1. Introduction
Rapamycin, also known as sirolimus, is a compound that has been extensively studied for its impact on life span and health span across multiple species [
1]. It primarily acts as an inhibitor of the ubiquitous signaling protein mammalian target of rapamycin (mTOR), which regulates growth and metabolism. There are many studies that have investigated the beneficial effect of rapamycin on life span extension, particularly in relation to cognitive decline and Alzheimer’s disease. These studies, summarized by Kaeberlein and Galvan [
2], show rapamycin treatment in various mouse models of Alzheimer’s disease can reduce amyloid-β deposition, pathogenic tau phosphorylation, and neurofibrillary tangles; restore cerebral blood flow; preserve the blood–brain barrier’s (BBB) integrity; and improve cognitive function.
Increasing central nervous system (CNS) insulin improves cognitive function as well. Brain insulin levels are dependent on BBB insulin transport. Therefore, if the basal BBB transport of insulin can be increased, CNS levels can be increased, leading to improvements in memory. Indeed, there are many intersections between metabolism, insulin signaling, and rapamycin [
3]. Rapamycin acts as a starvation mimetic, triggering a physiological unfed state. Starvation in mice increases insulin BBB transport [
4]. Rapamycin also increases plasma triglyceride levels following a 14-day sirolimus treatment in humans [
5] and in mice following longer rapamycin treatment [
6]. Plasma triglycerides are known to increase insulin BBB transport in mice [
4]. Cytokines can also increase insulin BBB transport [
7]. Additionally, changes in interactions of insulin at the BBB can indirectly impact signaling within the CNS. That is, the binding of insulin to the insulin receptor located on endothelial cells can affect the interactions of brain endothelial cells with other signaling factors by affecting cell adhesion molecules [
8]. Therefore, we hypothesized rapamycin would increase the amount of insulin BBB transport or affect the BBB interactions, which could be a mechanism for improved cognition. We randomly divided young male and female mice to receive either a vehicle or rapamycin daily injections for two weeks prior to the measurement of insulin signaling, vascular binding, and BBB pharmacokinetics. We also included a group of mice that received a single, acute injection of rapamycin to determine if there were any acute effects on insulin BBB transport.
2. Materials and Methods
2.1. Animals
Male and female CD-1 mice (7 weeks old) were purchased from Charles River Laboratories (Seattle, WA, USA). For chronic treatments, rapamycin intraperitoneal (IP) injections began at 8 weeks of age daily for 2 weeks. The mice were studied at 10 weeks of age. The mice had free access to water and food and were kept on a 12/12 h light/dark cycle. The mice were housed in groups of 4 mice per cage. Body weights were measured every two to three days. The local Institutional Animal Care and Use Committee (IACUC) at the Veteran Affairs Puget Sound approved all experimental animal procedures performed in this study. The study was performed at a facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC).
2.2. Chronic Rapamycin Injection Treatment
Rapamycin (LC Laboratories, Woburn, MA, USA) was prepared similar to the previously published studies [
9]. Briefly, rapamycin was dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich, St. Louis, MO, USA) to a stock concentration of 100 mg/ml. For the injectate, the 100 mg/mL rapamycin solution was diluted in a 5% Tween 80 (Sigma-Aldrich), 5% poly(ethylene glycol) 400 (PEG 400, Sigma-Aldrich) solution to create a final 1.2 mg/mL concentration. The vehicle was prepared by creating a 1.2% DMSO solution in 5% Tween 80, 5% PEG 400. The mice were randomly divided into two groups and received either a daily vehicle or rapamycin (8 mg/kg) IP injections for 2 weeks. The dosing was chosen based on what is currently used throughout the literature [
9] as well as reports regarding similarities on the average trough levels of rapamycin between mice and humans [
10].
2.3. Radioactive Labeling
Ten micrograms of human insulin (Sigma-Aldrich) were diluted in 40 µL 0.25 M chloride-free sodium phosphate buffer (PB) and labeled with 1 mCi 125I (PerkinElmer, Waltham, MA, USA) through the chloramine-T (Sigma-Aldrich) method. The reaction started with the addition of 10 μg chloramine T in 0.25 M PB and terminated 1 min later with the addition of 100 µg sodium metabisulfite. Albumin (1 mg, Sigma-Aldrich) was labeled with 1 mCi 99mTc (GE Healthcare, Seattle, WA, USA) after combination with 120 µg stannous tartrate and 20 μL 1M HCl in 500 µL deionized water for a 20 min reaction. Both 125I-insulin and 99mTc-albumin were purified via a column of Sephadex G-10 beads (Sigma-Aldrich) in 0.25 M PB. The protein labeling was characterized by 15% trichloroacetic acid (TCA) precipitation. Greater than 90% radioactivity in the precipitated fractions was consistently observed for insulin and albumin.
