 2016, 164 Pt B, 432437. [Google Scholar] [CrossRef]
- Tappy, L. Fructose-containing caloric sweeteners as a cause of obesity and metabolic disorders. J. Exp. Biol. 2018, 221 (Suppl. S1), jeb164202. [Google Scholar] [CrossRef]
- Tappy, L.; Lê, K.A. Health effects of fructose and fructose-containing caloric sweeteners: Where do we stand 10 years after the initial whistle blowings? Curr. Diab. Rep. 2015, 15, 54. [Google Scholar] [CrossRef]
- Bouzalmate Hajjaj, A.; Massó Guijarro, P.; Khan, K.S.; Bueno-Cavanillas, A.; Cano-Ibáñez, N. A systematic review and meta-analysis of weight loss in control group participants of lifestyle randomized trials. Sci. Rep. 2022, 12, 12252. [Google Scholar] [CrossRef]
- Lecoultre, V.; Carrel, G.; Egli, L.; Binnert, C.; Boss, A.; MacMillan, E.L.; Kreis, R.; Boesch, C.; Darimont, C.; Tappy, L. Coffee consumption attenuates short-term fructose-induced liver insulin resistance in healthy men. Am. J. Clin. Nutr. 2014, 99, 268–275. [Google Scholar] [CrossRef]
- Busnatu, S.S.; Salmen, T.; Pana, M.A.; Rizzo, M.; Stallone, T.; Papanas, N.; Popovic, D.; Tanasescu, D.; Serban, D.; Stoian, A.P. The Role of Fructose as a Cardiovascular Risk Factor: An Update. Metabolites 2022, 12, 67. [Google Scholar] [CrossRef]
- Stanhope, K.L.; Schwarz, J.M.; Keim, N.L.; Griffen, S.C.; Bremer, A.A.; Graham, J.L.; Hatcher, B.; Cox, C.L.; Dyachenko, A.; Zhang, W.; et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Investig. 2009, 119, 1322–1334. [Google Scholar] [CrossRef]
- Teysseire, F.; Bordier, V.; Beglinger, C.; Wölnerhanssen, B.K.; Meyer-Gerspach, A.C. Metabolic Effects of Selected Conventional and Alternative Sweeteners: A Narrative Review. Nutrients 2024, 16, 622. [Google Scholar] [CrossRef]
- Sylvetsky, A.C.; Greenberg, M.; Zhao, X.; Rother, K.I. What parents think about giving nonnutritive sweeteners to their children: A pilot study. Int. J. Pediatr. 2014, 2014, 819872. [Google Scholar] [CrossRef]
- Boulton, J.; Hashem, K.M.; Jenner, K.H.; Lloyd-Williams, F.; Bromley, H.; Capewell, S. How much sugar is hidden in drinks marketed to children? A survey of fruit juices, juice drinks and smoothies. BMJ Open 2016, 6, e010330. [Google Scholar] [CrossRef]
- Forshee, R.A.; Storey, M.L. Total beverage consumption and beverage choices among children and adolescents. Int. J. Food Sci. Nutr. 2003, 54, 297–307. [Google Scholar] [CrossRef]
- Giammattei, J.; Blix, G.; Marshak, H.H.; Wollitzer, A.O.; Pettitt, D.J. Television Watching and Soft Drink Consumption. Arch. Pediatr. Adolesc. Med. 2003, 157, 882. [Google Scholar] [CrossRef]
- Laverty, A.A.; Magee, L.; Monteiro, C.A.; Saxena, S.; Millett, C. Sugar and artificially sweetened beverage consumption and adiposity changes: National longitudinal study. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 137. [Google Scholar] [CrossRef]
- Katzmarzyk, P.T.; Broyles, S.T.; Champagne, C.M.; Chaput, J.P.; Fogelholm, M.; Hu, G.; Kuriyan, R.; Kurpad, A.; Lambert, E.V.; Maia, J.; et al. Relationship between Soft Drink Consumption and Obesity in 9–11 Years Old Children in a Multi-National Study. Nutrients 2016, 8, 770. [Google Scholar] [CrossRef]
- Sylvetsky, A.C.; Jin, Y.; Mathieu, K.; DiPietro, L.; Rother, K.I.; Talegawkar, S.A. Low-Calorie Sweeteners: Disturbing the Energy Balance Equation in Adolescents? Obesity 2017, 25, 2049–2054. [Google Scholar] [CrossRef]
- Filippi, B.M.; Abraham, M.A.; Yue, J.T.; Lam, T.K. Insulin and glucagon signaling in the central nervous system. Rev. Endocr. Metab. Disord. 2013, 14, 365–375. [Google Scholar] [CrossRef]
- Johnson, R.J.; Lanaspa, M.; Sanchez Lozada, L.G.; Tolan, D.; Nakagawa, T.; Ishimoto, T.; Andres-Hernando, A.; Rodriguez-Iturbe, B.; Stenvinkel, P. The fructose survival hypothesis for obesity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2023, 378, 20220230. [Google Scholar] [CrossRef]
- Johnson, R.J.; Sánchez-Lozada, L.G.; Lanaspa, M.A. The fructose survival hypothesis as a mechanism for unifying the various obesity hypotheses. Obesity 2024, 32, 12–22. [Google Scholar] [CrossRef]
