Neuroimaging of Traumatic Brain Injury
Abstract
:1. Introduction
1.1. Neuroanatomy
1.2. Severity of Traumatic Brain Injury
1.3. Criteria for Neuroimaging
1.4. Primary Traumatic Brain Injury vs. Secondary Traumatic Brain Injury
2. Conventional Diagnostic Imaging Techniques in Traumatic Brain Injury
3. Advanced Diagnostic Imaging Techniques in Traumatic Brain Injury
3.1. Perfusion Imaging
3.1.1. Clinical Considerations
3.1.2. Perfusion Imaging Techniques
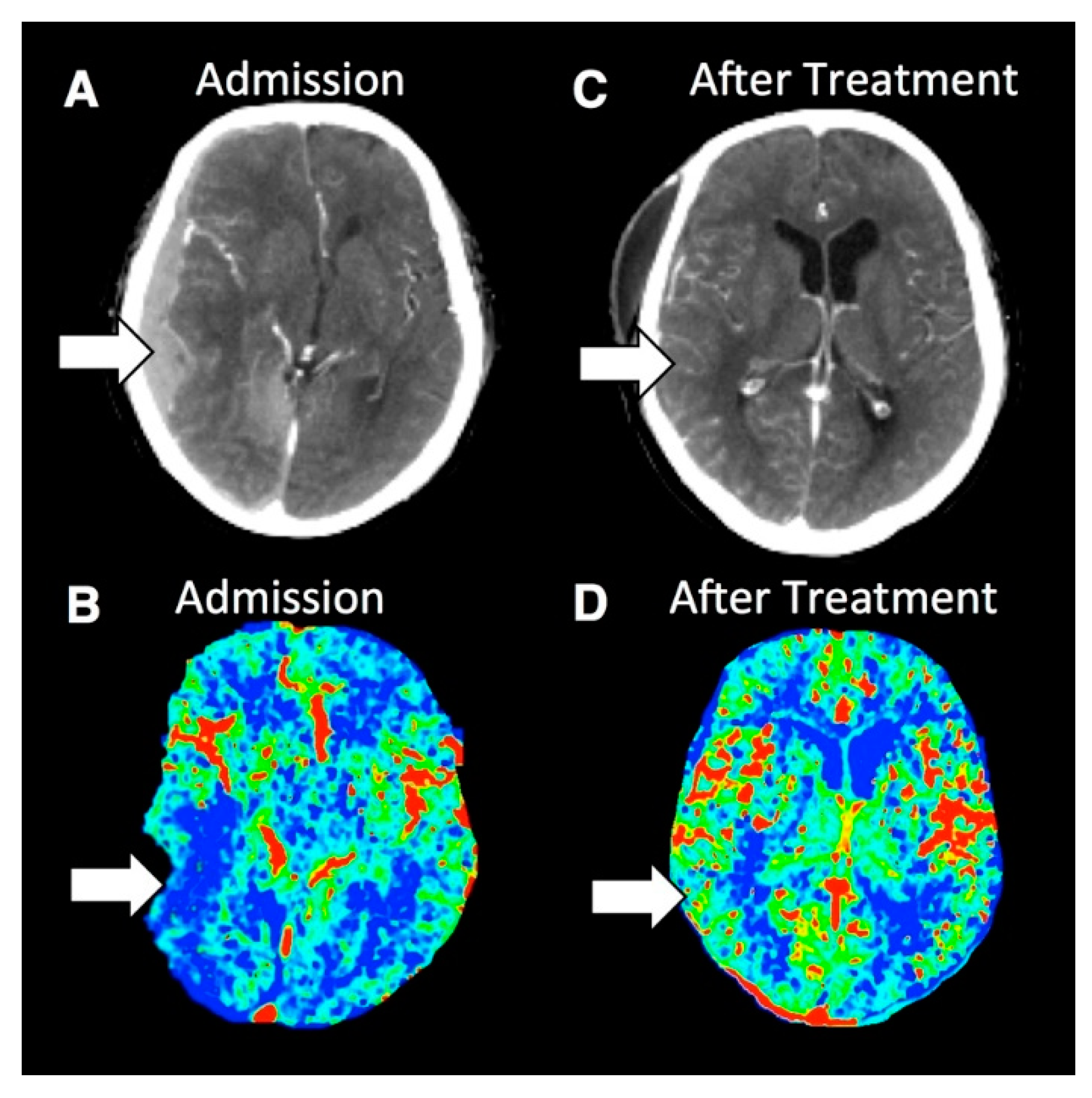
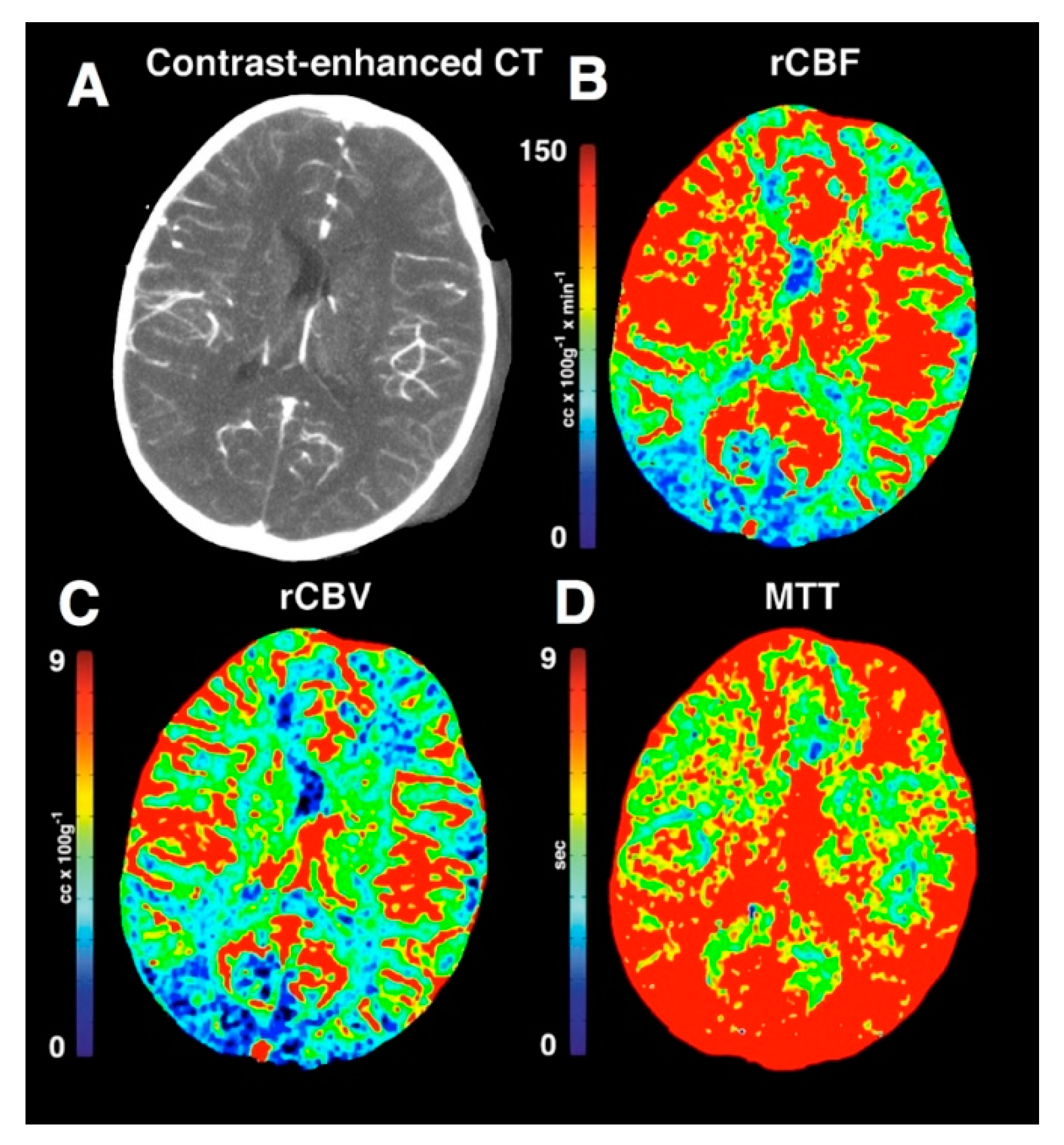
3.1.3. Results of Important Studies of Perfusion Imaging of Traumatic Brain Injury
3.1.4. Limitations of Perfusion Imaging in Traumatic Brain Injury
3.2. Diffusion Tensor Imaging
3.2.1. Clinical Considerations
3.2.2. Diffusion Tensor Imaging Techniques
3.2.3. Results of Important Studies of Diffusion Tensor Imaging of Traumatic Brain Injury
3.2.4. Limitations of Diffusion Tensor Imaging in Traumatic Brain Injury
4. Future Technologies
Machine Learning in Traumatic Brain Injury
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Marr, A.L.; Coronado, V.G. Central Nervous System Injury Surveillance Data Submission Standards—2002; Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: Atlanta, GA, USA, 2004.
- Faul, M. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths, 2002–2006; 1 Online Resource; National Center for Injury Prevention and Control (USA): Atlanta, GA, USA, 2010; Volume vii, 70p.
- Marin, J.R.; Weaver, M.D.; Yealy, D.M.; Mannix, R.C. Trends in visits for traumatic brain injury to emergency departments in the United States. Jama 2014, 311, 1917–1919. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Nonfatal traumatic brain injuries related to sports and recreation activities among persons aged ≤19 years-United States, 2001–2009. MMWR Morb. Mortal. Wkl. Rep. 2011, 60, 1337–1342. [Google Scholar]
- Bass, E.; Golding, H. The Veterans Health Administration’s Treatment of PTSD and Traumatic Brain Injury among Recent Combat Veterans; A CBO Study; 1 Online Resource; Congressional Budget Office: Washington, DC, USA, 2012; Volume ix, 39p.
- Mori, S.; Zhang, J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 2006, 51, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, G.; Jennett, B. Assessment of coma and impaired consciousness: A practical scale. Lancet 1974, 304, 81–84. [Google Scholar] [CrossRef]
- Shetty, V.S.; Reis, M.N.; Aulino, J.M.; Berger, K.L.; Broder, J.; Choudhri, A.F.; Kendi, A.T.; Kessler, M.M.; Kirsch, C.F.; Luttrull, M.D.; et al. ACR Appropriateness Criteria Head Trauma. J. Am. Coll. Radiol. 2016, 13, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Cushman, J.G.; Agarwal, N.; Fabian, T.C.; Garcia, V.; Nagy, K.K.; Pasquale, M.D.; Salotto, A.G. Practice management guidelines for the management of mild traumatic brain injury: The EAST practice management guidelines work group. J. Trauma Acute Care Surg. 2001, 51, 1016–1026. [Google Scholar] [CrossRef]
- Jagoda, A.S.; Bazarian, J.J.; Bruns, J.J., Jr.; Cantrill, S.V.; Gean, A.D.; Howard, P.K.; Ghajar, J.; Riggio, S.; Wright, D.W.; Wears, R.L. Clinical policy: neuroimaging and decisionmaking in adult mild traumatic brain injury in the acute setting. J. Emerg. Nurs. 2009, 35, e5–e40. [Google Scholar] [CrossRef]
- Tavender, E.J.; Bosch, M.; Green, S.; O’connor, D.; Pitt, V.; Phillips, K.; Bragge, P.; Gruen, R.L. Quality and consistency of guidelines for the management of mild traumatic brain injury in the emergency department. Acad. Emerg. Med. 2011, 18, 880–889. [Google Scholar] [CrossRef] [PubMed]
- National Collaborating Centre for Acute Care. Head Injury: Triage, Assessment, Investigation and Early Management of Head Injury in Infants, Children and Adults; National Institute for Health and Care Excellence: London, UK, 2007. [Google Scholar]
- Mower, W.R.; Hoffman, J.R.; Herbert, M.; Wolfson, A.B.; Pollack, C.V., Jr.; Zucker, M.I.; Nexus II Investigators. Nexus II Investigators. Developing a decision instrument to guide computed tomographic imaging of blunt head injury patients. J. Trauma Acute Care Surg. 2005, 59, 954–959. [Google Scholar] [CrossRef]
- Stiell, I.G.; Wells, G.A.; Vandemheen, K.; Clement, C.; Lesiuk, H.; Laupacis, A.; McKnight, R.D.; Verbeek, R.; Brison, R.; Cass, D.; et al. The Canadian CT Head Rule for patients with minor head injury. Lancet 2001, 357, 1391–1396. [Google Scholar] [CrossRef]
