Hemin Promotes Higher Effectiveness of Aminolevulinic-Photodynamic Therapy (ALA-PDT) in A549 Lung Cancer Cell Line by Interrupting ABCG2 Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Biochemicals
2.3. Cytotoxicity Assay
2.4. Hemin and ALA Treatment
2.5. High-Performance Liquid Chromatography (HPLC) to Measure PpIX Accumulation
2.6. Fluorescence Analysis of ROS and Intracellular PpIX
2.7. Analysis of Gene Expression
2.8. Western Blotting
2.9. Statistical Analysis
3. Results
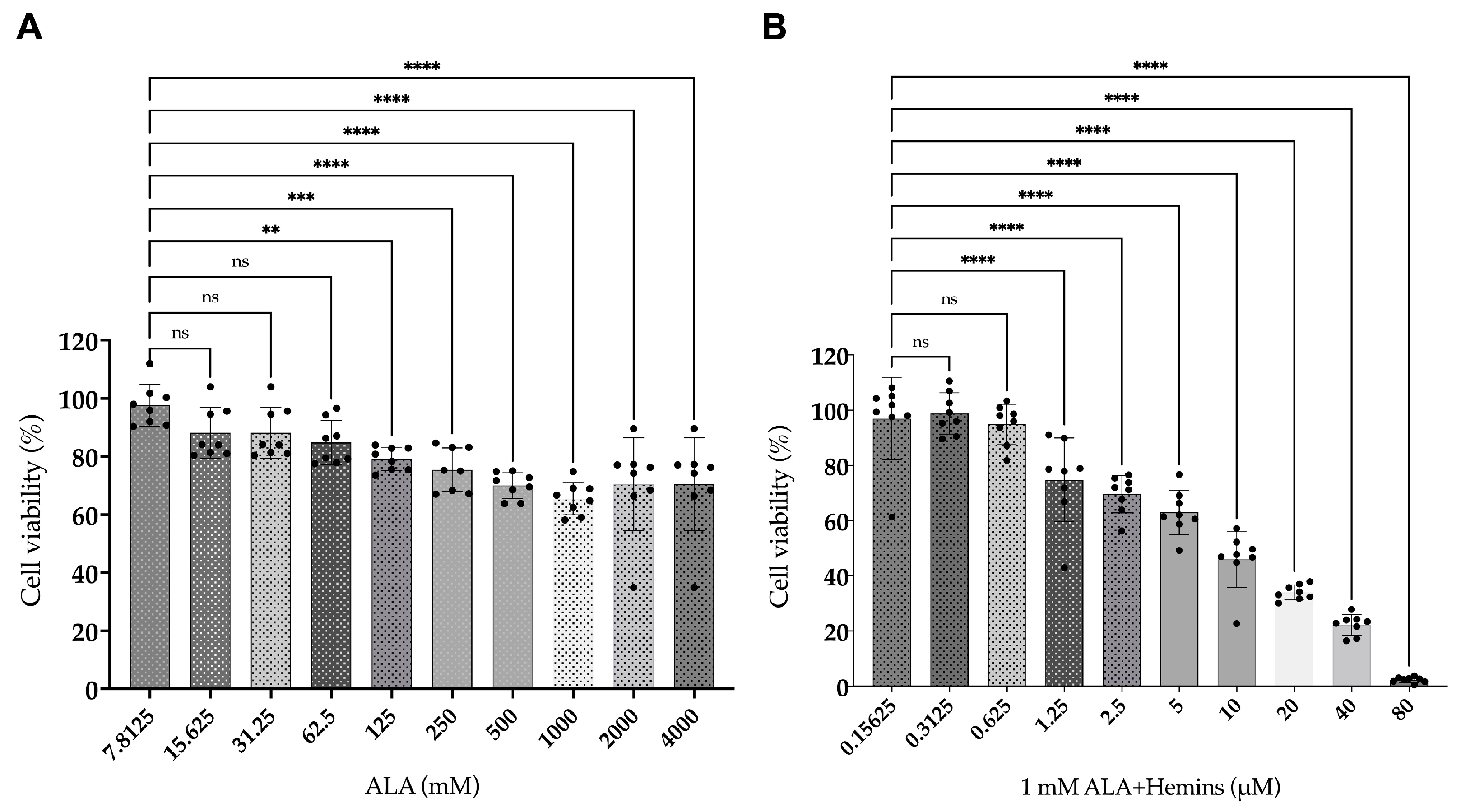
3.1. Reduced Viability of A549 After Combined Treatment of ALA and Hemin
3.2. Elevated PpIX Accumulation in A549 After Co-Treatment of ALA and Hemin
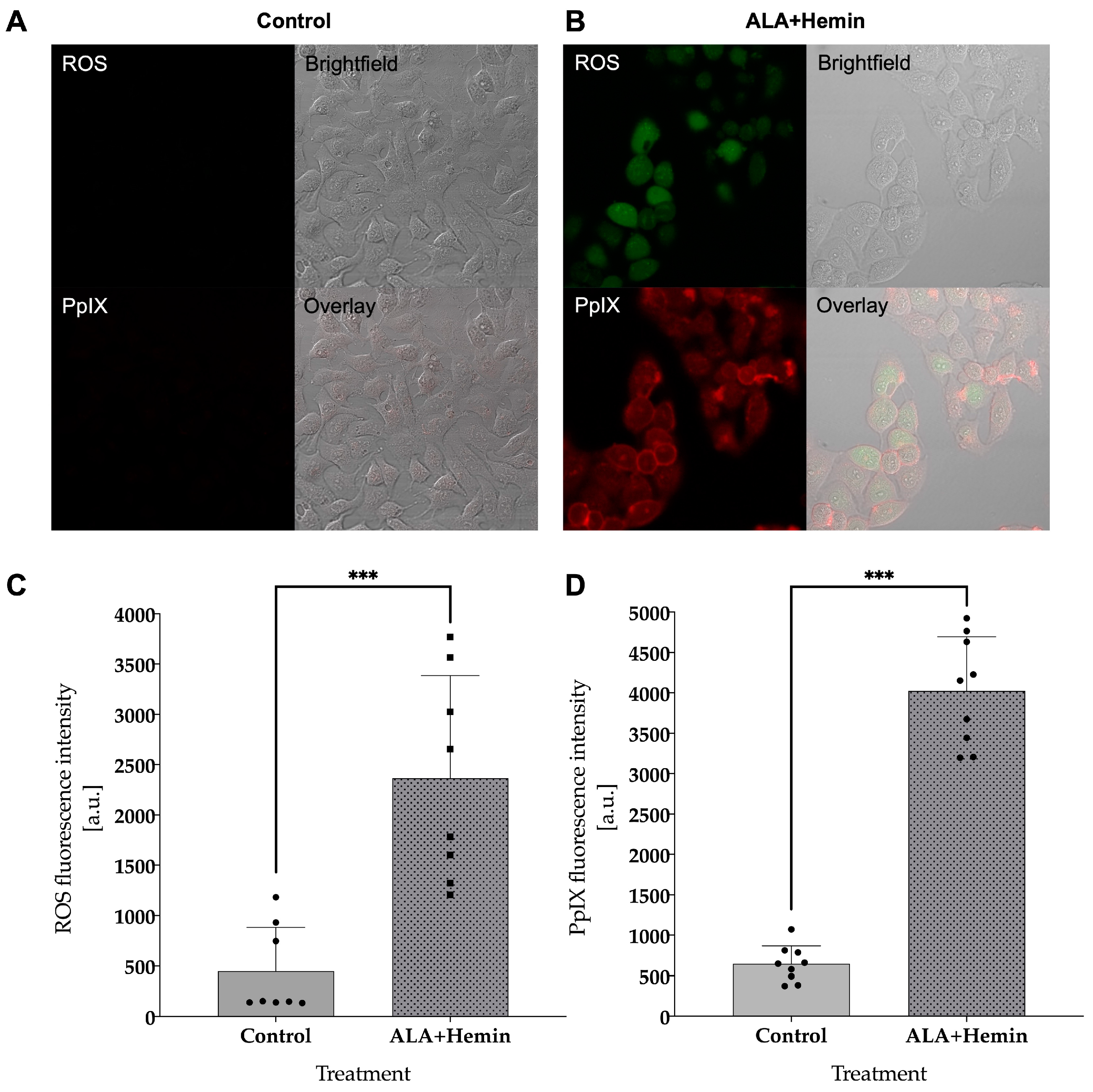
3.3. Combined ALA and Hemin Induces Cell Death via Intracellular ROS and PpIX Accumulation
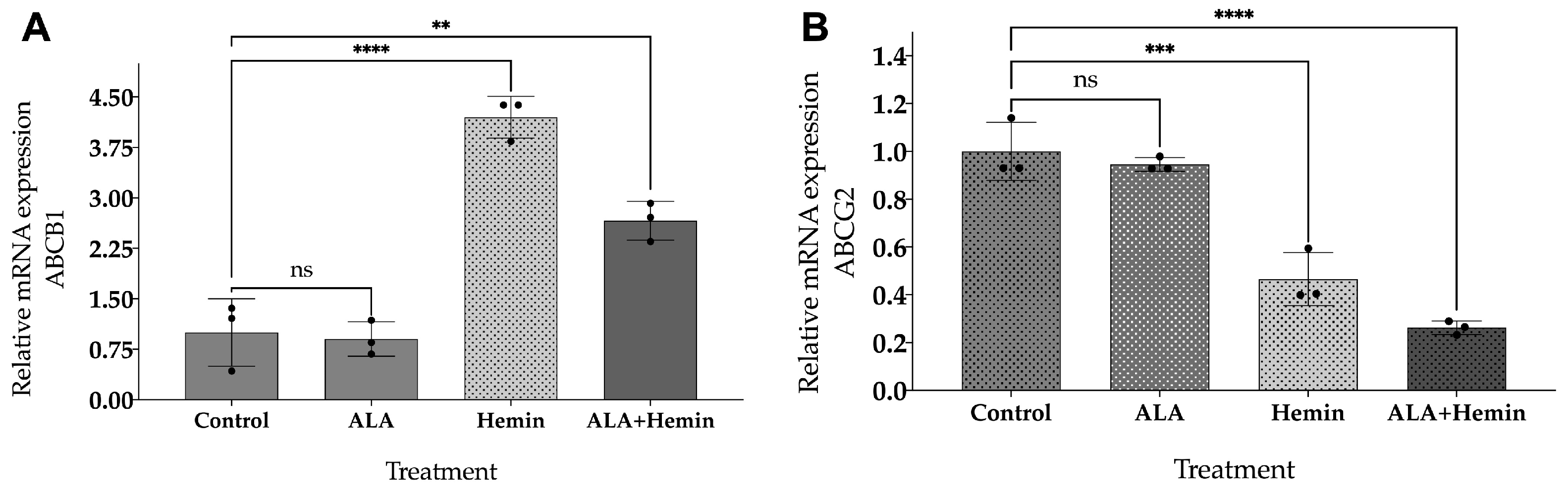
3.4. ALA and Hemin Influence the mRNA Expression of ABCG2 and ABCB1
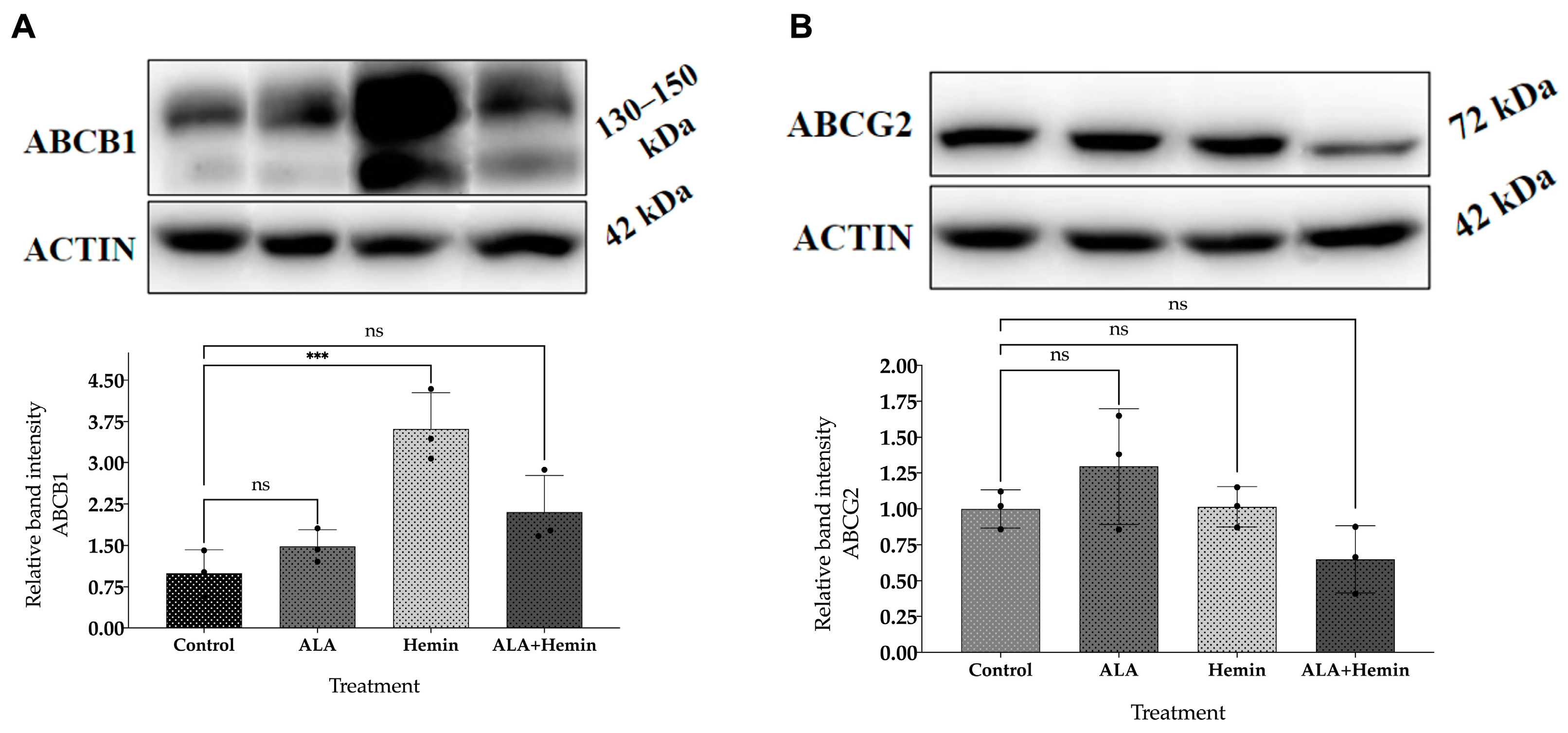
3.5. Changes in ABCG2 and ABCB1 Protein Expression by Co-Treatment of ALA and Hemin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boulanger, M.; Mitchell, C.; Zhong, J.; Hsu, M. Financial toxicity in lung cancer. Front. Oncol. 2022, 12, 1004102. [Google Scholar] [CrossRef] [PubMed]
- Au, P.C.M.; Lee, A.W.M.; Lee, V.H.F.; Wong, I.C.K.; Hui, R.Y.M.; Cheung, C.-L. The trends in lung cancer prevalence, incidence, and survival in Hong Kong over the past two decades (2002–2021): A population-based study. Lancet Reg. Health West. Pac. 2024, 45, 101030. [Google Scholar] [CrossRef] [PubMed]
- Horinouchi, H.; Kusumoto, M.; Yatabe, Y.; Aokage, K.; Watanabe, S.; Ishikura, S. Lung Cancer in Japan. J. Thorac. Oncol. 2022, 17, 353–361. [Google Scholar] [CrossRef]
