Epidemiology of Melanoma
Abstract
:1. Introduction
2. Epidemiology
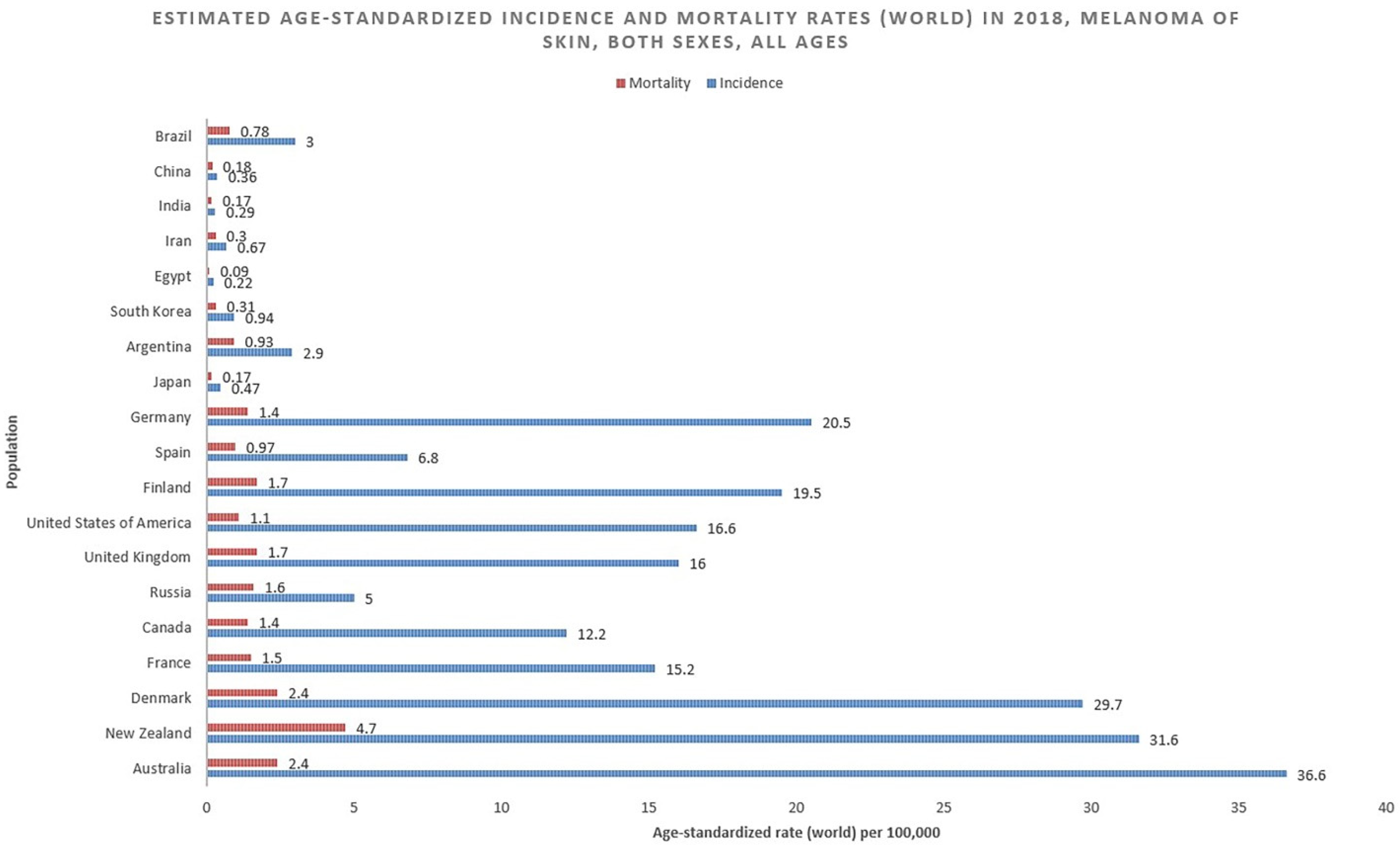
2.1. Incidence
2.2. Mortality
2.3. Survival
3. Risk Factors
3.1. Sun Exposure
3.2. Indoor Tanning
3.3. Immunosuppression
3.4. Moles (Nevi)
3.5. Family History
3.6. Obesity
4. Prevention
4.1. Primary Prevention and Education
4.2. Screening
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Cancer Institute Melanoma of the Skin-Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/melan.html (accessed on 10 May 2021).