- Haydel, M.J.; Preston, C.A.; Mills, T.J.; Luber, S.; Blaudeau, E.; DeBlieux, P.M. Indications for computed tomography in patients with minor head injury. N. Engl. J. Med. 2000, 343, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Kuppermann, N.; Holmes, J.F.; Dayan, P.S.; Hoyle, J.D.; Atabaki, S.M.; Holubkov, R.; Nadel, F.M.; Monroe, D.; Stanley, R.M.; Borgialli, D.A.; et al. Identification of children at very low risk of clinically-important brain injuries after head trauma: a prospective cohort study. Lancet 2009, 374, 1160–1170. [Google Scholar] [CrossRef]
- Trauma ACoSCo. ATLS® Student Manual, 9th ed.; American College of Surgeons: Chicago, IL, USA, 2012; Volume 6, pp. 7–8. [Google Scholar]
- Chesnut, R.; Ghajar, J.; Gordon, D. Surgical management of acute epidural hematomas. Neurosurgery 2006, 58, S2–S7. [Google Scholar]
- Bešenski, N. Traumatic injuries: Imaging of head injuries. Eur. Radiol. 2002, 12, 1237–1252. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Segar, D.J.; Asaad, W.F. Comprehensive assessment of isolated traumatic subarachnoid hemorrhage. J. Neurotrauma 2014, 31, 595–609. [Google Scholar] [CrossRef]
- Johnson, V.E.; Stewart, W.; Smith, D.H. Axonal pathology in traumatic brain injury. Exp. Neurol. 2013, 246, 35–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farkas, O.; Lifshitz, J.; Povlishock, J.T. Mechanoporation induced by diffuse traumatic brain injury: An irreversible or reversible response to injury? J. Neurosci. 2006, 26, 3130–3140. [Google Scholar] [CrossRef] [PubMed]
- Farkas, O.; Povlishock, J.T. Cellular and subcellular change evoked by diffuse traumatic brain injury: A complex web of change extending far beyond focal damage. Prog. Brain Res. 2007, 161, 43–59. [Google Scholar] [PubMed]
- Gennarelli, T.A.; Thibault, L.E.; Adams, J.H.; Graham, D.I.; Thompson, C.J.; Marcincin, R.P. Diffuse axonal injury and traumatic coma in the primate. Ann. Neurol. 1982, 12, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Levi, L.; Guilburd, J.N.; Lemberger, A.; Soustiel, J.F.; Feinsod, M. Diffuse axonal injury: Analysis of 100 patients with radiological signs. Neurosurgery 1990, 27, 429–432. [Google Scholar] [CrossRef]
- Young, A.M.; Donnelly, J.; Liu, X.; Guilfoyle, M.R.; Carew, M.; Cabeleira, M.; Cardim, D.; Garnett, M.R.; Fernandes, H.M.; Haubrich, C.; et al. Computed Tomography Indicators of Deranged Intracranial Physiology in Paediatric Traumatic Brain Injury. In Intracranial Pressure & Neuromonitoring XVI; Springer: Berlin, Germany, 2018; pp. 29–34. [Google Scholar]
- Servadei, M.T.; Nasi, G.; Giuliani, A.; Maria Cremonini, P.; Cenni, D.; Zappi, G.S.; Taylor, F. CT prognostic factors in acute subdural haematomas: The value of the ‘worst’ CT scan. Br. J. Neurosurg. 2000, 14, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Newberg, A. Neuroimaging in traumatic brain imaging. NeuroRx 2005, 2, 372–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celli, P.; Fruin, A.; Cervoni, L. Severe head trauma. Review of the factors influencing the prognosis. Minerva Chir. 1997, 52, 1467–1480. [Google Scholar] [PubMed]
- Lev, M.H.; Segal, A.Z.; Farkas, J.; Hossain, S.T.; Putman, C.; Hunter, G.J.; Budzik, R.; Harris, G.J.; Buonanno, F.S.; Ezzeddine, M.A.; et al. Utility of perfusion-weighted CT imaging in acute middle cerebral artery stroke treated with intra-arterial thrombolysis: Prediction of final infarct volume and clinical outcome. Stroke 2001, 32, 2021–2028. [Google Scholar] [CrossRef] [PubMed]
- Wintermark, M.; Reichhart, M.; Thiran, J.P.; Maeder, P.; Chalaron, M.; Schnyder, P.; Bogousslavsky, J.; Meuli, R. Prognostic accuracy of cerebral blood flow measurement by perfusion computed tomography, at the time of emergency room admission, in acute stroke patients. Ann. Neurol. 2002, 51, 417–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wintermark, M.; Reichhart, M.; Cuisenaire, O.; Maeder, P.; Thiran, J.P.; Schnyder, P.; Bogousslavsky, J.; Meuli, R. Comparison of admission perfusion computed tomography and qualitative diffusion- and perfusion-weighted magnetic resonance imaging in acute stroke patients. Stroke 2002, 33, 2025–2031. [Google Scholar] [CrossRef] [PubMed]
- Wintermark, M.; Maeder, P.; Thiran, J.P.; Schnyder, P.; Meuli, R. Quantitative assessment of regional cerebral blood flows by perfusion CT studies at low injection rates: A critical review of the underlying theoretical models. Eur. Radiol. 2001, 11, 1220–1230. [Google Scholar] [CrossRef]
