Hemodialysis without Systemic Anticoagulation: A Randomized Controlled Trial to Evaluate Five Strategies in Patients at a High Risk of Bleeding
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Groups
2.2. Interventions
2.3. Primary Outcomes
- No complete occlusion of air traps or dialyzer rendering HD impossible (grade 4 according to a semi-quantitative scale previously published) [6].
- No additional 0.9% saline flushes to prevent clotting.
- No exchange of dialyzer or bloodlines because of clotting.
- No premature termination (early rinse back) because of clotting.
2.4. Secondary Outcomes
2.5. Study Population
2.6. Statistical Methods
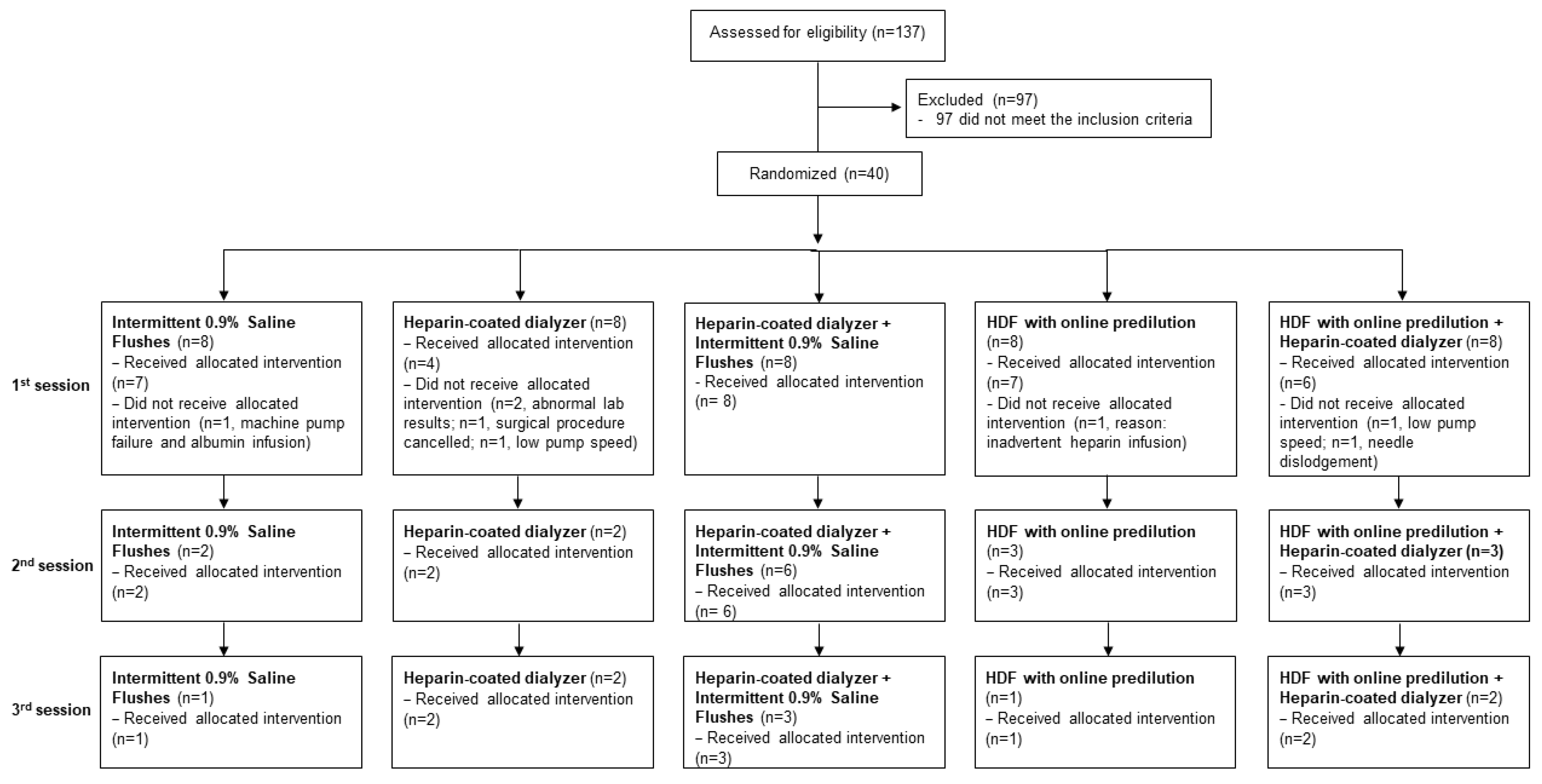
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kessler, M.; Moureau, F.; Nguyen, P. Anticoagulation in Chronic Hemodialysis: Progress Toward an Optimal Approach. Semin. Dial. 2015, 28, 474–489. [Google Scholar] [CrossRef] [PubMed]
- Guery, B.; Alberti, C.; Servais, A.; Harrami, E.; Bererhi, L.; Zins, B.; Touam, M.; Joly, D. Hemodialysis without systemic anticoagulation: A prospective randomized trial to evaluate 3 strategies in patients at risk of bleeding. PLoS ONE 2014, 9, e97187. [Google Scholar] [CrossRef] [PubMed]
- Kerr, P.; Perkovic, V.; Petrie, J.; Agar, J.; Disney, A. Caring for Australians with Renal I: The CARI guidelines. Dialysis adequacy (HD) guidelines. Nephrology 2005, 10 (Suppl. S4), S61–S80. [Google Scholar] [PubMed]
- European Best Practice Guidelines Expert Group on Hemodialysis, E.R.A. Section V. Chronic intermittent haemodialysis and prevention of clotting in the extracorporal system. Nephrol. Dial. Transplant. 2002, 17 (Suppl. S7), 63–71. [Google Scholar] [CrossRef] [PubMed]
- Lavaud, S.; Canivet, E.; Wuillai, A.; Maheut, H.; Randoux, C.; Bonnet, J.M.; Renaux, J.L.; Chanard, J. Optimal anticoagulation strategy in haemodialysis with heparin-coated polyacrylonitrile membrane. Nephrol. Dial. Transplant. 2003, 18, 2097–2104. [Google Scholar] [CrossRef] [PubMed]
- Laville, M.; Dorval, M.; Fort Ros, J.; Fay, R.; Cridlig, J.; Nortier, J.L.; Juillard, L.; Debska-Slizien, A.; Fernandez Lorente, L.; Thibaudin, D.; et al. Results of the HepZero study comparing heparin-grafted membrane and standard care show that heparin-grafted dialyzer is safe and easy to use for heparin-free dialysis. Kidney Int. 2014, 86, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.B.; Kim, B.; Lee, Y.H.; Yoon, S.J.; Kang, W.H.; Huh, W.; Kim, D.J.; Oh, H.Y.; Kim, Y.G. Hemodialysis using heparin-bound Hemophan in patients at risk of bleeding. Nephron Clin. Pract. 2004, 97, c5–c10. [Google Scholar] [CrossRef] [PubMed]