- Rabbie, R.; Ferguson, P.; Molina-Aguilar, C.; Adams, D.J.; Robles-Espinoza, C.D. Melanoma subtypes: Genomic profiles, prognostic molecular markers and therapeutic possibilities. J. Pathol. 2019, 247, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Manson, D.K.; Marr, B.P.; Carvajal, R.D. Treatment of uveal melanoma: Where are we now? Ther. Adv. Med. Oncol. 2018, 10, 1758834018757175. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. International Agency for Research on Cancer: Lyon, France. Available online: https://gco.iarc.fr/today (accessed on 10 May 2021).
- Whiteman, D.C.; Green, A.C.; Olsen, C.M. The Growing Burden of Invasive Melanoma: Projections of Incidence Rates and Numbers of New Cases in Six Susceptible Populations through. J. Investig. Dermatol. 2016, 136, 1161–1171. [Google Scholar] [CrossRef] [Green Version]
- Berk-Krauss, J.; Stein, J.A.; Weber, J.; Polsky, D.; Geller, A.C. New systematic therapies and trends in cutaneous melanoma deaths among US whites, 1986–2016. Am. J. Public Health 2020, 110, 731–733. [Google Scholar] [CrossRef]
- Bowden, N.A.; Ashton, K.A.; Avery-Kiejda, K.A.; Zhang, X.D.; Hersey, P.; Scott, R.J. Nucleotide excision repair gene expression after cisplatin treatment in melanoma. Cancer Res. 2010, 70, 7918–7926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishigori, C. Xeroderma Pigmentosum. Brain Nerve 2019, 71, 394–399. [Google Scholar] [PubMed]
- Paszkowska-Szczur, K.; Scott, R.J.; Serrano-Fernandez, P.; Mirecka, A.; Gapska, P.; Górski, B.; Cybulski, C.; Maleszka, R.; Sulikowski, M.; Nagay, L.; et al. Xeroderma pigmentosum genes and melanoma risk. Int. J. Cancer 2013, 133, 1094–1100. [Google Scholar] [CrossRef]
- Arisi, M.; Zane, C.; Caravello, S.; Rovati, C.; Zanca, A.; Venturini, M.; Calzavara-Pinton, P. Sun exposure and melanoma, certainties and weaknesses of the present knowledge. Front. Med. 2018, 5, 235. [Google Scholar] [CrossRef]
- Marionnet, C.; Tricaud, C.; Bernerd, F. Exposure to non-extreme solar UV daylight: Spectral characterization, effects on skin and photoprotection. Int. J. Mol. Sci. 2015, 16, 68–90. [Google Scholar] [CrossRef]
- Harris, R.S. Cancer mutation signatures, DNA damage mechanisms, and potential clinical implications. Genome Med. 2013, 5, 87. [Google Scholar] [CrossRef] [Green Version]
- Larsson, P.; Andersson, E.; Johansson, U.; Öllinger, K.; Rosdahl, I. Ultraviolet A and B affect human melanocytes and keratinocytes differently. A study of oxidative alterations and apoptosis. Exp. Dermatol. 2005, 14, 117–123. [Google Scholar] [CrossRef]
- Kappes, U.P.; Luo, D.; Potter, M.; Schulmeister, K.; Rünger, T.M. Short- and long-wave UV light (UVB and UVA) induce similar mutations in human skin cells. J. Investig. Dermatol. 2006, 126, 667–675. [Google Scholar] [CrossRef] [Green Version]
- Goel, V.K.; Lazar, A.J.F.; Warneke, C.L.; Redston, M.S.; Haluska, F.G. Examination of mutations in BRAF, NRAS, and PTEN in primary cutaneous melanoma. J. Investig. Dermatol. 2006, 126, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Thomas, N.E.; Edmiston, S.N.; Alexander, A.; Millikan, R.C.; Groben, P.A.; Hao, H.; Tolbert, D.; Berwick, M.; Busam, K.; Begg, C.B.; et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol. Biomark. Prev. 2007, 16, 991–997. [Google Scholar] [CrossRef] [Green Version]