- Shankar, J.; Vandorpe, R. CT perfusion for confirmation of brain death. Am. J. Neuroradiol. 2013, 34, 1175–1179. [Google Scholar] [CrossRef]
- Douglas, D.B.; Chaudhari, R.; Zhao, J.M.; Gullo, J.; Kirkland, J.; Douglas, P.K.; Wolin, E.; Walroth, J.; Wintermark, M. Perfusion Imaging in Acute Traumatic Brain Injury. Neuroimaging Clin. N. Am. 2017, 28, 55–65. [Google Scholar] [CrossRef]
- Garnett, M.R.; Blamire, A.M.; Corkill, R.G.; Rajagopalan, B.; Young, J.D.; Cadoux-Hudson, T.A.; Styles, P. Abnormal cerebral blood volume in regions of contused and normal appearing brain following traumatic brain injury using perfusion magnetic resonance imaging. J. Neurotrauma 2001, 18, 585–593. [Google Scholar] [CrossRef]
- Allen, C.J.; Baldor, D.J.; Hanna, M.M.; Namias, N.; Bullock, M.R.; Jagid, J.R.; Proctor, K.G. Early Craniectomy Improves Intracranial and Cerebral Perfusion Pressure after Severe Traumatic Brain Injury. Am. Surg. 2018, 84, 443–450. [Google Scholar] [PubMed]
- Menon, D.K. Brain ischaemia after traumatic brain injury: Lessons from 15O2 positron emission tomography. Curr. Opin. Crit. Care 2006, 12, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Coles, J.P. Regional ischemia after head injury. Curr. Opin. Crit. Care 2004, 10, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, A.S.; Salvador, R.; Coles, J.P.; Chatfield, D.A.; Bradley, P.G.; Johnston, A.J.; Steiner, L.A.; Fryer, T.D.; Aigbirhio, F.I.; Smielewski, P.; et al. Physiological thresholds for irreversible tissue damage in contusional regions following traumatic brain injury. Br. J. Neurol. 2005, 128, 1931–1942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGehee, B.E.; Pollock, J.M.; Maldjian, J.A. Brain perfusion imaging: How does it work and what should I use? J. Magn. Reson. Imaging 2012, 36, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.S.; Detre, J.A.; Leigh, J.S.; Koretsky, A.P. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc. Natl. Acad. Sci. USA 1992, 89, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Deibler, A.R.; Pollock, J.M.; Kraft, R.A.; Tan, H.; Burdette, J.H.; Maldjian, J.A. Arterial spin-labeling in routine clinical practice, part 1: technique and artifacts. AJNR Am. J. Neuroradiol. 2008, 29, 1228–1234. [Google Scholar] [CrossRef]
- Deibler, A.R.; Pollock, J.M.; Kraft, R.A.; Tan, H.; Burdette, J.H.; Maldjian, J.A. Arterial spin-labeling in routine clinical practice, part 2: Hypoperfusion patterns. AJNR Am. J. Neuroradiol. 2008, 29, 1235–1241. [Google Scholar] [CrossRef]
- Deibler, A.R.; Pollock, J.M.; Kraft, R.A.; Tan, H.; Burdette, J.H.; Maldjian, J.A. Arterial spin-labeling in routine clinical practice, part 3: hyperperfusion patterns. AJNR Am. J. Neuroradiol. 2008, 29, 1428–1435. [Google Scholar] [CrossRef]
- Wintermark, M.; Thiran, J.P.; Maeder, P.; Schnyder, P.; Meuli, R. Simultaneous measurement of regional cerebral blood flow by perfusion CT and stable xenon CT: A validation study. AJNR Am. J. Neuroradiol. 2001, 22, 905–914. [Google Scholar]
- Latchaw, R.E.; Yonas, H.; Hunter, G.J.; Yuh, W.T.; Ueda, T.; Sorensen, A.G.; Sunshine, J.L.; Biller, J.; Wechsler, L.; Higashida, R.; et al. Guidelines and recommendations for perfusion imaging in cerebral ischemia: A scientific statement for healthcare professionals by the writing group on perfusion imaging, from the Council on Cardiovascular Radiology of the American Heart Association. Stroke 2003, 34, 1084–1104. [Google Scholar] [CrossRef] [PubMed]
- Axel, L. Cerebral blood flow determination by rapid-sequence computed tomography: Theoretical analysis. Radiology 1980, 137, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Axel, L. A method of calculating brain blood flow with a CT dynamic scanner. Adv. Neurol. 1981, 30, 67–71. [Google Scholar] [PubMed]
- Axel, L. Tissue mean transit time from dynamic computed tomography by a simple deconvolution technique. Investig. Radiol. 1983, 18, 94–99. [Google Scholar] [CrossRef]
- Bivard, A.; Levi, C.; Spratt, N.; Parsons, M. Perfusion CT in acute stroke: a comprehensive analysis of infarct and penumbra. Radiology 2013, 267, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, B.; Wolfowitz, R.; Yeh, P.H.; Nathan, D.E.; Graner, J.; Tang, H.; Pan, H.; Harper, J.; Pham, D.; et al. Perfusion deficits in patients with mild traumatic brain injury characterized by dynamic susceptibility contrast MRI. NMR Biomed. 2013, 26, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Kochanek, P.M.; Hendrich, K.S.; Dixon, C.E.; Schiding, J.K.; Williams, D.S.; Ho, C. Cerebral blood flow at one year after controlled cortical impact in rats: Assessment by magnetic resonance imaging. J. Neurotrauma 2002, 19, 1029–1037. [Google Scholar] [CrossRef]