- Asmara, O.D.; Tenda, E.D.; Singh, G.; Pitoyo, C.W.; Rumende, C.M.; Rajabto, W.; Ananda, N.R.; Trisnawati, I.; Budiyono, E.; Thahadian, H.F.; et al. Lung Cancer in Indonesia. J. Thorac. Oncol. 2023, 18, 1134–1145. [Google Scholar] [CrossRef]
- Rivera-Concepcion, J.; Uprety, D.; Adjei, A.A. Challenges in the Use of Targeted Therapies in Non-Small Cell Lung Cancer. Cancer Res. Treat. 2022, 54, 315–329. [Google Scholar] [CrossRef]
- Shyam Sunder, S.; Sharma, U.C.; Pokharel, S. Adverse effects of tyrosine kinase inhibitors in cancer therapy: Pathophysiology, mechanisms and clinical management. Signal Transduct. Target. Ther. 2023, 8, 262. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Yang, J.; Zhang, L.; Zhang, Y.; Yan, G.; Zhang, H.; Liu, X.; Yang, J.; Wang, P.; Zhang, G.; et al. A prospective study of adverse reactions of ALA-PDT for acne vulgaris. Photodiagnosis Photodyn. Ther. 2022, 38, 102752. [Google Scholar] [CrossRef]
- Lai, H.W.; Takahashi, K.; Nakajima, M.; Tanaka, T.; Ogura, S. Efficiency of aminolevulinic acid (ALA)-photodynamic therapy based on ALA uptake transporters in a cell density-dependent malignancy model. J. Photochem. Photobiol. B 2021, 218, 112191. [Google Scholar] [CrossRef]
- Mohammadpour, H.; Fekrazad, R. Antitumor effect of combined Dkk-3 and 5-ALA mediated photodynamic therapy in breast cancer cell’s colony. Photodiagnosis Photodyn. Ther. 2016, 14, 200–203. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Müller, P.; Abdel Gaber, S.A.; Zimmermann, W.; Wittig, R.; Stepp, H. ABCG2 influence on the efficiency of photodynamic therapy in glioblastoma cells. J. Photochem. Photobiol. B 2020, 210, 111963. [Google Scholar] [CrossRef] [PubMed]