- Edlundh-Rose, E.; Egyházi, S.; Omholt, K.; Månsson-Brahme, E.; Platz, A.; Hansson, J.; Lundeberg, J. NRAS and BRAF mutations in melanoma tumours in relation to clinical characteristics: A study based on mutation screening by pyrosequencing. Melanoma Res. 2006, 16, 471–478. [Google Scholar] [CrossRef]
- Beadling, C.; Jacobson-Dunlop, E.; Hodi, F.S.; Le, C.; Warrick, A.; Patterson, J.; Town, A.; Harlow, A.; Cruz, F.; Azar, S.; et al. KIT gene mutations and copy number in melanoma subtypes. Clin. Cancer Res. 2008, 14, 6821–6828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Violanti, S.S.; Bononi, I.; Gallenga, C.E.; Martini, F.; Tognon, M.; Perri, P. New insights into molecular oncogenesis and therapy of uveal melanoma. Cancers 2019, 11, 694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitra, D.; Luo, X.; Morgan, A.; Wang, J.; Hoang, M.P.; Lo, J.; Guerrero, C.R.; Lennerz, J.K.; Mihm, M.C.; Wargo, J.A.; et al. An ultraviolet-radiation-independent pathway to melanoma carcinogenesis in the red hair/fair skin background. Nature 2012, 491, 449–453. [Google Scholar] [CrossRef]
- Fargnoli, M.C.; Gandini, S.; Peris, K.; Maisonneuve, P.; Raimondi, S. MC1R variants increase melanoma risk in families with CDKN2A mutations: A meta-analysis. Eur. J. Cancer 2010, 46, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Holman, D.M.; Ragan, K.R.; Julian, A.K.; Perna, F.M. The Context of Sunburn Among U.S. Adults: Common Activities and Sun Protection Behaviors. Am. J. Prev. Med. 2021, 60, e213–e220. [Google Scholar] [CrossRef]
- Lazovich, D.A.; Vogel, R.I.; Berwick, M.; Weinstock, M.A.; Anderson, K.E.; Warshaw, E.M. Indoor tanning and risk of melanoma: A case-control study in a highly exposed population. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2685. [Google Scholar] [CrossRef] [Green Version]
- Guy, G.P.J.; Berkowitz, Z.; Everett Jones, S.; Watson, M.; Richardson, L.C. Prevalence of Indoor Tanning and Association With Sunburn Among Youth in the United States. JAMA Dermatol. 2017, 153, 387–390. [Google Scholar] [CrossRef]
- Gordon, L.G.; Rodriguez-Acevedo, A.J.; Køster, B.; Guy, G.P., Jr.; Sinclair, C.; Van Deventer, E.; Green, A.C. Association of Indoor Tanning Regulations With Health and Economic Outcomes in North America and Europe. JAMA Dermatol. 2020, 156, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Moodycliffe, A.M.; Nghiem, D.; Clydesdale, G.; Ullrich, S.E. Immune suppression and skin cancer development: Regulation by NKT cells. Nat. Immunol. 2000, 1, 521–525. [Google Scholar] [CrossRef] [PubMed]
- González Maglio, D.H.; Paz, M.L.; Leoni, J. Sunlight Effects on Immune System: Is There Something Else in addition to UV-Induced Immunosuppression? Biomed. Res. Int. 2016, 2016, 1934518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halliday, G.M.; Lyons, J.G. Inflammatory doses of UV may not be necessary for skin carcinogenesis. Photochem. Photobiol. 2008, 84, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, T. 25 Years of UV-induced immunosuppression mediated by T Cells-From disregarded T suppressor cells to highly respected regulatory T cells. Photochem. Photobiol. 2008, 84, 10–18. [Google Scholar] [CrossRef]
- Alexander, W. The checkpoint immunotherapy revolution: What started as a trickle has become a flood, despite some daunting adverse effects, new drugs, indications, and combinations continue to emerge. P. T. 2016, 41, 185–191. [Google Scholar]
