Impaired Recovery from Influenza A/X-31(H3N2) Infection in Mice with 8-Lipoxygenase Deficiency
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Characterization of ALOX8 Gene Knockout
2.2. Influenza Virus
2.3. Surgery, Viral Inoculation, and Monitoring of the Infected Mice
2.4. Hematoxylin and Eosin Staining of Lung Sections
2.5. Measurement of Residual Viral RNA
2.6. Measurement of Cytokines and Chemokines
2.7. Statistical Analysis
3. Results
3.1. Characterization of ALOX8 Knockout Mice
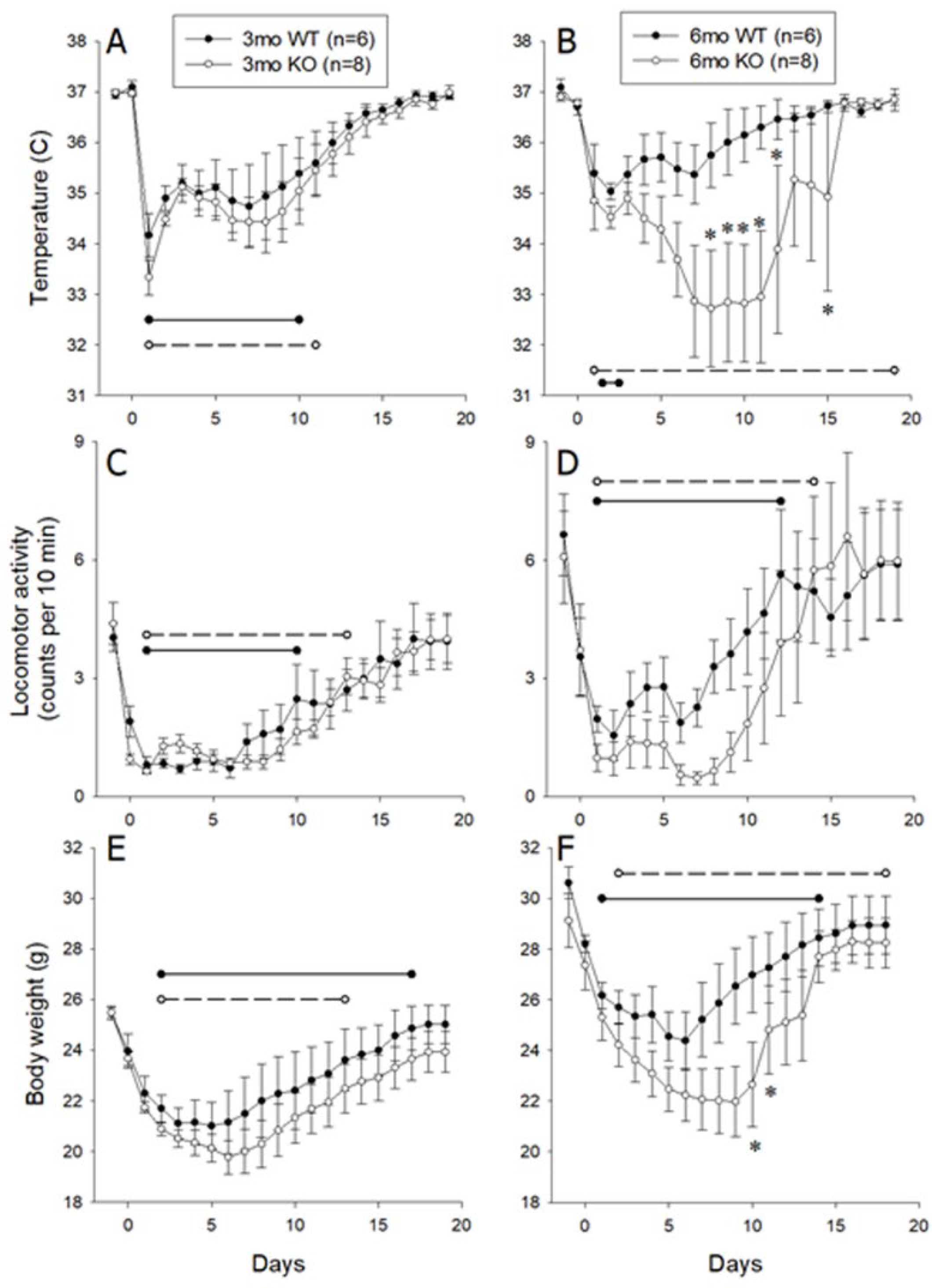
3.2. Responses Of 3- and 6-Month-Old Littermate ALOX8+/+ and ALOX8−/− After Inoculation with Influenza Virus as Measured by Intraperitoneal Transmitter
3.3. Responses of 3- and 6-Month-Old Littermate ALOX8+/+ and ALOX8−/− to Inoculation with Influenza Virus, Monitored Using Subcutaneous Chips
3.4. Residual Viral RNA in 6-Month-Old ALOX8−/− and ALOX8+/+ Mice 10 Days After Inoculation
3.5. Histopathology of Lungs of 6-Month-Old ALOX8−/− and ALOX8+/+ Mice 10 Days After Infections
3.6. Cytokine and Chemokine Levels in Lung of Infected and Uninfected 6-Month-Old ALOX8−/− and ALOX8+/+ Mice
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tam, V.C.; Quehenberger, O.; Oshansky, C.M.; Suen, R.; Armando, A.M.; Treuting, P.M.; Thomas, P.G.; Dennis, E.A.; Aderem, A. Lipidomic profiling of influenza infection identifies mediators that induce and resolve inflammation. Cell 2013, 154, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Nie, D.; Honn, K.V. Cyclooxygenase, lipoxygenase and tumor angiogenesis. Cell. Mol. Life Sci. 2002, 59, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Robbins, G.T.; Nie, D. PPAR gamma, bioactive lipids, and cancer progression. Front. Biosci. 2012, 17, 1816–1834. [Google Scholar] [CrossRef]
- Morita, M.; Kuba, K.; Ichikawa, A.; Nakayama, M.; Katahira, J.; Iwamoto, R.; Watanebe, T.; Sakabe, S.; Daidoji, T.; Nakamura, S.; et al. The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell 2013, 153, 112–125. [Google Scholar] [CrossRef]
- Kuhn, H.; O’Donnell, V.B. Inflammation and immune regulation by 12/15-lipoxygenases. Prog. Lipid Res. 2006, 45, 334–356. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Maddox, J.F.; Petasis, N.A.; Akritopoulou-Zanze, I.; Papayianni, A.; Brady, H.R.; Colgan, S.P.; Madara, J.L. Design of lipoxin A4 stable analogs that block transmigration and adhesion of human neutrophils. Biochemistry 1995, 34, 14609–14615. [Google Scholar] [CrossRef] [PubMed]
- Godson, C.; Mitchell, S.; Harvey, K.; Petasis, N.A.; Hogg, N.; Brady, H. R Cutting edge: Lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J. Immunol. 2000, 164, 1663–1667. [Google Scholar] [CrossRef]