- Pasqualli, T.; Chaves, P.E.E.; Pereira, C.L.D.V.; Serpa, É.A.; Oliveira, L.F.S.; Machado, M.M. Steviol, the active principle of the stevia sweetener, causes a reduction of the cells of the immunological system even consumed in low concentrations. Immunopharmacol. Immunotoxicol. 2020, 42, 504–508. [Google Scholar] [CrossRef]
- Uçar, A.; Yılmaz, S.; Yılmaz, Ş.; Kılıç, M.S. A research on the genotoxicity of stevia in human lymphocytes. Drug Chem. Toxicol. 2018, 41, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Goswami, U.; Pusalavidyasagar, S. Restless legs syndrome associated with use of stevia nonnutritive sweetener. J. Clin. Sleep. Med. 2020, 16, 1819–1821. [Google Scholar] [CrossRef] [PubMed]
- Shannon, M.; Rehfeld, A.; Frizzell, C.; Livingstone, C.; McGonagle, C.; Skakkebaek, N.E.; Wielogórska, E.; Connolly, L. In vitro bioassay investigations of the endocrine disrupting potential of steviol glycosides and their metabolite steviol, components of the natural sweetener Stevia. Mol. Cell Endocrinol. 2016, 427, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Momtazi-Borojeni, A.A.; Esmaeili, S.A.; Abdollahi, E.; Sahebkar, A. A Review on the Pharmacology and Toxicology of Steviol Glycosides Extracted from Stevia rebaudiana. Curr. Pharm. Des. 2017, 23, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Papazoglou, I.; Lee, J.H.; Cui, Z.; Li, C.; Fulgenzi, G.; Bahn, Y.J.; Staniszewska-Goraczniak, H.M.; Piñol, R.A.; Hogue, I.B.; Enquist, L.W.; et al. A distinct hypothalamus-to-β cell circuit modulates insulin secretion. Cell Metab. 2022, 34, 285–298.e7. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Pérez, S.; Orta-Méndez-Y-Sánchez, I.; García-Gómez, R.S.; Ordaz-Nava, G.; Gracia-Mora, M.I.; Macías-Rosales, L.; Rico-Morales, H.A.; Salas-Garrido, G.; Durán-Domínguez-de-Bazúa, M.D.C. Stevia rebaudiana Bertoni, an American plant used as sweetener: Study of its effects on body mass control and glycemia reduction in Wistar male and female rats. PLoS ONE 2024, 19, e0298251. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.V.; Small, D.M. Physiological mechanisms by which non-nutritive sweeteners may impact body weight and metabolism. Physiol. Behav. 2015, 152 Pt B, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Langhans, W.; Watts, A.G.; Spector, A.C. The elusive cephalic phase insulin response: Triggers, mechanisms, and functions. Physiol. Rev. 2023, 103, 1423–1485. [Google Scholar] [CrossRef] [PubMed]
- Glendinning, J.I. Oral Post-Oral Actions of Low-Calorie Sweeteners: A Tale of Contradictions and Controversies. Obesity 2018, 26 (Suppl. S3), S9–S17. [Google Scholar] [CrossRef]
- Haga, C.; Tolaymat, L.; Walker, A.; Hedges, M.; Yin, M.; McManus, M.; Dawson, N. Acute Adverse Effects Related to Consumption of Nonnutritive and Low-Calorie Sweeteners. South. Med. J. 2023, 116, 450–454. [Google Scholar] [CrossRef]
- Farup, P.G.; Lydersen, S.; Valeur, J. Are Nonnutritive Sweeteners Obesogenic? Associations between Diet, Faecal Microbiota, and Short-Chain Fatty Acids in Morbidly Obese Subjects. J. Obes. 2019, 2019, 4608315. [Google Scholar] [CrossRef] [PubMed]
- Higgins, K.A.; Mattes, R.D. A randomized controlled trial contrasting the effects of 4 low-calorie sweeteners and sucrose on body weight in adults with overweight or obesity. Am. J. Clin. Nutr. 2019, 109, 1288–1301. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, R.; Zhang, C.; Li, L.; Zhang, J.; Sunm, G. Consumption of Non-Nutritive Sweetener during Pregnancy and Weight Gain in Offspring: Evidence from Human Studies. Nutrients 2022, 14, 5098. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yu, Z.; Zhao, Y.; Yin, D. Obesogenic potentials of environmental artificial sweeteners with disturbances on both lipid metabolism and neural responses. Sci. Total Environ. 2024, 919, 170755. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Khalil, M.; Graziani, A.; Frühbeck, G.; Baffy, G.; Garruti, G.; Di Ciaula, A.; Bonfrate, L. Gut microbes in metabolic disturbances. Promising role for therapeutic manipulations? Eur. J. Intern. Med. 2024, 119, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Conz, A.; Salmona, M.; Diomede, L. Effect of Non-Nutritive Sweeteners on the Gut Microbiota. Nutrients 2023, 15, 1869. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Pastor-Villaescusa, B.; Rueda-Robles, A.; Abadia-Molina, F.; Ruiz-Ojeda, F.J. Plausible Biological Interactions of Low- and Non-Calorie Sweeteners with the Intestinal Microbiota: An Update of Recent Studies. Nutrients 2020, 12, 1153. [Google Scholar] [CrossRef] [PubMed]