- Schank, T.E.; Hassel, J.C. Immunotherapies for the treatment of uveal melanoma—history and future. Cancers 2019, 11, 1048. [Google Scholar] [CrossRef] [Green Version]
- Gandini, S.; Sera, F.; Cattaruzza, M.S.; Pasquini, P.; Abeni, D.; Boyle, P.; Melchi, C.F. Meta-analysis of risk factors for cutaneous melanoma: I. Common and atypical naevi. Eur. J. Cancer 2005, 41, 28–44. [Google Scholar] [CrossRef] [Green Version]
- Tsao, H.; Bevona, C.; Goggins, W.; Quinn, T. The transformation rate of moles (melanocytic nevi) into cutaneous melanoma: A population-based estimate. Arch. Dermatol. 2003, 139, 282–288. [Google Scholar] [CrossRef] [Green Version]
- Terushkin, V.; Ng, E.; Stein, J.A.; Katz, S.; Cohen, D.E.; Meehan, S.; Polsky, D. A prospective study evaluating the utility of a 2-mm biopsy margin for complete removal of histologically atypical (dysplastic) nevi. J. Am. Acad. Dermatol. 2017, 77, 1096–1099. [Google Scholar] [CrossRef]
- Berwick, M.; Erdei, E.; Hay, J. Melanoma Epidemiology and Public Health. Dermatol. Clin. 2009, 27, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Hara, K.; Nitta, Y.; Ikeya, T. Dysplastic nevus syndrome among Japanese: A case study and review of the Japanese literature. Am. J. Derm. 1992, 14, 24–31. [Google Scholar] [CrossRef]
- Greene, M.H.; Tucker, M.A.; Clark, W.H.; Kraemer, K.H.; Elder, D.E.; Fraser, M.C. Hereditary melanoma and the dysplastic nevus syndrome: The risk of cancers other than melanoma. J. Am. Acad. Derm. 1987, 16, 792–797. [Google Scholar] [CrossRef]
- Silva, J.H.; de Sá, B.C.; de Ávila, A.L.R.; Landman, G.; Neto, J.P.D. Atypical mole syndrome and dysplastic nevi: Identification of populations at risk for developing melanoma-review article. Clinics 2011, 66, 493–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paštar, Z.; Lipozenčić, J.; Kovačević, S.; Ćanović, S.; Didović-Torbarina, A.; Vukasović, A. Neurofibromatosis type 1 associated with dysplastic nevus syndrome. Acta Derm. Croat. 2009, 17, 118–122. [Google Scholar]
- Van Rooij, N.; Adams, A.; De’Ambrosis, B.; Nathan, V.; Hayward, N.; Whiteman, D. Cluster of pregnancy-associated melanoma: A case report and brief update. J. Dermatol. 2020, 47, 1054–1057. [Google Scholar] [CrossRef]
- Moustafa, D.; Blundell, A.R.; Hawryluk, E.B. Congenital melanocytic nevi. Curr. Opin. Pediatr. 2020, 32, 491–497. [Google Scholar] [CrossRef]
- Smith, L.K.; Arabi, S.; Lelliott, E.J.; McArthur, G.A.; Sheppard, K.E. Obesity and the impact on cutaneous melanoma: Friend or foe? Cancers 2020, 12, 1583. [Google Scholar] [CrossRef]
- De Pergola, G.; Silvestris, F. Obesity as a major risk factor for cancer. J. Obes. 2013, 2013, 291546. [Google Scholar] [CrossRef] [Green Version]
- Islami, F.; Goding Sauer, A.; Miller, K.D.; Siegel, R.L.; Fedewa, S.A.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Ma, J.; Soerjomataram, I.; et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA. Cancer J. Clin. 2018, 68, 31–54. [Google Scholar] [CrossRef]
- Green, A.C.; Williams, G.M.; Logan, V.; Strutton, G.M. Reduced melanoma after regular sunscreen use: Randomized trial follow-up. J. Clin. Oncol. 2011, 29, 257–263. [Google Scholar] [CrossRef] [Green Version]
- Choose the Right Sunscreen. Available online: https://www.cancer.org/latest-news/choose-the-right-sunscreen.html (accessed on 29 May 2021).