- McNicholas, K.; MacGregor, M.N.; Gleadle, J.M. In order for the light to shine so brightly, the darkness must be present—Why do cancers fluoresce with 5-aminolaevulinic acid? Br. J. Cancer 2019, 121, 631–639. [Google Scholar] [CrossRef]
- Pustimbara, A.; Li, C.; Ogura, S. Hemin enhances the 5-aminolevulinic acid-photodynamic therapy effect through the changes of cellular iron homeostasis. Photodiagnosis Photodyn. Ther. 2024, 48, 104253. [Google Scholar] [CrossRef]
- Vesel, M.; Rapp, J.; Feller, D.; Kiss, E.; Jaromi, L.; Meggyes, M.; Miskei, G.; Duga, B.; Smuk, G.; Laszlo, T.; et al. ABCB1 and ABCG2 drug transporters are differentially expressed in non-small cell lung cancers (NSCLC) and expression is modified by cisplatin treatment via altered Wnt signaling. Respir. Res. 2017, 18, 52. [Google Scholar] [CrossRef] [PubMed]
- Kobuchi, H.; Moriya, K.; Ogino, T.; Fujita, H.; Inoue, K.; Shuin, T.; Yasuda, T.; Utsumi, K.; Utsumi, T. Mitochondrial Localization of ABC Transporter ABCG2 and Its Function in 5-Aminolevulinic Acid-Mediated Protoporphyrin IX Accumulation. PLoS ONE 2012, 7, e50082. [Google Scholar] [CrossRef] [PubMed]
- Palasuberniam, P.; Yang, X.; Kraus, D.; Jones, P.; Myers, K.A.; Chen, B. ABCG2 transporter inhibitor restores the sensitivity of triple negative breast cancer cells to aminolevulinic acid-mediated photodynamic therapy. Sci. Rep. 2015, 5, 13298. [Google Scholar] [CrossRef]
- Imi, Y.; Shibata, K. Nutritional Factors That Affect the Formation of 5-Aminolevulinic Acid, a Key Intermediate of Heme Biosynthesis. J. Nutr. Sci. Vitaminol. 2021, 67, 339–350. [Google Scholar] [CrossRef]