2.4. Intravenous Injection Study
Following 2 weeks of daily the vehicle or rapamycin IP injections, the mice were anesthetized with 0.15 mL of 40% urethane and the right jugular vein and left carotid artery were exposed. The blood glucose was measured via the tail vein (AlphaTrak2, Abbott Laboratories, Chicago, IL, USA). The mice received an intravenous (i.v.) injection of a 0.2 mL solution containing 1 × 10
6 cpm of
125I-insulin and 5 × 10
5 cpm of
99mTc-albumin in 1% bovine serum albumin in lactated Ringer’s (BSA-LR) directly into the jugular vein. The radioactivity circulated for times between 1–10 min, after which the carotid artery was cut to collect blood. The mice were immediately decapitated and the whole brain was removed and weighed. The blood was centrifuged at 5400 g for 10 min and the amount of radioactivity in the serum (50 µL) and the whole brain was counted in a gamma counter (Wizard2, PerkinElmer, Waltham, MA, USA). Two separate cohorts of mice were combined in the final analyses. In a separate cohort of mice, the effect of a single, acute rapamycin injection was studied. Naïve male mice were anesthetized as described. The vehicle or rapamycin (400 ng) was injected intravenously and allowed to circulate for 10 min. This dose was chosen based to approximately match a previous study reporting blood rapamycin levels following an 8 mg/kg body weight IP injection (100 ng/mL) [
9]. Following the 10 min circulation period, the
125I-insulin and
99mTc-albumin solution was injected as described above.
2.5. Transcardiac Brain Perfusion
Following the rapamycin treatment for 2 weeks, the thoracic cavity was opened, the heart was exposed, both jugulars were severed, and the descending thoracic aorta was clamped. A 26-gauge butterfly needle was inserted into the left ventricle of the heart and a freshly prepared Zlokovic’s buffer (7.19 g/L NaCl, 0.3 g/L KCl, 0.28 g/L CaCl2, 2.1 g/L NaHCO3, 0.16 g/L KH2PO4, 0.17 g/L anhydrous MgCl2, 0.99 g/L D-glucose, and 1% BSA) containing 2 × 105 cpm 125I-insulin was infused at a rate of 2 mL/min for 1–5 min. Perfusate was collected throughout the study to determine the average cpm/µL of perfusate. After perfusion, the whole brain was weighed and the amount of radioactivity was counted in a gamma counter (Wizard2, PerkinElmer). The brain/perfusate ratios were calculated by dividing the cpm in a gram of brain by the cpm in a µL of perfusate to yield the units of µL/g.
2.6. Multiple-Time Regression Analysis
A multiple-time regression analysis was used as detailed previously [
11,
12] to calculate the rate of unidirectional influx for
125I-insulin. For the i.v. study, the brain/serum (B/S) ratios for
125I-insulin are corrected for vascular space by subtracting the corresponding ratio for
99mTc-albumin, yielding a delta B/S ratio. The exposure time is calculated by the formula:
where Cp(t) is the level of radioactivity (cpm) in the serum at time (t). The exposure time corrects for the clearance of peptide from the blood. The B/S ratios are plotted against the exposure time to calculate the influx of
125I-insulin using the same formula described by Blasberg et al. [
11]:
where Am is the level of radioactivity (cpm) per g of brain tissue at time t, Cpt is the level of radioactivity (cpm) per μL serum at time t, K
i (μL/g-min) is the unidirectional solute influx from blood to brain, and V
i (μL/g) is the level of rapid and reversible binding for the brain vasculature. The slope of the linearity measures the K
i and is reported with its standard error term. The y-intercept of the linearity measures the V
i, the initial volume of distribution in brain at t = 0 [
11]. For cardiac perfusion studies, the latter formula is employed, using the brain/perfusate ratios for
125I-insulin and the clock time is used in place of the exposure time.
2.7. Biochemical Tissue Processing
A separate cohort of male and female mice (n = 4/group) were treated for 2 weeks with vehicle or 8 mg/kg body weight rapamycin as described above. Following a 6 h fast, blood glucose was taken. The mice were anesthetized as described above, the thoracic cavity was opened, and blood was collected from the descending aorta. The heart was exposed, both jugulars were severed, and the descending thoracic aorta was clamped. The brain vasculature was washed out by perfusing 20 mL of ice cold LR into the left ventricle of the heart. The brain was removed and split into the right and left hemispheres. The aorta was also removed. The blood was centrifuged at 5400 g for 10 min and the serum was collected. All the tissues were snap frozen in liquid nitrogen and stored at −80 °C until further processing.