- Dobbinson, S.J.; Wakefield, M.A.; Jamsen, K.M.; Herd, N.L.; Spittal, M.J.; Lipscomb, J.E.; Hill, D.J. Weekend Sun Protection and Sunburn in Australia. Trends (1987–2002) and Association with SunSmart Television Advertising. Am. J. Prev. Med. 2008, 34, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Giles-Corti, B.; English, D.R.; Costa, C.; Milne, E.; Cross, D.; Johnston, R. Creating SunSmart schools. Health Educ. Res. 2004, 19, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Kyle, J.W.; Hammitt, J.K.; Lim, H.W.; Geller, A.C.; Hall-Jordan, L.H.; Maibach, E.W.; De Fabo, E.C.; Wagner, M.C. Economic evaluation of the US environmental protection agency’s sunwise program: Sun protection education for young children. Pediatrics 2008, 121, e1074–e1084. [Google Scholar] [CrossRef]
- Bibbins-Domingo, K.; Grossman, D.C.; Curry, S.J.; Davidson, K.W.; Ebell, M.; Epling, J.W.; García, F.A.R.; Gillman, M.W.; Kemper, A.R.; Krist, A.H.; et al. Screening for skin cancer US preventive services task force recommendation statement. JAMA-J. Am. Med. Assoc. 2016, 22, 652–665. [Google Scholar]
- Brunssen, A.; Waldmann, A.; Eisemann, N.; Katalinic, A. Impact of skin cancer screening and secondary prevention campaigns on skin cancer incidence and mortality: A systematic review. J. Am. Acad. Dermatol. 2017, 76, 129–139. [Google Scholar] [CrossRef]
- Hoorens, I.; Vossaert, K.; Pil, L.; Boone, B.; De Schepper, S.; Ongenae, K.; Annemans, L.; Chevolet, I.; Brochez, L. Total-body examination vs lesion-directed skin cancer screening. JAMA Dermatol. 2016, 152, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, M.A.; Risica, P.M.; Martin, R.A.; Rakowski, W.; Smith, K.J.; Berwick, M.; Goldstein, M.G.; Upegui, D.; Lasater, T. Reliability of assessment and circumstances of performance of thorough skin self-examination for the early detection of melanoma in the Check-It-Out Project. Prev. Med. (Baltim) 2004, 38, 761–765. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Barsouk, A. Epidemiology of Melanoma. Med. Sci. 2021, 9, 63. https://doi.org/10.3390/medsci9040063
Saginala K, Barsouk A, Aluru JS, Rawla P, Barsouk A. Epidemiology of Melanoma. Medical Sciences. 2021; 9(4):63. https://doi.org/10.3390/medsci9040063
Chicago/Turabian StyleSaginala, Kalyan, Adam Barsouk, John Sukumar Aluru, Prashanth Rawla, and Alexander Barsouk. 2021. "Epidemiology of Melanoma" Medical Sciences 9, no. 4: 63. https://doi.org/10.3390/medsci9040063
APA StyleSaginala, K., Barsouk, A., Aluru, J. S., Rawla, P., & Barsouk, A. (2021). Epidemiology of Melanoma. Medical Sciences, 9(4), 63. https://doi.org/10.3390/medsci9040063