- Zimbudzi, E. Intermittent saline flushes or continuous saline infusion: What works better when heparin-free dialysis is recommended? Int. J. Nephrol. Renov. Dis. 2013, 6, 65–69. [Google Scholar] [CrossRef]
- Vandenbosch, I.; Dejongh, S.; Claes, K.; Bammens, B.; De Vusser, K.; Van Craenenbroeck, A.; Kuypers, D.; Evenepoel, P.; Meijers, B. Strategies for asymmetrical triacetate dialyser heparin-free effective haemodialysis: The SAFE study. Clin. Kidney J. 2021, 14, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
- Meijers, B.; Metalidis, C.; Vanhove, T.; Poesen, R.; Kuypers, D.; Evenepoel, P. A noninferiority trial comparing a heparin-grafted membrane plus citrate-containing dialysate versus regional citrate anticoagulation: Results of the CiTED study. Nephrol. Dial. Transplant. 2017, 32, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Francois, K.; De Clerck, D.; Tonnelier, A.; Cambier, M.L.; Orlando, C.; Jochmans, K.; Cools, W.; Wissing, K.M. Dialyzer Performance During Hemodialysis Without Systemic Anticoagulation Using a Heparin-Grafted Dialyzer Combined With a Citrate-Enriched Dialysate: Results of the Randomized Crossover Noninferiority EvoCit Study. Am. J. Kidney Dis. 2022, 79, 79–87.e71. [Google Scholar] [CrossRef] [PubMed]
- Francois, K.; Wissing, K.M.; Jacobs, R.; Boone, D.; Jacobs, K.; Tielemans, C. Avoidance of systemic anticoagulation during intermittent haemodialysis with heparin-grafted polyacrilonitrile membrane and citrate-enriched dialysate: A retrospective cohort study. BMC Nephrol. 2014, 15, 104. [Google Scholar] [CrossRef] [PubMed]
- Krummel, T.; Cellot, E.; Thiery, A.; De Geyer, G.; Keller, N.; Hannedouche, T. Hemodialysis without anticoagulation: Less clotting in conventional hemodialysis than in predilution hemodiafiltration. Hemodial. Int. 2019, 23, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Lucchi, L.; Ligabue, G.; Marietta, M.; Delnevo, A.; Malagoli, M.; Perrone, S.; Stipo, L.; Grandi, F.; Albertazzi, A. Activation of coagulation during hemodialysis: Effect of blood lines alone and whole extracorporeal circuit. Artif. Organs 2006, 30, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Klingel, R.; Schaefer, M.; Schwarting, A.; Himmelsbach, F.; Altes, U.; Uhlenbusch-Korwer, I.; Hafner, G. Comparative analysis of procoagulatory activity of haemodialysis, haemofiltration and haemodiafiltration with a polysulfone membrane (APS) and with different modes of enoxaparin anticoagulation. Nephrol. Dial. Transplant. 