The right hemisphere was crushed with a mortar and pestle in liquid nitrogen and approximately 100 mg of tissue was homogenized in radioimmunoprecipitation assay (RIPA) buffer (150 mM NaCl, 0.5% deoxycholic acid, 0.1% sodium dodecyl sulfate (SDS), 20 mM Tris HCl, and 2 mM ethylenediaminetetraacetic acid (EDTA)) plus freshly added 1/100 dilutions of a protease inhibitor cocktail (Sigma-Aldrich), 1mM sodium orthovanadate, and a phosphatase inhibitor (1 tablet PhosSTOP per 10 mL buffer) (Roche, Basel, Switzerland). The aortas were homogenized in the same lysis buffer. The samples were sonicated at 40% amplitude prior to centrifugation at 12,000× g for 10 min at 4 °C. The supernatants were collected and frozen at −80 °C. The protein was measured using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Rockford, IL, USA).
2.8. Measurement of Hormone Levels
A panel of 8 metabolic hormones was measured in the brain lysate and serum using a Bio-Plex Pro Mouse Diabetes Assay (Bio-Rad Laboratories, Hercules, CA, USA): ghrelin, glucose-dependent insulinotropic peptide (GIP), glucagon-like peptide-1 (GLP-1), glucagon, insulin, leptin, plasminogen activator inhibitor-1 (PAI-1), and resistin. The serum was diluted 1:4 with the kit diluent. Twenty µg/µL protein lysate samples were measured from the right hemisphere. The samples were measured according to the manufacturer and read on a Bio-Plex 200 (Bio-Rad Laboratories).
2.9. Western Immunoblotting
The protein lysates were solubilized and denatured in NuPAGE sample buffer (Invitrogen, Grand Island, NY, USA), warmed for 10 min at 70 °C, and resolved on a 4–12% Bis-Tris gel (Invitrogen). The protein was then transferred to nitrocellulose membranes using the iBlot transfer system (Invitrogen). The membranes were blocked with 5% BSA in 0.1% Tween-20 in tris-buffered saline (TBS-T) (BSA/TBS-T) for 1 h. The membranes were incubated in primary antibodies from Cell Signaling Technologies (Danvers, MA, USA) for modifications in insulin signaling (phosphorylated protein kinase B [pAkt Ser473], total protein kinase B [Akt], mitogen-activated protein kinase [MAPK], and β-actin) prepared in BSA/TBS-T overnight at 4 °C. The membranes were washed in TBS-T and then probed with the respective secondary antibodies conjugated to horseradish peroxidase (Jackson Labs, West Grove, PA, USA) for 1 h at room temperature. The membranes were washed with TBS-T and then illuminated with ECL Prime Western Blotting Detection Reagent (Amersham, GE Life Sciences, Piscataway, NJ, USA). Following the band visualization on the ImageQuant LAS4000 CCD imaging system (GE Life Sciences), the blots were stripped with Restore Western Blot Stripping Buffer (Thermo Scientific) and re-probed for the total protein levels or β-actin. A densitometric analysis of bands was performed using ImageQuant TL (IQTL) software (GE Life Sciences, Piscataway, NJ, USA). The band intensities of phosphorylated proteins were normalized to the band intensities of the corresponding total protein levels, and the ratios of phosphorylated/total protein were normalized to the male vehicle group.
2.10. Measurement of Serum Cytokines
A panel of 23 cytokines was measured in the brain protein lysate and serum using a mouse Bio-Plex Pro Mouse Cytokine Assay (Bio-Rad Laboratories): interleukin (IL)-1α; IL-1β; IL-2; IL-3; IL-4; IL-5; IL-6; IL-9; IL-10; IL-12(p40); IL-12(p70); IL-13; IL-17; eotaxin (CCL11); granulocyte colony-stimulating factor (G-CSF); granulocyte-macrophage colony-stimulating factor (GM-CSF); interferon (IFN)-γ; keratinocyte chemoattractant (KC) (CXCL1); monocyte chemoattractant protein (MCP)-1 (CCL2); macrophage inflammatory protein (MIP)-1α (CCL3); MIP-1β (CCL4); and regulated on activation, normal T cell expressed and secreted (RANTES; CCL5) and tumor necrosis factor (TNF)-α). The serum samples were diluted 1:4 with the kit sample diluent. The samples were measured according to the manufacturer and read on a Bio-Plex 200 (Bio-Rad Laboratories).