- Ge, Y.; Patel, M.B.; Chen, Q.; Grossman, E.J.; Zhang, K.; Miles, L.; Babb, J.S.; Reaume, J.; Grossman, R.I. Assessment of thalamic perfusion in patients with mild traumatic brain injury by true FISP arterial spin labelling MR imaging at 3T. Brain Inj. 2009, 23, 666–674. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Whyte, J.; Patel, S.; Avants, B.; Europa, E.; Wang, J.; Slattery, J.; Gee, J.C.; Coslett, H.B.; Detre, J.A. Resting cerebral blood flow alterations in chronic traumatic brain injury: an arterial spin labeling perfusion FMRI study. J. Neurotrauma 2010, 27, 1399–1411. [Google Scholar] [CrossRef]
- Doshi, H.; Wiseman, N.; Liu, J.; Wang, W.; Welch, R.D.; O’Neil, B.J.; Zuk, C.; Wang, X.; Mika, V.; Szaflarski, J.P.; et al. Cerebral hemodynamic changes of mild traumatic brain injury at the acute stage. PLoS ONE 2015, 10, e0118061. [Google Scholar] [CrossRef]
- Zeineh, M.D.D.; Parekh, M.; Wilson, E.; Parivash, S.; Mitchell, L.; Boldt, B.; Anderson, S.; Hoffman, A.; Bian, W.; Grant, G.; et al. Alteration of cerebral blood flow in contact-sport athletes. In Proceedings of the American Society of Neuroradiology Annual Conference, Chicago, IL, USA, 29 April 2015. [Google Scholar]
- Wintermark, M.; van Melle, G.; Schnyder, P.; Revelly, J.P.; Porchet, F.; Regli, L.; Meuli, R.; Maeder, P.; Chioléro, R. Admission perfusion CT: Prognostic value in patients with severe head trauma. Radiology 2004, 232, 211–220. [Google Scholar] [CrossRef]
- Honda, M.; Ichibayashi, R.; Suzuki, G.; Yokomuro, H.; Seiki, Y.; Sase, S.; Kishi, T. Consideration of the intracranial pressure threshold value for the initiation of traumatic brain injury treatment: a xenon CT and perfusion CT study. Neurocrit. Care 2017, 27, 308–315. [Google Scholar] [CrossRef]
- Soustiel, J.F.; Mahamid, E.; Goldsher, D.; Zaaroor, M. Perfusion-CT for early assessment of traumatic cerebral contusions. Neuroradiology 2008, 50, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Metting, Z.; Rödiger, L.A.; de Jong, B.M.; Stewart, R.E.; Kremer, B.P.; van der Naalt, J. Acute cerebral perfusion CT abnormalities associated with posttraumatic amnesia in mild head injury. J. Neurotrauma 2010, 27, 2183–2189. [Google Scholar] [CrossRef] [PubMed]
- Bindu, T.S.; Vyas, S.; Khandelwal, N.; Bhatia, V.; Dhandapani, S.; Kumar, A.; Ahuja, C.K. Role of whole-brain computed tomography perfusion in head injury patients to predict outcome. Indian J. Radiol. Imaging 2017, 27, 268. [Google Scholar] [PubMed]
- Bendinelli, C.; Bivard, A.; Nebauer, S.; Parsons, M.W.; Balogh, Z.J. Brain CT perfusion provides additional useful information in severe traumatic brain injury. Injury 2013, 44, 1208–1212. [Google Scholar] [CrossRef] [PubMed]
- Bendinelli, C.; Cooper, S.; Evans, T.; Bivard, A.; Pacey, D.; Parson, M.; Balogh, Z.J. Perfusion Abnormalities are Frequently Detected by Early CT Perfusion and Predict Unfavourable Outcome Following Severe Traumatic Brain Injury. World J. Surg. 2017, 41, 2512–2520. [Google Scholar] [CrossRef] [PubMed]
- Wintermark, M.; Maeder, P.; Verdun, F.R.; Thiran, J.P.; Valley, J.F.; Schnyder, P.; Meuli, R. Using 80 kVp versus 120 kVp in perfusion CT measurement of regional cerebral blood flow. AJNR Am. J. Neuroradiol. 2000, 21, 1881–1884. [Google Scholar]
- Radiology ACo. ACR Manual on Contrast Media; American College of Radiology: Reston, VA, USA, 2015. [Google Scholar]
- Golby, A.J.; Kindlmann, G.; Norton, I.; Yarmarkovich, A.; Pieper, S.; Kikinis, R. Interactive diffusion tensor tractography visualization for neurosurgical planning. Neurosurgery 2011, 68, 496–505. [Google Scholar] [CrossRef]
- Gong, G.; He, Y.; Concha, L.; Lebel, C.; Gross, D.W.; Evans, A.C.; Beaulieu, C. Mapping anatomical connectivity patterns of human cerebral cortex using in vivo diffusion tensor imaging tractography. Cereb. Cortex 2009, 19, 524–536. [Google Scholar] [CrossRef]
- Basser, P.J. New histological and physiological stains derived from diffusion-tensor MR images. Ann. N. Y. Acad. Sci. 1997, 820, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Basser, P.J.; Mattiello, J.; LeBihan, D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J. Magn. Reson. Ser. B 1994, 103, 247–254. [Google Scholar] [CrossRef]
