Wound Healing and Therapy in Soft Tissue Defects of the Hand and Foot from a Surgical Point of View
Abstract
:1. Introduction
2. Pathways of Wound Healing
2.1. Healing by Primary Intention
2.2. Healing by Secondary Intention
2.3. Healing by Tertiary Intention
3. Anatomical Characteristics of Soft Tissue on the Hand and Foot
4. Wound Treatment of Hand and Foot Defects
4.1. Conservative Wound Management
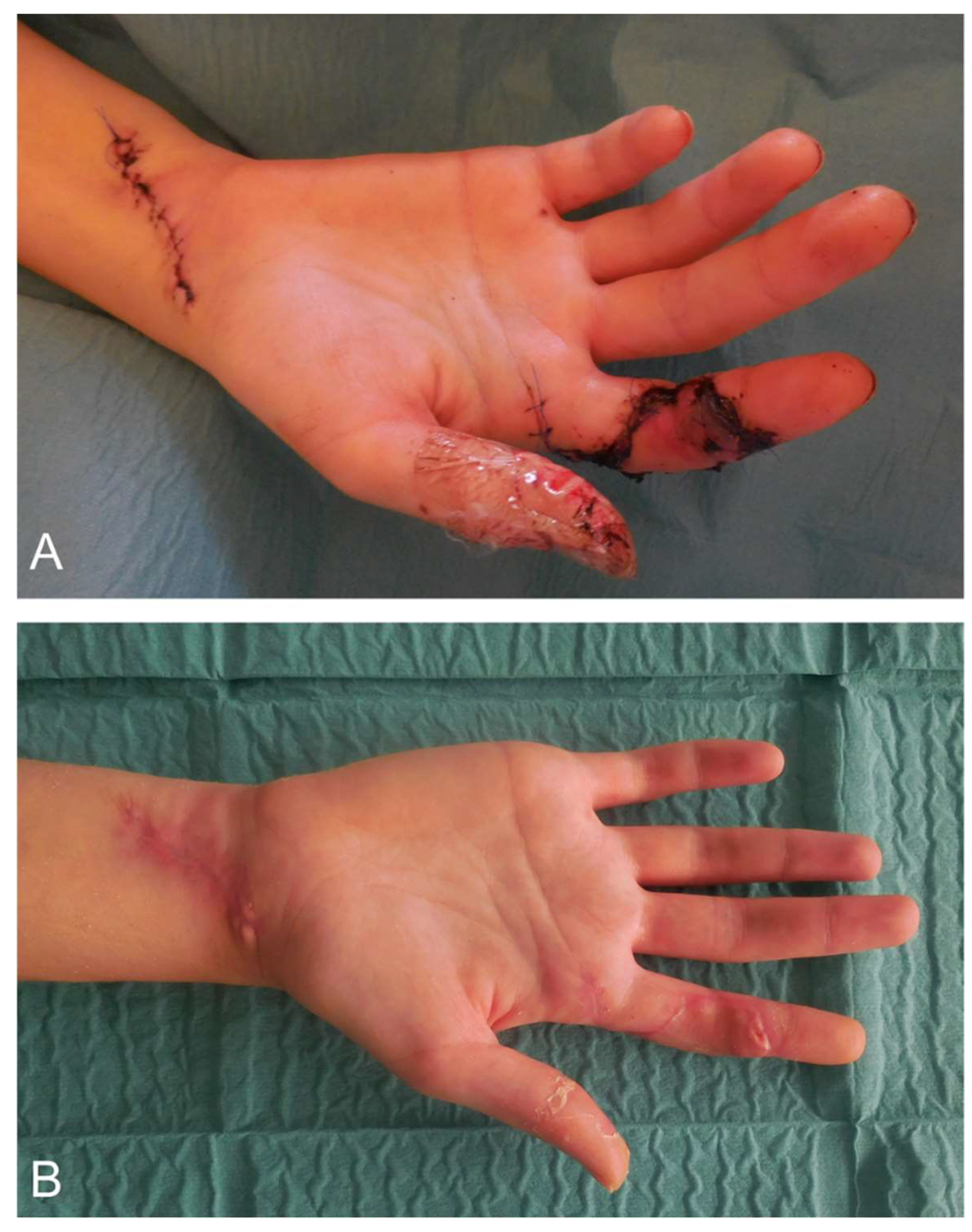
4.2. Surgical Wound Management of the Hand
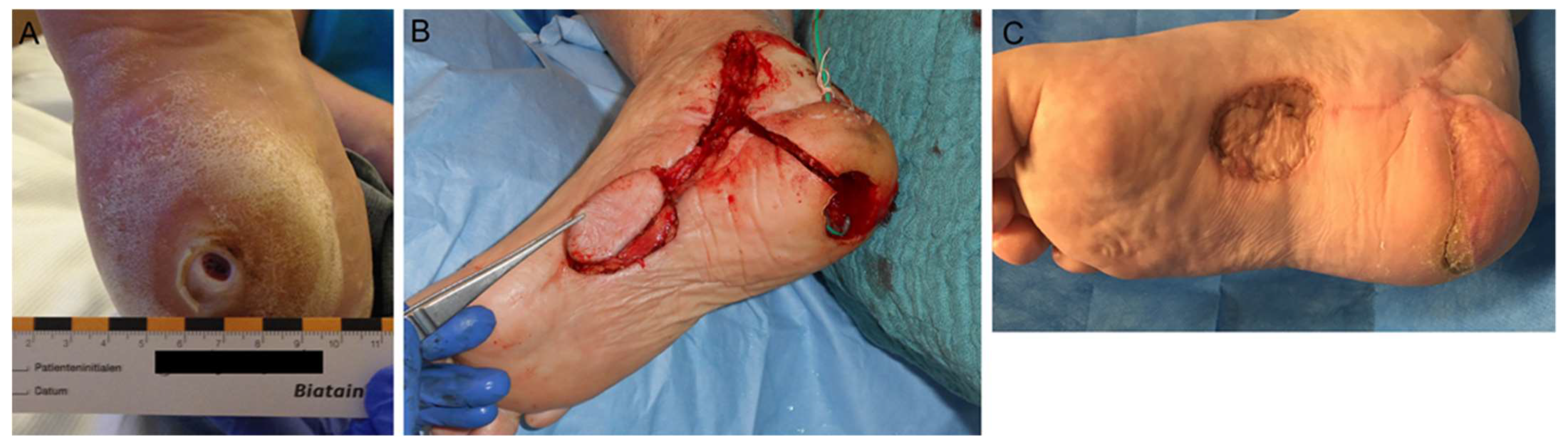
4.3. Surgical Wound Management of the Foot
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barron, J.N. The structure and function of the skin of the hand. Hand 1970, 2, 93–96. [Google Scholar] [CrossRef]
- Homann, H.H.; Tilkorn, D.; Hauser, J.; Lehnhardt, M. Behandlungsoptionen bei zusätzlichem Weichteilschaden. Trauma Berufskrankh. 2007, 9, 308–314. [Google Scholar] [CrossRef]
- Löfstrand, J.G.; Lin, C.H. Reconstruction of defects in the weight-bearing plantar area using the innervated free medial plantar (Instep) Flap. Ann. Plast. Surg. 2018, 80, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Viseux, F.J.F. The sensory role of the sole of the foot: Review and update on clinical perspectives. Neurophysiol. Clin. 2020, 50, 55–68. [Google Scholar] [CrossRef]
- Farahani, M.; Shafiee, A. Wound healing: From passive to smart dressings. Adv. Healthc. Mater. 2021, 10, e2100477. [Google Scholar] [CrossRef]
- Trybus, M.; Lorkowski, J.; Brongel, L.; Hladki, W. Causes and consequences of hand injuries. Am. J. Surg. 2006, 192, 52–57. [Google Scholar] [CrossRef]
- Orsted, H.L.; Keast, D.; Forest-Lalande, L.; Mégie, M.F. Basic principles of wound healing. Wound Care Can. 2015, 9, 4–12. [Google Scholar]
- Molnar, J.A.; Vlad, L.G.; Gumus, T. Nutrition and chronic wounds: Improving clinical outcomes. Plast. Reconstr. Surg. 2016, 138, 71S–81S. [Google Scholar] [CrossRef]
- Andrews, K.L.; Houdek, M.T.; Kiemele, L.J. Wound management of chronic diabetic foot ulcers: From the basics to regenerative medicine. Prosthet. Orthot. Int. 2015, 39, 29–39. [Google Scholar] [CrossRef]
- Fuchs, E.; Blau, H.M. Tissue stem cells: Architects of their niches. Cell Stem Cell 2020, 27, 532–556. [Google Scholar] [CrossRef]