- Harada, Y.; Murayama, Y.; Takamatsu, T.; Otsuji, E.; Tanaka, H. 5-Aminolevulinic Acid-Induced Protoporphyrin IX Fluorescence Imaging for Tumor Detection: Recent Advances and Challenges. Int. J. Mol. Sci. 2022, 23, 6478. [Google Scholar] [CrossRef]
- Pacioni, S.; D’alessandris, Q.G.; Giannetti, S.; Della Pepa, G.M.; Offi, M.; Giordano, M.; Caccavella, V.M.; Falchetti, M.L.; Lauretti, L.; Pallini, R. 5-Aminolevulinic Acid (5-ALA)-Induced Protoporphyrin IX Fluorescence by Glioma Cells—A Fluorescence Microscopy Clinical Study. Cancers 2022, 14, 2844. [Google Scholar] [CrossRef]
- Jiang, M.; Hong, K.; Mao, Y.; Ma, H.; Chen, T.; Wang, Z. Natural 5-Aminolevulinic Acid: Sources, Biosynthesis, Detection and Applications. Front. Bioeng. Biotechnol. 2022, 10, 841443. [Google Scholar] [CrossRef]
- Inoue, K.; Fukuhara, H.; Yamamoto, S.; Karashima, T.; Kurabayashi, A.; Furihata, M.; Hanazaki, K.; Lai, H.W.; Ogura, S. Current status of photodynamic technology for urothelial cancer. Cancer Sci. 2022, 113, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Kumbham, S.; Rahman, K.M.M.; Bosmajian, C.; Bist, G.; Foster, B.A.; Woo, S.; You, Y. Enhancing PDT efficacy in NMIBC: Efflux inhibitor mediated improvement of PpIX levels and efficacy of the combination of PpIX-PDT and SO-cleavable prodrugs. Photochem. Photobiol. 2024, 100, 1636–1646. [Google Scholar] [CrossRef] [PubMed]
- Hagiya, Y.; Endo, Y.; Yonemura, Y.; Takahashi, K.; Ishizuka, M.; Abe, F.; Tanaka, T.; Okura, I.; Nakajima, M.; Ishikawa, T.; et al. Pivotal roles of peptide transporter PEPT1 and ATP-binding cassette (ABC) transporter ABCG2 in 5-aminolevulinic acid (ALA)-based photocytotoxicity of gastric cancer cells in vitro. Photodiagnosis