- Le Bihan, D.; Mangin, J.F.; Poupon, C.; Clark, C.A.; Pappata, S.; Molko, N.; Chabriat, H. Diffusion tensor imaging: concepts and applications. J. Magn. Reson. Imaging 2001, 13, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Le Bihan, D.; Mangin, J.F.; Poupon, C.; Clark, C.A.; Pappata, S.; Molko, N.; Chabriat, H. Looking into the functional architecture of the brain with diffusion MRI. Nat. Rev. Neurosci. 2003, 4, 469–480. [Google Scholar] [CrossRef]
- Basser, P.J.; Jones, D.K. Diffusion-tensor MRI: Theory, experimental design and data analysis-a technical review. NMR Biomed. 2002, 15, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Mori, S. Introduction to Diffusion Tensor Imaging; Elsiever: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Johansen-Berg, H.; Behrens, T.E. Diffusion MRI: From Quantitative Measurements to In Vivo Neuroanatomy; Academic Press: London, UK, 2009. [Google Scholar]
- Douglas, D.B.; Iv, M.; Douglas, P.K.; Anderson, A.; Vos, S.B.; Bammer, R.; Zeineh, M.; Wintermark, M. Diffusion Tensor Imaging of TBI: Potentials and Challenges. Top. Magn. Reson. Imaging 2015, 24, 241–251. [Google Scholar] [CrossRef]
- Mori, S.; Wakana, S.; Van Zijl, P.C.M; Nagae-Poetscher, L.M. MRI Atlas of Human White Matter; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Jones, D.K.; Leemans, A. Diffusion tensor imaging. Methods Mol. Biol. 2011, 711, 127–144. [Google Scholar]
- Skare, S.; Hedehus, M.; Moseley, M.E.; Li, T.Q. Condition number as a measure of noise performance of diffusion tensor data acquisition schemes with MRI. J. Magn. Reson. 2000, 147, 340–352. [Google Scholar] [CrossRef]
- Kaplan, P.E. Encyclopedia of Clinical Neuropsychology; Springer: Berlin, Germany, 2011. [Google Scholar]
- Wheeler-Kingshott, C.A.; Cercignani, M. About “axial” and “radial” diffusivities. Magnetic resonance in medicine. Soc. Magn. Reson. Med. Soc. Magn. Reson. Med. 2009, 61, 1255–1260. [Google Scholar] [CrossRef]
- Jones, D.K. Challenges and limitations of quantifying brain connectivity in vivo with diffusion MRI. Imaging Med. 2010, 2, 341. [Google Scholar] [CrossRef]
- Jeurissen, B.; Leemans, A.; Tournier, J.D.; Jones, D.K.; Sijbers, J. Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Hum. Brain Mapp. 2013, 34, 2747–2766. [Google Scholar] [CrossRef] [PubMed]
- Behrens, T.E.; Berg, H.J.; Jbabdi, S.; Rushworth, M.F.; Woolrich, M.W. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? NeuroImage 2007, 34, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Schneider, T.; Wheeler-Kingshott, C.A.; Alexander, D.C. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage 2012, 61, 1000–1016. [Google Scholar] [CrossRef] [PubMed]
- DeCarlo, L.T. On the Meaning and Use of Kurtosis. Psychol. Methods 1997, 2, 292–307. [Google Scholar] [CrossRef]
- Lazar, M.; Jensen, J.H.; Xuan, L.; Helpern, J.A. Estimation of the orientation distribution function from diffusional kurtosis imaging. Magn. Reson. Med. 2008, 60, 774–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudrapatna, S.U.; Wieloch, T.; Beirup, K.; Ruscher, K.; Mol, W.; Yanev, P.; Leemans, A.; van der Toorn, A.; Dijkhuizen, R.M. Can diffusion kurtosis imaging improve the sensitivity and specificity of detecting microstructural alterations in brain tissue chronically after experimental stroke? Comparisons with diffusion tensor imaging and histology. NeuroImage 2014, 97, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Van, A.T.; Granziera, C.; Bammer, R. An introduction to model-independent diffusion magnetic resonance imaging. Top. Magn. Reson. Imaging 2010, 21, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.K.; Horsfield, M.A.; Simmons, A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn. Reson. Med. 1999, 42, 515–525. [Google Scholar] [CrossRef] [Green Version]
- Wedeen, V.J.; Hagmann, P.; Tseng, W.Y.I.; Reese, T.G.; Weisskoff, R.M. Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magn. Reson. Med. 2005, 54, 1377–1386. [Google Scholar] [CrossRef] [Green Version]
- Tian, L.; Yan, H.; Zhang, D. Diffusion spectrum magnetic resonance imaging. J. Peking Univ. Health Sci. 2009, 41, 716–720. [Google Scholar]
- Kuo, L.W.; Chen, J.H.; Wedeen, V.J.; Tseng, W.Y. Optimization of diffusion spectrum imaging and q-ball imaging on clinical MRI system. NeuroImage 2008, 41, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Krueger, G. Application Guide EP2D DSI Work-in-Progress Package for Diffusion Spectrum Imaging in Siemens. 2008. [Google Scholar]