2004, 19, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Evenepoel, P.; Dejagere, T.; Verhamme, P.; Claes, K.; Kuypers, D.; Bammens, B.; Vanrenterghem, Y. Heparin-coated polyacrylonitrile membrane versus regional citrate anticoagulation: A prospective randomized study of 2 anticoagulation strategies in patients at risk of bleeding. Am. J. Kidney Dis. 2007, 49, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hongying, N. Efficacy and safety of regional citrate anticoagulation in critically ill patients undergoing continuous renal replacement therapy. Intensive Care Med. 2012, 38, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Singer, R.F.; Williams, O.; Mercado, C.; Chen, B.; Talaulikar, G.; Walters, G.; Roberts, D.M. Regional citrate anticoagulation in hemodialysis: An observational study of safety, efficacy, and effect on calcium balance during routine care. Can. J. Kidney Health Dis. 2016, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Roach, R.E.; Lijfering, W.M.; Rosendaal, F.R.; Cannegieter, S.C.; le Cessie, S. Sex difference in risk of second but not of first venous thrombosis: Paradox explained. Circulation 2014, 129, 51–56. [Google Scholar] [CrossRef]
- Sahota, S.; Rodby, R. Inpatient hemodialysis without anticoagulation in adults. Clin. Kidney J. 2014, 7, 552–556. [Google Scholar] [CrossRef]
- Delgado Cordova, M.; Blanco, N.; Azana, C. Hypotension in hemodialysis secondary to a reaction to synthetic membranes. Nefrologia 2018, 38, 329–330. [Google Scholar] [CrossRef]
- Gonzalez Sanchidrian, S.; Labrador Gomez, P.J.; Marin Alvarez, J.P.; Jimenez Herrero, M.C.; Castellano Cervino, I.; Gallego Dominguez, S.; Sanchez-Montalban, J.M.; Deira Lorenzo, J.; Davin Carrero, E.; Polanco Candelario, S.; et al. Reaction to synthetic membranes in hemodialysis. Nefrologia 2016, 36, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Skagerlind, M.S.E.; Stegmayr, B.G. An evaluation of four modes of low-dose anticoagulation during intermittent haemodialysis. Eur. J. Clin. Pharmacol. 2018, 74, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Hoenich, N.A.; Levin, R.; Pearce, C. Clinical waste generation from renal units: Implications and solutions. Semin. Dial. 2005, 18, 396–400. [Google Scholar] [CrossRef] [PubMed]