Photodyn. Ther. 2012, 9, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.W.; Steadman, K.; Polgar, O.; Bates, S.E. ABCG2-mediated transport of photosensitizers: Potential impact on photodynamic therapy. Cancer Biol. Ther. 2005, 4, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, E.; Chelakkot, V.S.; Licursi, M.; Rutihinda, S.G.; Som, J.; Derwish, L.; King, J.J.; Pongnopparat, T.; Mearow, K.; Larijani, M.; et al. Enhancement of Cancer-Specific Protoporphyrin IX Fluorescence by Targeting Oncogenic Ras/MEK Pathway. Theranostics 2018, 8, 2134–2146. [Google Scholar] [CrossRef]
- Yang, H.; Wei, Y.-C.; Li, W.-C.; Chen, H.-Y.; Lin, H.-Y.; Chiang, C.-P.; Chen, H.-M. Natural Compounds Modulate Drug Transporter Mediated Oral Cancer Treatment. Biomolecules 2020, 10, 1335. [Google Scholar] [CrossRef]
- Lai, H.W.; Tani, Y.; Sukatta, U.; Rugthaworn, P.; Thepyos, A.; Yamamoto, S.; Fukuhara, H.; Inoue, K.; Yuasa, H.; Nakamura, H.; et al. Mangostin enhances efficacy of aminolevulinic acid-photodynamic therapy against cancer through inhibition of ABCG2 activity. Photodiagnosis Photodyn. Ther. 2023, 44, 103798. [Google Scholar] [CrossRef]
- Coló, G.P.; Schweitzer, K.; Oresti, G.M.; Alonso, E.G.; Chávez, L.F.; Mascaró, M.; Giorgi, G.; Curino, A.C.; Facchinetti, M.M. Proteomic analysis of the effect of hemin in breast cancer. Sci. Rep. 2023, 13, 10091. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, M.; Zhang, Y.; Li, L.; Peng, Y.; Li, J.; Zhou, W. Hemin-incorporating DNA nanozyme enabling catalytic oxygenation and GSH depletion for enhanced photodynamic therapy and synergistic tumor ferroptosis. J. Nanobiotechnol. 2022, 20, 410. [Google Scholar] [CrossRef]