- Martyn, D.; Darch, M.; Roberts, A.; Lee, H.Y.; Yaqiong Tian, T.; Kaburagi, N.; Belmar, P. Low-/No-Calorie Sweeteners: A Review of Global Intakes. Nutrients 2018, 10, 357. [Google Scholar] [CrossRef] [PubMed]
- Logue, C.; Dowey, L.C.; Strain, J.J.; Verhagen, H.; Gallagher, A.M. The potential application of a biomarker approach for the investigation of low-calorie sweetener exposure. Proc. Nutr. Soc. 2016, 75, 216–225. [Google Scholar] [CrossRef]
- Wang, Y.; O’Neil, A.; Jiao, Y.; Wang, L.; Huang, J.; Lan, Y.; Zhu, Y.; Yu, C. Sex differences in the association between diabetes and risk of cardiovascular disease, cancer, and all-cause and cause-specific mortality: A systematic review and meta-analysis of 5,162,654 participants. BMC Med. 2019, 17, 136. [Google Scholar] [CrossRef]
- Peters, S.A.; Huxley, R.R.; Woodward, M. Diabetes as a risk factor for stroke in women compared with men: A systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet 2014, 383, 1973–1980. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.A.; Huxley, R.R.; Woodward, M. Diabetes as risk factor for incident coronary heart disease in women compared with men: A systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia 2014, 57, 1542–1551. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.J.; Shan, S.B.; Zhou, Y.H.; Zhong, L.Y. Diabetes mellitus and the risk of gastrointestinal cancer in women compared with men: A meta-analysis of cohort studies. BMC Cancer 2018, 18, 422. [Google Scholar] [CrossRef] [PubMed]
- Mathur, K.; Agrawal, R.K.; Nagpure, S.; Deshpande, D. Effect of artificial sweeteners on insulin resistance among type-2 diabetes mellitus patients. J. Family Med. Prim. Care 2020, 9, 69–71. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antasouras, G.; Dakanalis, A.; Chrysafi, M.; Papadopoulou, S.K.; Trifonidi, I.; Spanoudaki, M.; Alexatou, O.; Pritsa, A.; Louka, A.; Giaginis, C. Could Insulin Be a Better Regulator of Appetite/Satiety Balance and Body Weight Maintenance in Response to Glucose Exposure Compared to Sucrose Substitutes? Unraveling Current Knowledge and Searching for More Appropriate Choices. Med. Sci. 2024, 12, 29. https://doi.org/10.3390/medsci12020029
Antasouras G, Dakanalis A, Chrysafi M, Papadopoulou SK, Trifonidi I, Spanoudaki M, Alexatou O, Pritsa A, Louka A, Giaginis C. Could Insulin Be a Better Regulator of Appetite/Satiety Balance and Body Weight Maintenance in Response to Glucose Exposure Compared to Sucrose Substitutes? Unraveling Current Knowledge and Searching for More Appropriate Choices. Medical Sciences. 2024; 12(2):29. https://doi.org/10.3390/medsci12020029
Chicago/Turabian StyleAntasouras, Georgios, Antonios Dakanalis, Maria Chrysafi, Sousana K. Papadopoulou, Ioulia Trifonidi, Maria Spanoudaki, Olga Alexatou, Agathi Pritsa, Aikaterini Louka, and Constantinos Giaginis. 2024. "Could Insulin Be a Better Regulator of Appetite/Satiety Balance and Body Weight Maintenance in Response to Glucose Exposure Compared to Sucrose Substitutes? Unraveling Current Knowledge and Searching for More Appropriate Choices" Medical Sciences 12, no. 2: 29. https://doi.org/10.3390/medsci12020029
APA StyleAntasouras, G., Dakanalis, A., Chrysafi, M., Papadopoulou, S. K., Trifonidi, I., Spanoudaki, M., Alexatou, O., Pritsa, A., Louka, A., & Giaginis, C. (2024). Could Insulin Be a Better Regulator of Appetite/Satiety Balance and Body Weight Maintenance in Response to Glucose Exposure Compared to Sucrose Substitutes? Unraveling Current Knowledge and Searching for More Appropriate Choices. Medical Sciences, 12(2), 29. https://doi.org/10.3390/medsci12020029