- Teipel, S.J.; Stahl, R.; Dietrich, O.; Schoenberg, S.O.; Perneczky, R.; Bokde, A.L.; Reiser, M.F.; Möller, H.J.; Hampel, H. Multivariate network analysis of fiber tract integrity in Alzheimer’s disease. NeuroImage 2007, 34, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.K.; Cercignani, M. Twenty-five pitfalls in the analysis of diffusion MRI data. NMR Biomed. 2010, 23, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Wilde, E.A.; Ramos, M.A.; Yallampalli, R.; Bigler, E.D.; McCauley, S.R.; Chu, Z.; Wu, T.C.; Hanten, G.; Scheibel, R.S.; Li, X.; et al. Diffusion tensor imaging of the cingulum bundle in children after traumatic brain injury. Dev. Neuropsychol. 2010, 35, 333–351. [Google Scholar] [CrossRef] [PubMed]
- Arfanakis, K.; Haughton, V.M.; Carew, J.D.; Rogers, B.P.; Dempsey, R.J.; Meyerand, M.E. Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am. J. Neuroradiol. 2002, 23, 794–802. [Google Scholar] [PubMed]
- Kumar, R.; Gupta, R.K.; Husain, M.; Chaudhry, C.; Srivastava, A.; Saksena, S.; Rathore, R.K. Comparative evaluation of corpus callosum DTI metrics in acute mild and moderate traumatic brain injury: Its correlation with neuropsychometric tests. Brain Inj. 2009, 23, 675–685. [Google Scholar] [CrossRef]
- Newcombe, V.F.; Williams, G.B.; Nortje, J.; Bradley, P.G.; Chatfield, D.A.; Outtrim, J.G.; Harding, S.G.; Coles, J.P.; Maiya, B.; Gillard, J.H.; et al. Concordant biology underlies discordant imaging findings: Diffusivity behaves differently in grey and white matter post acute neurotrauma. Acta Neurochir. Suppl. 2008, 102, 247–251. [Google Scholar]
- Miles, L.; Grossman, R.I.; Johnson, G.; Babb, J.S.; Diller, L.; Inglese, M. Short-term DTI predictors of cognitive dysfunction in mild traumatic brain injury. Brain Inj. 2008, 22, 115–122. [Google Scholar] [CrossRef]
- Newcombe, V.F.J.; Williams, G.B.; Nortje, J.; Bradley, P.G.; Harding, S.G.; Smielewski, P.; Coles, J.P.; Maiya, B.; Gillard, J.H.; Hutchinson, P.J.; et al. Analysis of acute traumatic axonal injury using diffusion tensor imaging. Br. J. Neurosurg. 2007, 21, 340–348. [Google Scholar] [CrossRef]
- Wozniak, J.R.; Lim, K.O. Advances in white matter imaging: A review of in vivo magnetic resonance methodologies and their applicability to the study of development and aging. Neurosci. Biobehav. Rev. 2006, 30, 762–774. [Google Scholar] [CrossRef] [Green Version]
- Wozniak, J.R.; Krach, L.; Ward, E.; Mueller, B.A.; Muetzel, R.; Schnoebelen, S.; Kiragu, A.; Lim, K.O. Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: a diffusion tensor imaging (DTI) study. Arch. Clin. Neuropsychol. 2007, 22, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; Inokuchi, R.; Gunshin, M.; Yahagi, N.; Suwa, H. Diffusion tensor imaging studies of mild traumatic brain injury: A meta-analysis. J. Neurol. Neurosurg. Psychiatry 2012, 83, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Brandstack, N.; Kurki, T.; Tenovuo, O. Quantitative diffusion-tensor tractography of long association tracts in patients with traumatic brain injury without associated findings at routine MR imaging. Radiology 2013, 267, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Davenport, N.D.; Lim, K.O.; Armstrong, M.T.; Sponheim, S.R. Diffuse and spatially variable white matter disruptions are associated with blast-related mild traumatic brain injury. NeuroImage 2012, 59, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.R.; Ling, J.M.; Yang, Z.; Pena, A.; Yeo, R.A.; Klimaj, S. Diffusion abnormalities in pediatric mild traumatic brain injury. J. Neurosci. 2012, 32, 17961–17969. [Google Scholar] [CrossRef]
- Ling, J.M.; Pena, A.; Yeo, R.A.; Merideth, F.L.; Klimaj, S.; Gasparovic, C.; Mayer, A. R Biomarkers of increased diffusion anisotropy in semi-acute mild traumatic brain injury: A longitudinal perspective. Brain J. Neurol. 2012, 135, 1281–1292. [Google Scholar] [CrossRef]
- Wilde, E.A.; McCauley, S.R.; Hunter, J.V.; Bigler, E.D.; Chu, Z.; Wang, Z.J.; Hanten, G.R.; Troyanskaya, M.; Yallampalli, R.; Li, X.; et al. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology 2008, 70, 948–955. [Google Scholar] [CrossRef]
- Chu, Z.; Wilde, E.A.; Hunter, J.V.; McCauley, S.R.; Bigler, E.D.; Troyanskaya, M.; Yallampalli, R.; Chia, J.M.; Levin, H.S. Voxel-based analysis of diffusion tensor imaging in mild traumatic brain injury in adolescents. AJNR Am. J. Neuroradiol. 2010, 31, 340–346. [Google Scholar] [CrossRef]