| Group 1 Intermittent 0.9% Saline Flushes | Group 2 Evodial® | Group 3 Evodial® + Intermittent 0.9% Saline Flushes | Group 4 HDF | Group 5 HDF + Evodial® | |
|---|---|---|---|---|---|
| n = 7 | n = 4 | n = 8 | n = 7 | n = 6 | |
| Age | 62 (36–67) | 72 (62–78) | 69 (51–77) | 57 (38–59) | 58 (46–79) |
| Male gender | 3 (43%) | 3 (75%) | 7 (88%) | 4 (57%) | 5 (83%) |
| Dialysis vintage (years), (n = 30) | |||||
| 2005–2014 | 2 (29%) | 2 (50%) | 5 (63%) | 1 17%) | 0 (0%) |
| 2015–2020 | 5 (71%) | 2 (50%) | 3 (38%) | 5 (83%) | 5 (100%) |
| Primary renal disease | |||||
| Glomerulonephritis | 1 (14%) | 0 (0%) | 1 (13%) | 1 (14%) | 0 (0%) |
| Diabetic nephropathy | 4 (57%) | 2 (50%) | 2 (25%) | 1 (14%) | 2 (33%) |
| Hypertension | 0 (0%) | 1 (25%) | 1 (13%) | 1 (14%) | 0 (0%) |
| Polycystic kidney disease | 1 (14%) | 0 (0%) | 1 (13%) | 0 (0% | 0 (0%) |
| Unknown | 0 (0%) | 1 (25%) | 0 (0%) | 1 (14%) | 1 (17%) |
| Other * | 1 (14%) | 0 (0%) | 3 (38%) | 3 (43%) | 3 (50%) |
| Main comorbidities | |||||
| Hypertension | 4 (57%) | 4 (100%) | 4 (50%) | 5 (71%) | 5 (83%) |
| Diabetes | 4 (57%) | 3 (75%) | 2 (25%) | 2 (29%) | 4 (67%) |
| Ischemic heart disease | 2 (29%) | 2 (50%) | 3 (38%) | 3 (43%) | 1 (17%) |
| Peripheral arterial disease | 0 (0%) | 2 (50%) | 2 (25%) | 1 (14%) | 3 (50%) |
| Dyslipidemia | 1 (14%) | 1 (25%) | 3 (38%) | 3 (43%) | 2 (33%) |
| Previous DVT and/or PE | 0 (0%) | 1 (25%) | 2 (25%) | 1 (14%) | 1 (17%) |
| Concomitant medications | |||||
| Antacid preparations * | 4 (57%) | 2 (50%) | 7 (88%) | 5 (71%) | 3 (50%) |
| Oral hypoglycemic agents | 3 (43%) | 2 (50%) | 2 (25%) | 1 (14%) | 3 (50%) |
| IV iron | 4 (57%) | 3 (75%) | 6 (75%) | 5 (71%) | 6 (100%) |
| Calcium carbonate | 3 (43%) | 3 (75%) | 5 (63%) | 4 (57%) | 6 (100%) |
| Diuretics | 2 (29%) | 1 (25%) | 0 (0%) | 1 (14%) | 3 (50%) |
| Beta-blockers | 3 (43%) | 1 (25%) | 3 (38%) | 5 (71%) | 3 (50%) |
| Renin–angiotensin system antagonists | 2 (29%) | 0 (0%) | 2 (25%) | 2 (29%) | 1 (17%) |
| Erythropoietin agent | 6 (86%) | 0 (0%) | 4 (50%) | 4 (57%) | 6 (100%) |
| Acetylsalicylic acid | 3 (43%) | 3 (75%) | 7 (88%) | 2 (29%) | 6 (100%) |
| Pathology results prior to the heparin-free session | |||||
| Hemoglobin (g/L) | 102 (83–114) | 126 (102–132) | 95.5 (93–100) | 103 (97–110) | 103 (93–108) |