- Almahi, W.A.; Yu, K.N.; Mohammed, F.; Kong, P.; Han, W. Hemin enhances radiosensitivity of lung cancer cells through ferroptosis. Exp. Cell Res. 2022, 410, 112946. [Google Scholar] [CrossRef]
- Pignatelli, P.; Umme, S.; D’Antonio, D.L.; Piattelli, A.; Curia, M.C. Reactive Oxygen Species Produced by 5-Aminolevulinic Acid Photodynamic Therapy in the Treatment of Cancer. Int. J. Mol. Sci. 2023, 24, 8964. [Google Scholar] [CrossRef] [PubMed]
- Mansi, M.; Howley, R.; Chandratre, S.; Chen, B. Inhibition of ABCG2 transporter by lapatinib enhances 5-aminolevulinic acid-mediated protoporphyrin IX fluorescence and photodynamic therapy response in human glioma cell lines. Biochem. Pharmacol. 2022, 200, 115031. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, P.; Xie, T.; Schuetz, J.D. The role of transporters in cellular heme and porphyrin homeostasis. Pharmacol. Ther. 2007, 114, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Skinner, K.T.; Palkar, A.M.; Hong, A.L. Genetics of ABCB1 in Cancer. Cancers 2023, 15, 4236. [Google Scholar] [CrossRef] [PubMed]
- Adorni, M.P.; Galetti, M.; La Monica, S.; Incerti, M.; Ruffoni, A.; Elviri, L.; Zanotti, I.; Papotti, B.; Cavallo, D.; Alfieri, R.; et al. A New ABCB1 Inhibitor Enhances the Anticancer Effect of Doxorubicin in Both In Vitro and In Vivo Models of NSCLC. Int. J. Mol. Sci. 2023, 24, 989. [Google Scholar] [CrossRef]
- Chelakkot, V.S.; Liu, K.; Yoshioka, E.; Saha, S.; Xu, D.; Licursi, M.; Dorward, A.; Hirasawa, K. MEK reduces cancer-specific PpIX accumulation through the RSK-ABCB1 and HIF-1α-FECH axes. Sci. Rep. 2020, 10, 22124. [Google Scholar] [CrossRef]

| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| ABCG2 | 5′ GCAACCATCAATTCAGGTCAAGA ‘3 | 5′ GAAACACAACACTTGGCTGTAGCA ‘3 |
| ABCB1 | 5′ GGAAGCCAATGCCTATGACTTTA’3 | 5′ GATGACGTCAGCATTACGAACTGTA ‘3 |
| ACTIN | 5′ TGGCACCCAGCACAATGAA ‘3 | 5′ CTAAGTCATAGTCCGCCTAGAAGCA ‘3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pustimbara, A.; Umami, R.W.; Prakoso, N.M.; Rozaliyani, A.; Zaini, J.; Dwiranti, A.; Ogura, S.-i.; Bowolaksono, A. Hemin Promotes Higher Effectiveness of Aminolevulinic-Photodynamic Therapy (ALA-PDT) in A549 Lung Cancer Cell Line by Interrupting ABCG2 Expression. Med. Sci. 2024, 12, 66. https://doi.org/10.3390/medsci12040066
Pustimbara A, Umami RW, Prakoso NM, Rozaliyani A, Zaini J, Dwiranti A, Ogura S-i, Bowolaksono A. Hemin Promotes Higher Effectiveness of Aminolevulinic-Photodynamic Therapy (ALA-PDT) in A549 Lung Cancer Cell Line by Interrupting ABCG2 Expression. Medical Sciences. 2024; 12(4):66. https://doi.org/10.3390/medsci12040066
Chicago/Turabian StylePustimbara, Anantya, Rahma Wirdatul Umami, Nurul Muhammad Prakoso, Anna Rozaliyani, Jamal Zaini, Astari Dwiranti, Shun-ichiro Ogura, and Anom Bowolaksono. 2024. "Hemin Promotes Higher Effectiveness of Aminolevulinic-Photodynamic Therapy (ALA-PDT) in A549 Lung Cancer Cell Line by Interrupting ABCG2 Expression" Medical Sciences 12, no. 4: 66. https://doi.org/10.3390/medsci12040066
APA StylePustimbara, A., Umami, R. W., Prakoso, N. M., Rozaliyani, A., Zaini, J., Dwiranti, A., Ogura, S.-i., & Bowolaksono, A. (2024). Hemin Promotes Higher Effectiveness of Aminolevulinic-Photodynamic Therapy (ALA-PDT) in A549 Lung Cancer Cell Line by Interrupting ABCG2 Expression. Medical Sciences, 12(4), 66. https://doi.org/10.3390/medsci12040066