2.11. Statistics
A regression analysis and other statistical analyses were performed with the use of Prism 8.0 (GraphPad Software Inc., San Diego, CA, USA). Body weight means were reported with their standard error terms and compared by a mixed-effects analysis followed by Sidak’s post hoc test. All the other basic biochemical comparisons were performed using a two-way ANOVA followed by Sidak’s post hoc test to compare the effect of sex and rapamycin treatment. Two-way ANOVA analyses are listed in the manuscript text while post hoc differences are designated on the figures with symbols and in the figure legends. For the pharmacokinetic studies, the slope of the linear regression lines (K
i), reported with their correlation coefficients (r), and y-intercepts (V
i) were compared statistically with the Prism 8.0 software package, as described [
13].
4. Discussion
We investigated the effects of rapamycin treatment on the basal levels of insulin vascular binding, receptor signaling, and BBB pharmacokinetics in male and female mice. While we found an acute spike in the blood rapamycin levels had no impact on insulin BBB transport, it did decrease vascular binding in male mice. We also found a short-term chronic, daily treatment with rapamycin led to alterations in insulin signaling in endothelial cells. Chronic rapamycin differentially affected body weight gain, serum glucose, and the serum levels of other metabolic hormones and cytokine levels due to sex. There was no significant effect on the BBB insulin pharmacokinetics due to chronic rapamycin treatment in the presence or absence of serum factors. There were also no changes in the serum triglyceride levels following the rapamycin treatment paradigm.
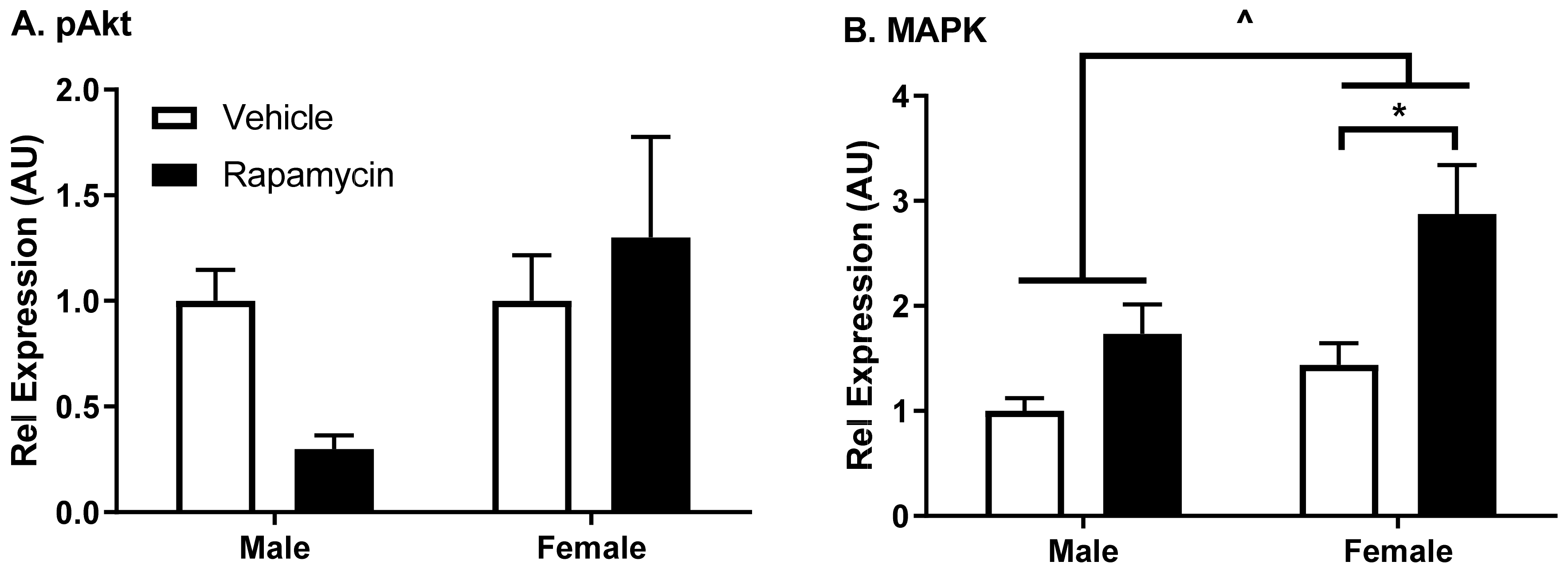
An acute increase in the blood rapamycin levels led to a small, but significant decrease in insulin vascular binding in the whole brain. A decrease in binding could lead to changes in the intracellular insulin receptor signaling, which is known to affect expression the levels of various proteins, including cell adhesion molecules [
8]. Changes in these alternative proteins within the brain endothelial cells could affect signals relayed to the CNS. We observed changes in insulin receptor signaling following chronic rapamycin treatment in a predominantly endothelial cell tissue, the aorta. Aortic and brain endothelial cells are regulated differentially but the aorta can be a predictor of what is occurring in the brain endothelial cells. While not quite reaching statistical significance using a two-way ANOVA, there was a trend in decreased pAkt Ser
473 protein levels compared to the total Akt in males in the aorta. This protein difference in males due to the rapamycin treatment could be due to the decreased body weight gain present in males treated with rapamycin compared to the vehicle controls. There was a significant increase in the MAPK protein levels relative to β-actin due to the rapamycin treatment as well as in the females compared to the males, suggesting a dysregulation of this signaling molecule. The inhibition of mTOR using rapamycin has been shown to have an impact on the MAPK pathway [
15,
16]. These studies suggest rapamycin has a sexual dimorphic effect on MAPK signaling in endothelial cells, with a greater effect elicited in females.