| Haematocrit (L/L) | 0.31 (0.22–0.38) | 0.37 (0.28–0.41) | 0.29 (0.21–0.36) | 0.30 (0.26–0.35) | 0.32 (0.25–0.40) |
| aPTT * (seconds), (n = 29) | 29 (28–34) | 35 (30–42) | 28.5 (26.5–34.5) | 33 (29–40) | 29 (29–30) |
| INR *, (n = 28) | 1 (1–1.2) | 1 (1–1.1) | 1.05 (1–1.1) | 1 (0.9–1.1) | 1 (0.9–1.1) |
| Creatinine (µmol/L) | 419 (307–585) | 854 (667–947) | 550 (505–680) | 661 (424–886) | 752 (542–824) |
| Urea (mmol/L) | 13.2 (8.6–18.6) | 28.2 (19.5–39.9) | 16.1 (13.8–19.3) | 16.5 (9.5–25) | 18.5 (14.4–25.2) |
| Albumin (g/L) | 32 (24–36) | 30 (25–35) | 30 (28.5–33.5) | 34 (28–35) | 29.5 (29–36) |
| Corrected calcium (mmol/L) | 2.41 (2.3–2.47) | 2.22 (1.96–2.53) | 2.44 (2.32–2.52) | 2.37 (2.33–2.5) | 2.45 (2.31–2.59) |
| Phosphate (mmol/L) | 1.62 (0.66–1.74) | 2.11 (1.72–2.7) | 1.45 (1.24–1.96) | 2.36 (1.29–2.95) | 1.57 (1.28–2.32) |
| CRP (mg/L), (n = 18) | 117 (22–171) | 44.7 (6.4–83) | 21 (5.9–28) | 13 (4–43) | 35 (14–56) |
| Platelet count (×109/L) | 198 (165–285) | 275.5 (221–309) | 258 (234–290) | 210 (186–257) | 181 (143–276) |
| Access type | |||||
| AV fistula | 4 (57%) | 0 (0%) | 6 (75%) | 3 (43%) | 3 (50%) |
| Tunneled catheter | 3 (43%) | 4 (100%) | 2 (25%) | 4 (57%) | 3 (50%) |
| Average pump speed—first session (mL/min) | 300 (300–350) | 268 (250–305) | 298 (250–350) | 294 (250–350) | 303 (270–350) |
| Indications for heparin-free hemodialysis | |||||
| Active bleeding | 0 (0%) | 0 (0%) | 3 (38%) | 1 (14%) | 1 (17%) |
| Bleeding wound | 2 (29%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Pre- and post-operative | 4 (57%) | 4 (100%) | 3 (38%) | 3 (43%) | 3 (50%) |
| Recent biopsy | 0 (0%) | 0 (0%) | 1 (13%) | 0 (0%) | 0 (0%) |
| Tunneled catheter insertion | 1 (14%) | 0 (0%) | 0 (0%) | 2 (29%) | 2 (33%) |
| Recent stroke | 0 (0%) | 0 (0%) | 1 (13%) | 0 (0%) | 0 (0%) |
| Hypertensive crisis | 0 (0%) | 0 (0%) | 0 (0%) | 1 (14%) | 0 (0%) |
| Group 1 Intermittent 0.9% Saline Flushes | Group 2 Evodial® | Group 3 Evodial® + Intermittent 0.9% Saline Flushes | Group 4 HDF | Group 5 HDF + Evodial® | p-Value | |
|---|---|---|---|---|---|---|
| n = 7 | n = 4 | n = 8 | n = 7 | n = 6 | ||
| Successful HD/HDF * | 5 (71%) | 2 (50%) | 8 (100%) | 5 (71%) | 5 (83%) | 0.30 |
| Interrupted session | 2 (29%) | 2 (50%) | 0 (0%) | 2 (29%) | 1 (17%) | |
| Exchange of dialyzer due to clotting | 1 (50%) | 0 (0%) | 0 (0%) | 2 (100%) | 1 (100%) | |