- Mayer, A.R.; Ling, J.; Mannell, M.V.; Gasparovic, C.; Phillips, J.P.; Doezema, D.; Reichard, R.; Yeo, R.A. A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology 2010, 74, 643–650. [Google Scholar] [CrossRef] [Green Version]
- Mac Donald, C.L.; Johnson, A.M.; Cooper, D.; Nelson, E.C.; Werner, N.J.; Shimony, J.S.; Snyder, A.Z.; Raichle, M.E.; Witherow, J.R.; Fang, R.; et al. Detection of blast-related traumatic brain injury in USA military personnel. N. Engl. J. Med. 2011, 364, 2091–2100. [Google Scholar] [CrossRef]
- Hart, J.; Kraut, M.A.; Womack, K.B.; Strain, J.; Didehbani, N.; Bartz, E.; Conover, H.; Mansinghani, S.; Lu, H.; Cullum, C.M. Neuroimaging of cognitive dysfunction and depression in aging retired National Football League players: A cross-sectional study. JAMA Neurol. 2013, 70, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Wallace, E.J.; Mathias, J.L.; Ward, L. Diffusion tensor imaging changes following mild, moderate and severe adult traumatic brain injury: A meta-analysis. Brain Imaging Behav. 2018, 1–15, currently published online, but not yet assigned to a volume or issue. [Google Scholar] [CrossRef] [PubMed]
- Shenton, M.E.; Hamoda, H.M.; Schneiderman, J.S.; Bouix, S.; Pasternak, O.; Rathi, Y.; Vu, M.A.; Purohit, M.P.; Helmer, K.; Koerte, I.; et al. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 2012, 6, 137–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niogi, S.N.; Mukherjee, P. Diffusion tensor imaging of mild traumatic brain injury. J. Head Trauma Rehabil. 2010, 25, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.B.; Hart, T.; Whyte, J.; Rabinowitz, A.; Detre, J.A.; Kim, J. Inter-subject variability of axonal injury in diffuse traumatic brain injury. J. Neurotrauma 2017, 34, 2243–2253. [Google Scholar] [CrossRef] [PubMed]
- Sbardella, E.; Tona, F.; Petsas, N.; Pantano, P. DTI measurements in multiple sclerosis: Evaluation of brain damage and clinical implications. Mult. Scler. Int. 2013. [Google Scholar] [CrossRef] [PubMed]
- Saatman, K.E.; Duhaime, A.C.; Bullock, R.; Maas, A.I.; Valadka, A.; Manley, G.T. Classification of traumatic brain injury for targeted therapies. J. Neurotrauma 2008, 25, 719–738. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.K. Neuroprotection in traumatic brain injury. Drug Discov. Today 2008, 13, 1082–1089. [Google Scholar] [CrossRef]
- Bullock, M.R.; Lyeth, B.G.; Muizelaar, J.P. Current status of neuroprotection trials for traumatic brain injury: Lessons from animal models and clinical studies. Neurosurgery 1999, 45, 207–217. [Google Scholar] [CrossRef]
- Narayan, R.K.; Michel, M.E.; Ansell, B.; Baethmann, A.; Biegon, A.; Bracken, M.B.; Bullock, M.R.; Choi, S.C.; Clifton, G.L.; Contant, C.F.; et al. Clinical trials in head injury. J. Neurotrauma 2002, 19, 503–557. [Google Scholar] [CrossRef]
- Tolias, C.M.; Bullock, M.R. Critical appraisal of neuroprotection trials in head injury: What have we learned? NeuroRx 2004, 1, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaloostian, P.; Robertson, C.; Gopinath, S.P.; Stippler, M.; King, C.C.; Qualls, C.; Yonas, H.; Nemoto, E.M. Outcome prediction within twelve hours after severe traumatic brain injury by quantitative cerebral blood flow. J. Neurotrauma 2012, 29, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Loane, D.J.; Faden, A.I. Neuroprotection for traumatic brain injury: Translational challenges and emerging therapeutic strategies. Trends Pharmacol. Sci. 2010, 31, 596–604. [Google Scholar] [CrossRef] [PubMed]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436. [Google Scholar] [CrossRef] [PubMed]
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. Imagenet classification with deep convolutional neural networks. Adv. Neural. Inf. Process. Syst. 2012, 25, 1097–1105. [Google Scholar] [CrossRef]
- Larson, D.B.; Chen, M.C.; Lungren, M.P.; Halabi, S.S.; Stence, N.V.; Langlotz, C.P. Performance of a deep-learning neural network model in assessing skeletal maturity on pediatric hand radiographs. Radiology 2017, 287, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Gong, E.; Pauly, J.M.; Wintermark, M.; Zaharchuk, G. Deep learning enables reduced gadolinium dose for contrast-enhanced brain MRI. J. Magn. Reson. Imaging 2018. [Google Scholar] [CrossRef]
- Ueda, D.; Yamamoto, A.; Nishimori, M.; Shimono, T.; Doishita, S.; Shimazaki, A.; Katayama, Y.; Fukumoto, S.; Choppin, A.; Shimahara, Y.; et al. Deep learning for MR angiography: Automated detection of cerebral aneurysms. Radiology 2018, 180901. [Google Scholar] [CrossRef]