- Hankenson, F.C.; Ruskoski, N.; van Saun, M.; Ying, G.S.; Oh, J.; Fraser, N.W. Weight loss and reduced body temperature determine humane endpoints in a mouse model of ocular herpesvirus infection. J. Am. Assoc. Lab. Anim. Sci. 2013, 52, 277–285. [Google Scholar]
- Toth, L.A.; Hughes, L.F. Sleep and temperature responses of inbred mice with Candida albicans-induced pyelonephritis. Comp. Med. 2006, 56, 252–261. [Google Scholar] [PubMed]
- Trammell, R.A.; Toth, L.A. Behavioral perturbation and sleep in healthy and virus-infected inbred mice. Comp. Med. 2014, 64, 283–292. [Google Scholar]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008, 2008, pdb-prot4986. [Google Scholar] [CrossRef]
- Erben, U.; Loddenkemper, C.; Doerfel, K.; Spieckermann, S.; Haller, D.; Heimesaat, M.M.; Zeitz, M.; Siegmund, B.; Kühl, A.A. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int. J. Clin. Exp. Pathol. 2014, 7, 4557–4576. [Google Scholar] [PubMed]
- Furuse, Y.; Suzuki, A.; Kamigaki, T.; Oshitani, H. Evolution of the M gene of the influenza A virus in different host species: Large-scale sequence analysis. Virol. J. 2009, 6, 67. [Google Scholar] [CrossRef]
- Wang, Z.; Daum, L.T.; Vora, G.J.; Metzgar, D.; Walter, E.A.; Canas, L.C.; Malanoski, A.P.; Lin, B.; Stenger, D.A. Identifying influenza viruses with resequencing microarrays. Emerg. Infect. Dis. 2006, 12, 638–646. [Google Scholar] [CrossRef]
- Ngaosuwankul, N.; Noisumdaeng, P.; Komolsiri, P.; Pooruk, P.; Chokephaibulkit, K.; Chotpitayasunondh, T.; Sangsajja, C.; Chuchottaworn, C.; Farrar, J.; Puthavathana, P. Influenza A viral loads in respiratory samples collected from patients infected with pandemic H1N1, seasonal H1N1 and H3N2 viruses. Virol. J. 2010, 7, 75. [Google Scholar] [CrossRef]
- Jisaka, M.; Kim, R.B.; Boeglin, W.E.; Brash, A.R. Identification of amino acid determinants of the positional specificity of mouse 8S-lipoxygenase and human 15S-lipoxygenase-2. J. Biol. Chem. 2000, 275, 1287–1293. [Google Scholar] [CrossRef]
- Trammell, R.A.; Toth, L.A. Effects of sleep fragmentation and chronic latent viral infection on behavior and inflammation in mice. Comp. Med. 2015, 65, 173–185. [Google Scholar]
- Toth, L.A.; Rehg, J.E.; Webster, R.G. Strain differences in sleep and other pathophysiological sequelae of influenza virus infection in naive and immunized mice. J. Neuroimmunol. 1995, 58, 89–99. [Google Scholar] [CrossRef]
- Olivadoti, M.D.; Weinberg, J.B.; Toth, L.A.; Opp, M.R. Sleep and fatigue in mice infected with murine gammaherpesvirus 68. Brain Behav. Immun. 2011, 25, 696–705. [Google Scholar] [CrossRef]
- Rodriguez, L.; Nogales, A.; Martinez-Sobrido, L. Influenza a virus studies in a mouse model of infection. J. Vis. Exp. 2017, 127, e55898. [Google Scholar] [CrossRef]