| Early rinse back due to clotting | 1 (50%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Additional flushes to prevent clotting | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Complete occlusion of air traps | 0 (0%) | 2 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Timing of HD/HDF interruption, (n = 7) | ||||||
| Hour 3 | 1 (50%) | 1 (50%) | 0 (0%) | 1 (50%) | 1 (100%) | 1.00 |
| Hour 4 | 1 (50%) | 0 (0%) | 0 (0%) | 1 (50%) | 0 (0%) | |
| Hour 5 | 0 (0%) | 1 (50%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Group 1 Intermittent 0.9% Saline Flushes | Group 2 Evodial® | Group 3 Evodial® + Intermittent 0.9% Saline Flushes | Group 4 HDF | Group 5 HDF + Evodial® | p-Value | ||
|---|---|---|---|---|---|---|---|
| n = 7 | n = 4 | n = 8 | n = 7 | n = 6 | |||
| Online KT/V, (n = 16) | 1.47 (1.4–1.53) | 1.3 (1–1.3) | 1.45 (1.18–1.5) | 1.38 (1.12–1.63) | 1.08 (0.84–1.5) | 0.51 | |
| Clotting score (grades) * | |||||||
| Hour 1, (n = 30) | 1 | 6 (100%) | 2 (67%) | 7 (88%) | 6 (86%) | 6 (100%) | 0.65 |
| 2 | 0 (0%) | 1 (33%) | 1 (13%) | 1 (14%) | 0 (0%) | ||
| Hour 2, (n = 30) | 1 | 3 (50%) | 1 (33%) | 6 (75%) | 6 (86%) | 4 (67%) | 0.50 |
| 2 | 3 (50%) | 2 (67%) | 2 (25%) | 1 (14%) | 2 (33%) | ||
| Hour 3, (n = 29) | 1–2 | 2 (40%) | 3 (100%) | 6 (75%) | 6 (86%) | 5 (83%) | 0.43 |
| 3 | 3 (60%) | 0 (0%) | 2 (25%) | 1 (14%) | 1 (17%) | ||
| Hour 4, (n = 25) | 1–2 | 2 (50%) | 2 (67%) | 5 (63%) | 5 (83%) | 4 (100%) | 0.57 |
| 3–4 | 2 (50%) | 1 (33%) | 3 (38%) | 1 (17%) | 0 (0%) | ||
| Group 1 Intermittent 0.9% Saline Flushes | Group 2 Evodial® | Group 3 Evodial® + Intermittent 0.9% Saline Flushes | Group 4 HDF | Group 5 HDF + Evodial® | ||
|---|---|---|---|---|---|---|
| n = 11 | n = 8 | n = 17 | n = 11 | n = 11 | ||
| Successful HD/HDF * | 7 (64%) | 3 (38%) | 17 (100%) | 6 (55%) | 10 (91%) | |
| Interrupted sessions | 3 (30%) | 5 (63%) | 0 (0%) | 5 (45%) | 1 (9%) | |
| Exchange of dialyzer due to clotting | 2 (67%) | 1 (20%) | 0 (0%) | 3 (60%) | 1 (100%) | |
| Early rinse back due to clotting | 1 (33%) | 2 (40%) | 0 (0%) | 1 (20%) | 0 (0%) | |
| Additional flushes to prevent clotting | 0 (0%) | 0 (0%) | 0 (0%) | 1 (20%) | 0 (0%) | |
| Complete occlusion of air traps | 0 (0%) | 2 (40%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Timing of HD/HDF interruption, (n = 15) | ||||||
| Hour 1 | 0 (0%) | 1 (20%) | 0 (0%) | 1 (20%) | 0 (0%) | |
| Hour 2 | 0 (0%) | 0 (0%) | 0 (0%) | 1 (20%) | 0 (0%) | |
| Hour 3 | 3 (75%) | 1 (20%) | 0 (0%) | 1 (20%) | 1 (100%) | |