- Krafts, K.P. Tissue repair: The hidden drama. Organogenesis 2010, 6, 225–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinke, J.M.; Sorg, H. Wound repair and regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in wound repair: Molecular and cellular mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef]
- Tziotzios, C.; Profyris, C.; Sterling, J. Cutaneous scarring: Pathophysiology, molecular mechanisms, and scar reduction therapeutics Part II. Strategies to reduce scar formation after dermatologic procedures. J. Am. Acad. Dermatol. 2012, 66, 13–24. [Google Scholar] [CrossRef]
- Profyris, C.; Tziotzios, C.; Do Vale, I. Cutaneous scarring: Pathophysiology, molecular mechanisms, and scar reduction therapeutics Part, I. The molecular basis of scar formation. J. Am. Acad. Dermatol. 2012, 66, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Koh, T.J.; Di Pietro, L.A. Inflammation and wound healing: The role of the macrophage. Expert. Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef] [Green Version]
- Martin, P. Wound healing—Aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef]
- Lau, K.; Paus, R.; Tiede, S.; Day, P.; Bayat, A. Exploring the role of stem cells in cutaneous wound healing. Exp. Dermatol. 2009, 18, 921–933. [Google Scholar] [CrossRef]
- Miller, S.J.; Burke, E.M.; Rader, M.D.; Coulombe, P.A.; Lavker, R.M. Re-epithelialization of porcine skin by the sweat apparatus. J. Investig. Dermatol. 1998, 110, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Roh, C.; Lyle, S. Cutaneous stem cells and wound healing. Pediatr. Res. 2006, 59, 100R–103R. [Google Scholar] [CrossRef] [Green Version]
- Bauer, S.M.; Bauer, R.J.; Velazquez, O.C. Angiogenesis, vasculogenesis, and induction of healing in chronic wounds. Vasc. Endovascular. Surg. 2005, 39, 293–306. [Google Scholar] [CrossRef]
- Arnold, F.; West, D.C. Angiogenesis in wound healing. Pharmacol. Ther. 1991, 52, 407–422. [Google Scholar] [CrossRef]
- Madden, J.W.; Peacock, E.E., Jr. Studies on the biology of collagen during wound healing. 3. Dynamic metabolism of scar collagen and remodeling of dermal wounds. Ann. Surg. 1971, 174, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.C.; Steed, D.L.; Franz, M.G. Wound healing: Biologic features and approaches to maximize healing trajectories. Curr. Probl. Surg. 2001, 38, 72–140. [Google Scholar] [CrossRef]