While others have reported a decrease in body weight in male mice with rapamycin treatment [
6,
9], a decrease in body weight for C57BL6/J female mice has also been reported [
9]. We observed a decrease in body weight only in males. The lack of difference in our study between sexes could be due to the differences in mouse strains (CD-1 vs. C57BL6/J) or the rapamycin treatment length. Similar to our findings, rapamycin has previously been shown to impair glucose tolerance following 2 weeks of daily injections of a fourfold lower concentration of rapamycin than the one used in the current study [
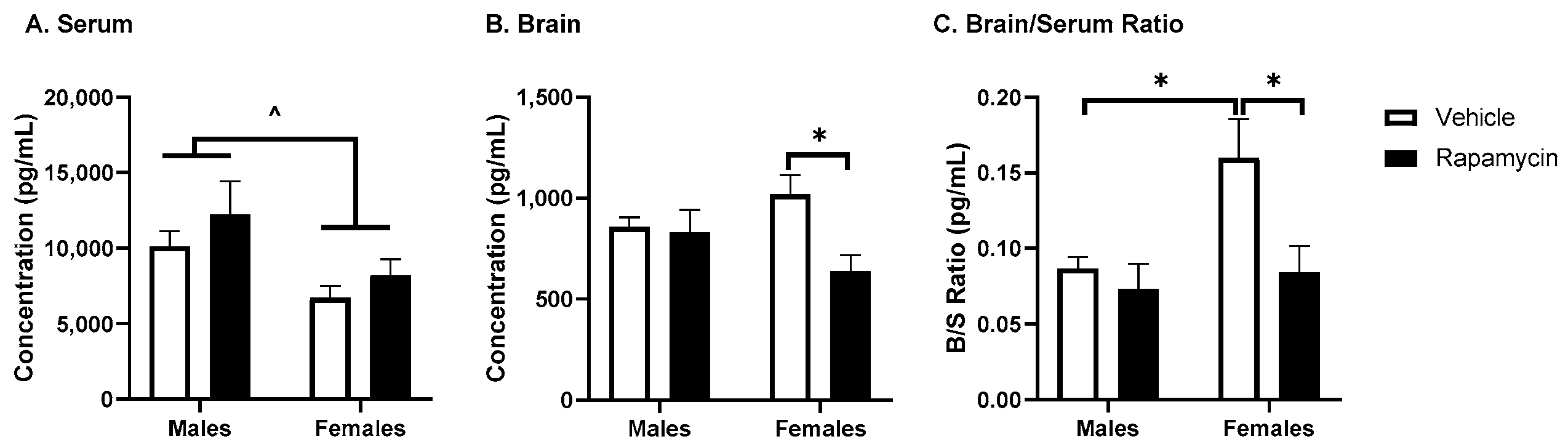
17]. Five weeks of daily rapamycin treatment also led to an increase in the serum glucose levels of mice fasted overnight [
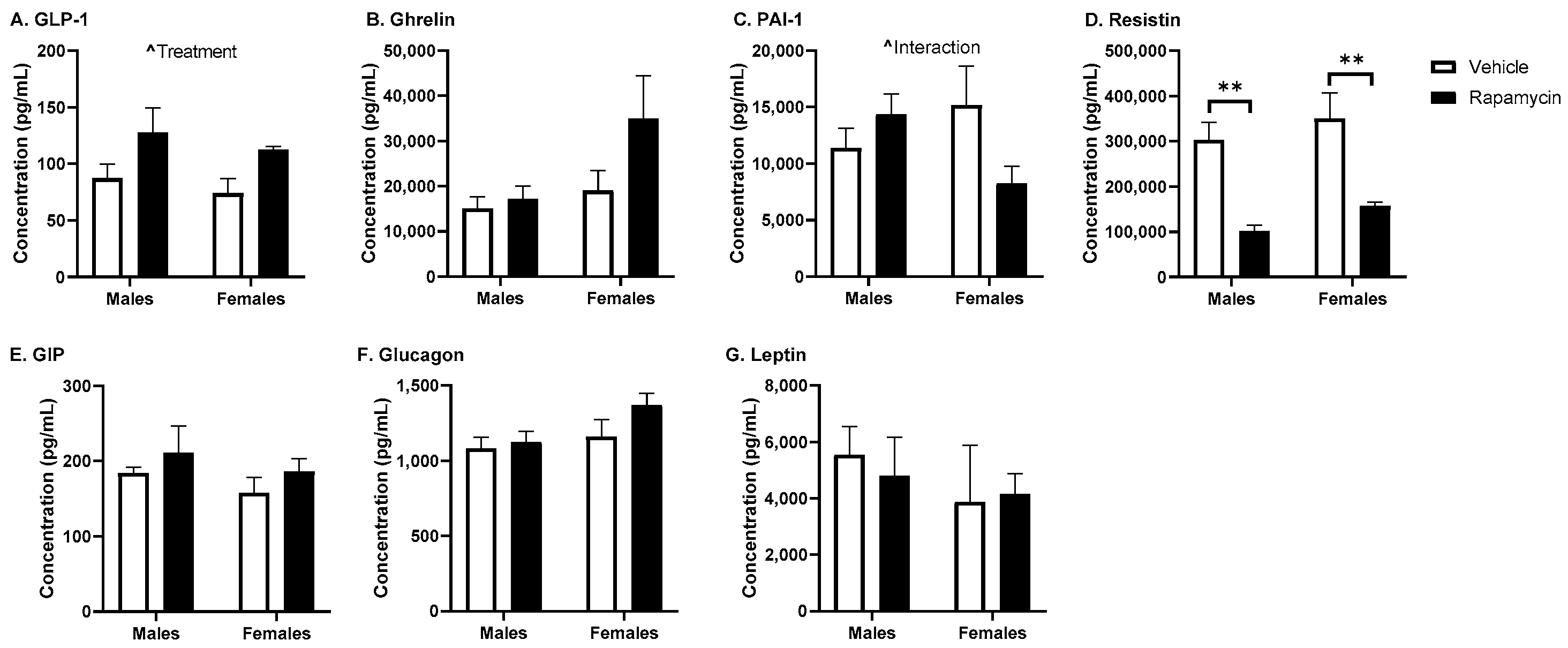
17]. While we did not observe a significant effect of rapamycin on the fasted serum insulin levels, we did observe differences in the brain insulin levels in females only. Although this could be due to changes in insulin BBB transport, it is more likely due to changes in insulin brain degradation, based on our results below investigating BBB transport. An increase in the brain/serum ratio for insulin in the vehicle females was unexpected. Sex differences in the insulin brain/serum ratios have not been investigated. However, it is known women are at an increased risk for Alzheimer’s disease, which could be linked to alterations in CNS insulin signaling and thus differences in the brain/serum insulin ratios. The serum resistin levels were significantly decreased due to the rapamycin treatment, independent of sex. Acute caloric restriction, which is thought to act similar to rapamycin treatment, decreases resistin levels [
18]. The serum GLP-1 levels were significantly increased due to the rapamycin treatment which is similar to what occurs with fasting [
19]. PAI-1 is often considered a marker of insulin resistance. However, it is interesting that we observed an interaction due to sex and the rapamycin treatment on the serum levels of PAI-1. The serum insulin and glucose levels were higher in the males which could account for the differences observed in males versus females for the PAI-1 levels.
As rapamycin is used in the clinic as an immunosuppressant and since cytokines can affect insulin BBB transport, we wanted to measure the serum cytokine levels following our rapamycin treatment paradigm. We found the rapamycin treatment was able to increase the IL-2, IL-3, and IL-17 levels. These cytokines are all produced by T cells. IL-2 is known to impact the Akt/mTOR and MAPK/ERK pathways and works in conjunction with IL-3. IL-17 is a pro-inflammatory cytokine that is known to aid in the release of chemokines, recruiting immune cells and working in conjunction with TNF-α, for which there was a trend towards increased levels due to the rapamycin treatment. However, the degree of change for these cytokines was unable to alter insulin BBB transport, as discussed below, suggesting a greater level of cytokine production is needed or a different combination of cytokine increases is necessary to alter insulin BBB transport.