| Hour 4 | 1 (25%) | 1 (20%) | 0 (0%) | 2 (40%) | 0 (0%) | |
| Hour 5 | 0 (0%) | 2 (40%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Clotting score (grades) ⁑ | ||||||
| Hour 1, (n = 54) | 1 | 10 (100%) | 5 (83%) | 16 (94%) | 7 (70%) | 11 (100%) |
| 2 | 0 (0%) | 1 (17%) | 1 (6%) | 3 (30%) | 0 (0%) | |
| Hour 2, (n = 54) | 1 | 10 (100%) | 6 (100%) | 17 (100%) | 9 (90%) | 11 (100%) |
| 2 | 0 (0%) | 0 (0%) | 0 (0%) | 1 (10%) | 0 (0%) | |
| Hour 3, (n = 52) | 1–2 | 5 (63%) | 5 (83%) | 15 (88%) | 8 (80%) | 10 (91%) |
| 3 | 3 (38%) | 1 (17%) | 2 (12%) | 2 (20%) | 1 (9%) | |
| Hour 4, (n = 45) | 1–2 | 2 (33%) | 3 (50%) | 13 (76%) | 7 (88%) | 8 (100%) |
| 3–4 | 4 (67%) | 3 (50%) | 4 (24%) | 1 (13%) | 0 (0%) | |
| Online KT/V, (n = 30) | 1.47 (1.4–1.53) | 1.15 (0.99–1.3) | 1.49 (1.28–1.58) | 1.3 (1.12–1.63) | 1.12 (0.99–1.21) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gois, P.H.F.; McIntyre, D.; Ratanjee, S.; Pelecanos, A.; Scuderi, C.; Janoschka, C.L.; Summers, K.; Wu, H.; Elford, B.; Ranganathan, D.; et al. Hemodialysis without Systemic Anticoagulation: A Randomized Controlled Trial to Evaluate Five Strategies in Patients at a High Risk of Bleeding. Med. Sci. 2024, 12, 38. https://doi.org/10.3390/medsci12030038
Gois PHF, McIntyre D, Ratanjee S, Pelecanos A, Scuderi C, Janoschka CL, Summers K, Wu H, Elford B, Ranganathan D, et al. Hemodialysis without Systemic Anticoagulation: A Randomized Controlled Trial to Evaluate Five Strategies in Patients at a High Risk of Bleeding. Medical Sciences. 2024; 12(3):38. https://doi.org/10.3390/medsci12030038
Chicago/Turabian StyleGois, Pedro H. Franca, David McIntyre, Sharad Ratanjee, Anita Pelecanos, Carla Scuderi, Chungun L. Janoschka, Kara Summers, Haibing Wu, Belinda Elford, Dwarakanathan Ranganathan, and et al. 2024. "Hemodialysis without Systemic Anticoagulation: A Randomized Controlled Trial to Evaluate Five Strategies in Patients at a High Risk of Bleeding" Medical Sciences 12, no. 3: 38. https://doi.org/10.3390/medsci12030038
APA StyleGois, P. H. F., McIntyre, D., Ratanjee, S., Pelecanos, A., Scuderi, C., Janoschka, C. L., Summers, K., Wu, H., Elford, B., Ranganathan, D., & Healy, H. G. (2024). Hemodialysis without Systemic Anticoagulation: A Randomized Controlled Trial to Evaluate Five Strategies in Patients at a High Risk of Bleeding. Medical Sciences, 12(3), 38. https://doi.org/10.3390/medsci12030038