- Hernandez-Vargas, E.A.; Wilk, E.; Canini, L.; Toapanta, F.R.; Binder, S.C.; Uvarovskii, A.; Ross, T.M.; Guzmán, C.A.; Perelson, A.S.; Meyer-Hermann, M. Effects of aging on influenza virus infection dynamics. J. Virol. 2014, 88, 4123–4131. [Google Scholar] [CrossRef]
- Pernet, E.; Downey, J.; Coulombe, F.; Allard, B.; Martin, J.; Divangahi, M. Lipoxygenase-derived lipid mediators effectively regulate influenza-induced immunopathology. J. Immunol. 2016, 196, 78.5. [Google Scholar]
- Gazit, R.; Gruda, R.; Elboim, M.; Arnon, T.I.; Katz, G.; Achdout, H.; Hanna, J.; Qimron, U.; Landau, G.; Greenbaum, E.; et al. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat. Immunol. 2006, 7, 517–523. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Moki, T.; Takizawa, T.; Shiratsuchi, A.; Nakanishi, Y. Evidence for phagocytosis of influenza virus-infected, apoptotic cells by neutrophils and macrophages in mice. J. Immunol. 2007, 178, 2448–2457. [Google Scholar] [CrossRef]
- De Jong, M.D.; Simmons, C.P.; Thanh, T.T.; Hien, V.M.; Smith, G.J.; Chau, T.N.B.; Hoang, D.M.; Chau, N.V.; Khanh, T.H.; Dong, V.C.; et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 2006, 12, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Van Heeckeren, A.M.; Tscheikuna, J.; Walenga, R.W.; Konstan, M.W.; Davis, P.B.; Erokwu, B.; Haxhiu, M.A.; Ferkol, T.W. Effect of Pseudomonas infection on weight loss, lung mechanics, and cytokines in mice. Am. J. Respir. Crit. Care Med. 2000, 161, 271–279. [Google Scholar] [CrossRef]
- Metcalf, D. The granulocyte-macrophage colony-stimulating factors. Science 1985, 229, 16–22. [Google Scholar] [CrossRef]
- Thomas, J.; Liu, F.; Link, D.C. Mechanisms of mobilization of hematopoietic progenitors with granulocyte colony-stimulating factor. Curr. Opin. Hematol. 2002, 9, 183–189. [Google Scholar] [CrossRef]
- Dufour, J.H.; Dziejman, M.; Liu, M.T.; Leung, J.H.; Lane, T.E.; Luster, A.D. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J. Immunol. 2002, 168, 3195–3204. [Google Scholar] [CrossRef]
- Wareing, M.D.; Lyon, A.; Inglis, C.; Giannoni, F.; Charo, I.; Sarawar, S.R. Chemokine regulation of the inflammatory response to a low-dose influenza infection in CCR2-/- mice. J. Leukoc. Biol. 2007, 81, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Krönke, G.; Katzenbeisser, J.; Uderhardt, S.; Zaiss, M.M.; Scholtysek, C.; Schabbauer, G.; Zarbock, A.; Koenders, M.I.; Axmann, R.; Zwerina, J.; et al. 12/15-lipoxygenase counteracts inflammation and tissue damage in arthritis. J. Immunol. 2009, 183, 3383–3389. [Google Scholar] [CrossRef]
- Miller, R.A.; Nadon, N.L. Principles of animal use for gerontological research. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2000, 55, B117–B123. [Google Scholar] [CrossRef]