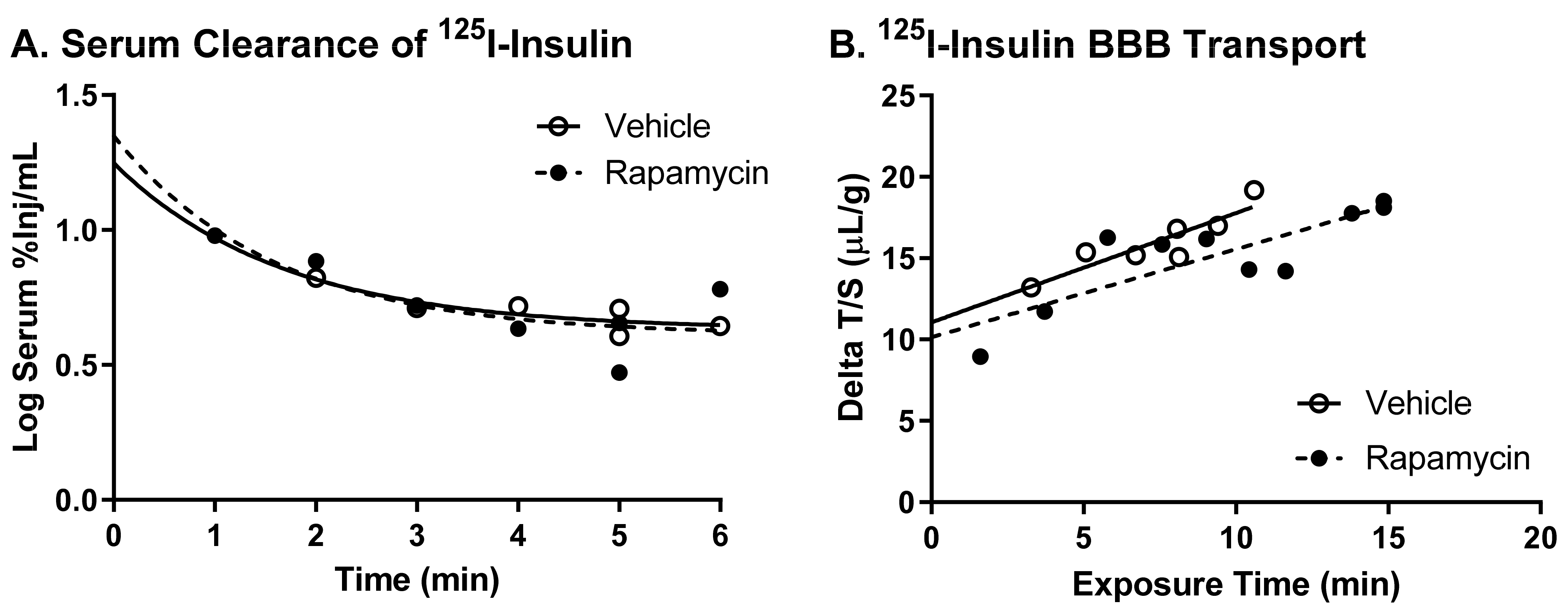
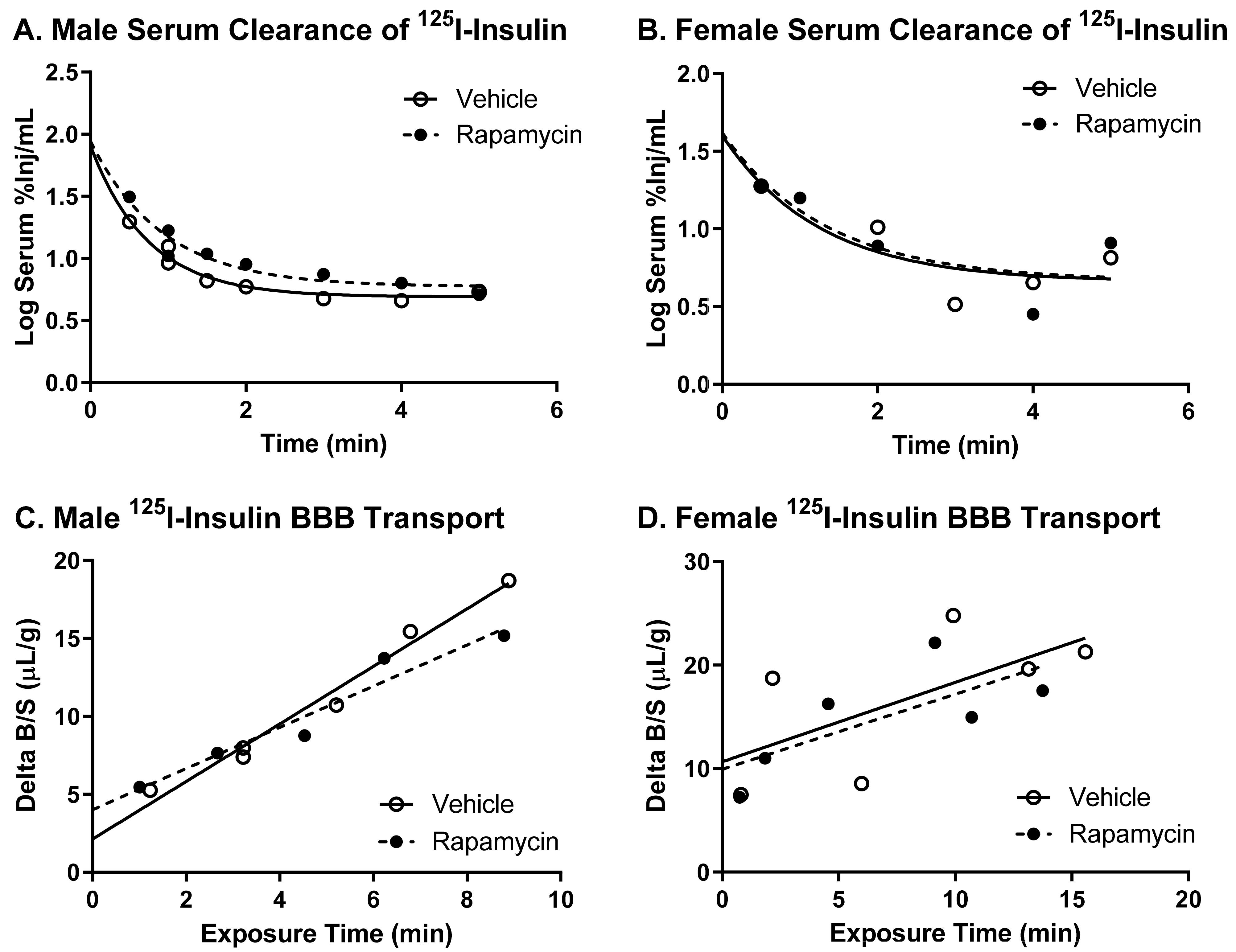
There was no difference in the transport rate of insulin across the BBB following two weeks of rapamycin treatment. In the males, there was a trend in a decreased transport rate due to rapamycin (
p = 0.055). However, there was no effect in females. Additionally, the level of insulin vascular binding was similar between the two treatment groups. The lack of differences in insulin vascular binding here compared to the acute administration of rapamycin could be due to a couple different factors. First, the brain endothelial cells could have adjusted to the chronic exposure of rapamycin administration and therefore did not have a strong impact on vascular binding. Second, acute administration of rapamycin could lead to differences in the levels of serum factors that could also impact insulin binding. The trending difference in males could be due to the metabolic changes observed in the male mice due to the rapamycin treatment. Various physiological factors are known to alter insulin BBB transport [
20]. However, it was anticipated that rapamycin would increase BBB transport rather than decrease transport based on previous studies showing similarities between the beneficial effects of rapamycin and insulin on cognition. While changes in BBB transport can be masked by serum factors, there was still no effect of rapamycin treatment on insulin BBB transport in the absence of serum factors.
Triglycerides were one mechanism we hypothesized rapamycin could impact insulin BBB transport. However, our rapamycin treatment regimen did not alter the serum triglyceride levels which could be one reason we did not observe significant differences in insulin BBB transport. Previous reports in growth hormone receptor knockout male mice showed that a 4 mg/kg rapamycin treatment administered every other day for 20 weeks was able to increase the serum triglyceride levels by approximately 12% [
6]. A study in humans reported an increase in the serum triglyceride levels by approximately 47% following a 10 mg/day dose of sirolimus for 42 days as tolerated [
5]. It should be noted this patient population was chosen for this longer treatment period due to an initial hyperlipidemic response to a short-term treatment of sirolimus. Lastly, in one final study looking at the serum triglyceride levels in mice fed rapamycin orally, no differences were observed [
21]. These studies suggest the genetic background and propensity to respond to rapamycin can alter the serum triglyceride levels differently.
Our findings suggest that rapamycin can alter the metabolic factors and insulin receptor signaling and binding on endothelial cells in a sexually dimorphic way. However, chronic rapamycin treatment is unable to alter insulin BBB transport. The differences in metabolism between males and females should be taken into consideration in planning future studies. Longer treatments (13+ weeks) with rapamycin have been shown to improve cognition [
22]. Since evidence for testing rapamycin treatment in humans with Alzheimer’s disease is becoming greater [
2], mechanisms by which rapamycin could improve cognition in pre-clinical models should continue to be investigated. Therefore, investigating the impact of long-term rapamycin treatment on insulin BBB transport, especially in mouse models of Alzheimer’s disease, still warrants further investigation as a mechanism for contributing to the cognitive improvements due to rapamycin.