- Molaei, S.; Korley, F.K.; Soroushmehr, S.R.; Falk, H.; Sair, H.; Ward, K.; Najarian, K. A machine learning based approach for identifying traumatic brain injury patients for whom a head CT scan can be avoided. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016. [Google Scholar]
- Arbabshirani, M.R.; Fornwalt, B.K.; Mongelluzzo, G.J.; Suever, J.D.; Geise, B.D.; Patel, A.A.; Moore, G.J. Advanced machine learning in action: Identification of intracranial hemorrhage on computed tomography scans of the head with clinical workflow integration. npj Digit. Med. 2018, 1, 9. [Google Scholar] [CrossRef]
- Mitra, J.; Shen, K.K.; Ghose, S.; Bourgeat, P.; Fripp, J.; Salvado, O.; Pannek, K.; Taylor, D.J.; Mathias, J.L.; Rose, S. Statistical machine learning to identify traumatic brain injury (TBI) from structural disconnections of white matter networks. NeuroImage 2016, 129, 247–259. [Google Scholar] [CrossRef]
- Vergara, V.M.; Mayer, A.R.; Damaraju, E.; Kiehl, K.A.; Calhoun, V. Detection of mild traumatic brain injury by machine learning classification using resting state functional network connectivity and fractional anisotropy. J. Neurotrauma 2017, 34, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Ji, S. Combining Deep Learning Networks with Permutation Tests to Predict Traumatic Brain Injury Outcome. In International Workshop on Brainlesion: Glioma, Multiple Sclerosis, Stroke and Traumatic Brain Injuries; Springer: Berlin, Germany, 2016; pp. 259–270. [Google Scholar]
- Keshavamurthy, K.N.; Leary, O.P.; Merck, L.H.; Kimia, B.; Collins, S.; Wright, D.W.; Allen, J.W.; Brock, J.F.; Merck, D. Machine learning algorithm for automatic detection of CT-identifiable hyperdense lesions associated with traumatic brain injury. In Medical Imaging 2017: Computer-Aided Diagnosis; International Society for Optics and Photonics: Bellingham, WA, USA, 2017. [Google Scholar]
- Savjani, R.R.; Taylor, B.A.; Acion, L.; Wilde, E.A.; Jorge, R.E. Accelerated changes in cortical thickness measurements with age in military service members with traumatic brain injury. J. Neurotrauma 2017, 34, 3107–3116. [Google Scholar] [CrossRef] [PubMed]
- Mourao-Miranda, J.; Bokde, A.L.; Born, C.; Hampel, H.; Stetter, M. Classifying brain states and determining the discriminating activation patterns: Support Vector Machine on functional MRI data. NeuroImage 2005, 28, 980–995. [Google Scholar] [CrossRef] [PubMed]
- De Martino, F.; Valente, G.; Staeren, N.; Ashburner, J.; Goebel, R.; Formisano, E. Combining multivariate voxel selection and support vector machines for mapping and classification of fMRI spatial patterns. NeuroImage 2008, 43, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J. Modalities, modes, and models in functional neuroimaging. Science 2009, 326, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Burges, J.C. A Tutorial on Support Vector Machines for Pattern Recognition. Data Min. Knowl. Discov. 1998, 2, 121–167. [Google Scholar] [CrossRef]
- Wintermark, M.; Coombs, L.; Druzgal, T.J.; Field, A.S.; Filippi, C.G.; Hicks, R.; Horton, R.; Lui, Y.W.; Law, M.; Mukherjee, P.; et al. Traumatic brain injury imaging research roadmap. AJNR Am. J. Neuroradiol. 2015, 36, E12–E23. [Google Scholar] [CrossRef] [PubMed]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Douglas, D.B.; Ro, T.; Toffoli, T.; Krawchuk, B.; Muldermans, J.; Gullo, J.; Dulberger, A.; Anderson, A.E.; Douglas, P.K.; Wintermark, M. Neuroimaging of Traumatic Brain Injury. Med. Sci. 2019, 7, 2. https://doi.org/10.3390/medsci7010002
Douglas DB, Ro T, Toffoli T, Krawchuk B, Muldermans J, Gullo J, Dulberger A, Anderson AE, Douglas PK, Wintermark M. Neuroimaging of Traumatic Brain Injury. Medical Sciences. 2019; 7(1):2. https://doi.org/10.3390/medsci7010002
Chicago/Turabian StyleDouglas, David B., Tae Ro, Thomas Toffoli, Bennet Krawchuk, Jonathan Muldermans, James Gullo, Adam Dulberger, Ariana E. Anderson, Pamela K. Douglas, and Max Wintermark. 2019. "Neuroimaging of Traumatic Brain Injury" Medical Sciences 7, no. 1: 2. https://doi.org/10.3390/medsci7010002
APA StyleDouglas, D. B., Ro, T., Toffoli, T., Krawchuk, B., Muldermans, J., Gullo, J., Dulberger, A., Anderson, A. E., Douglas, P. K., & Wintermark, M. (2019). Neuroimaging of Traumatic Brain Injury. Medical Sciences, 7(1), 2. https://doi.org/10.3390/medsci7010002