- Lynch, H.E.; Goldberg, G.L.; Chidgey, A.; Van den Brink, M.R.; Boyd, R.; Sempowski, G.D. Thymic involution and immune reconstitution. Trends Immunol. 2009, 30, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, B.; Tang, S.; Yang, P.; Doll, A.; Aumüeller, G.; Newman, R.A.; Tang, D.G. Cell-autonomous induction of functional tumor suppressor 15-lipoxygenase 2 (15-LOX2) contributes to replicative senescence of human prostate progenitor cells. Oncogene 2005, 24, 3583–3595. [Google Scholar] [CrossRef] [PubMed]



| Genotype | ||
|---|---|---|
| Age | ALOX8+/+ | ALOX8−/− |
| 3 months | 0.001 ± 0.000 | 0.002 ± 0.001 |
| 6 months | 0.017 ± 0.008 | 0.018 ± 0.002 * |
| ALOX8+/+ | ALOX8−/− | |
|---|---|---|
| Inflammation Score | ||
| Uninfected | 0 ± 0 (n = 3) | 0.6667 ± 0.57735 (n = 3) |
| Infected | 0.8333 ± 0.75277 (n = 6) | 2.375 ± 0.91613 (n = 8)* |
| Architectural Pathology Score | ||
| Uninfected | 0 ± 0 (n = 3) | 0.3333 ± 0.57735 (n = 3) |
| Infected | 0.50 ± 0.54722 (n = 6) | 1.50 ± 0.75593 (n = 8) * † |
| Cytokines | ALOX8+/+ | ALOX8−/− |
|---|---|---|
| IL-1β | ||
| Uninfected | 1.38 ± 0.15 | 1.36 ± 0.10 |
| Infected | 1.26 ± 0.11 | 1.23 ± 0.09 |
| IL-5 | ||
| Uninfected | 0.22 ± 0.19 | 0.14 ± 0.16 |
| Infected | 0.14 ± 0.04 | 0.54 ± 0.07 |
| G-CSF | ||
| Uninfected | 0.75 ± 0.05 | 0.75 ± 0.06 |
| Infected | 1.95 ± 0.25 * | 1.89 ± 0.10 * |
| IL-6 | ||
| Uninfected | 0.82 ± 0.09 | 0.75 ± 0.03 |
| Infected | 1.32 ± 0.16 * | 1.73 ± 0.05 * † |
| IP-10 (CXCL10) | ||
| Uninfected | 1.75 ± 0.03 | 1.84 ± 0.03 |
| Infected | 3.09 ± 0.08 * | 3.23 ± 0.02 * |
| KC (CXCL1) | ||
| Uninfected | 1.49 ± 0.07 | 1.50 ± 0.04 |
| Infected | 1.78 ± 0.05 * | 1.97 ± 0.04 * † |
| MCP-1 (CCL2) | ||
| Uninfected | 1.61 ± 0.19 | 1.55 ± 0.14 |
| Infected | 2.65 ± 0.20 * | 2.31 ± 0.06 * |
| MIG (CXCL9) | ||
| Uninfected | 1.73 ± 0.03 | 1.78 ± 0.04 |
| Infected | 2.99 ± 0.05 * | 3.07 ± 0.02 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfardan, R.; Guo, C.; Toth, L.A.; Nie, D. Impaired Recovery from Influenza A/X-31(H3N2) Infection in Mice with 8-Lipoxygenase Deficiency. Med. Sci. 2019, 7, 60. https://doi.org/10.3390/medsci7040060
Alfardan R, Guo C, Toth LA, Nie D. Impaired Recovery from Influenza A/X-31(H3N2) Infection in Mice with 8-Lipoxygenase Deficiency. Medical Sciences. 2019; 7(4):60. https://doi.org/10.3390/medsci7040060
Chicago/Turabian StyleAlfardan, Rana, Changxiong Guo, Linda A. Toth, and Daotai Nie. 2019. "Impaired Recovery from Influenza A/X-31(H3N2) Infection in Mice with 8-Lipoxygenase Deficiency" Medical Sciences 7, no. 4: 60. https://doi.org/10.3390/medsci7040060
APA StyleAlfardan, R., Guo, C., Toth, L. A., & Nie, D. (2019). Impaired Recovery from Influenza A/X-31(H3N2) Infection in Mice with 8-Lipoxygenase Deficiency. Medical Sciences, 7(4), 60. https://doi.org/10.3390/medsci7040060