- Strodtbeck, F. Physiology of wound healing. Newborn Infant. Nurs. Rev. 2001, 1, 43–52. [Google Scholar] [CrossRef]
- Nauta, A.; Gurtner, G.; Longaker, M.T. Wound healing and regenerative strategies. Oral. Dis. 2011, 17, 541–549. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, D.G. The role of apoptosis in wound healing. Int. J. Biochem. Cell Biol. 1998, 30, 1019–1030. [Google Scholar] [CrossRef]
- Childs, D.R.; Murthy, A.S. Overview of wound healing and management. Surg. Clin. N. Am. 2017, 97, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Siewert, R.J. Basiswissen Chirurgie; Springer: Heildeberg, Germany, 2007; 30–31p. [Google Scholar]
- Peterson, A.S.; Aument, W.L. The “Golden period” for wound repair. J. Lanc. Gen. Hosp. 2010, 5, 134–135. [Google Scholar]
- Chhabra, S.; Chhabra, N.; Kaur, A.; Gupta, N. Wound healing concepts in clinical practice of OMFS. J. Maxillofac. Oral. Surg. 2017, 16, 403–423. [Google Scholar] [CrossRef]
- Rudigier, J.; Meier, R. Kurzgefasste Handchirurgie Klinik und Praxis, 6th ed.; Thieme: Stuttgart, Germany, 2014; 52p. [Google Scholar]
- Schmidt, H.M.; Lanz, U. Surgical Anatomy of the Hand; Thieme: Stuttgart, Germany, 2004; 19–20p. [Google Scholar]
- Zhang, P.; Lu, J.; Jing, Y.; Tang, S.; Zhu, D.; Bi, Y. Global epidemiology of diabetic foot ulceration: A systematic review and meta-analysis. Ann. Med. 2017, 49, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.Y.; Roh, S.G.; Sharaf, B.; Lee, N.H. Risk of major limb amputation in diabetic foot ulcer and accompanying disease: A meta-analysis. J. Plast. Reconstr. Aesthet. Surg. 2017, 70, 1681–1688. [Google Scholar] [CrossRef] [PubMed]
- Apelqvist, J. Diagnostics and treatment of the diabetic foot. Endocrine 2012, 41, 384–397. [Google Scholar] [CrossRef]
- Prompers, L.; Schaper, N.; Apelqvist, J.; Edmonds, M.; Jude, E.; Mauricio, D.; Uccioli, L.; Urbancic, V.; Bakker, K.; Holstein, P.; et al. Prediction of outcome in individuals with diabetic foot ulcers: Focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE Study. Diabetologia 2008, 51, 747–755. [Google Scholar] [CrossRef] [Green Version]
- Gershater, M.A.; Löndahl, M.; Nyberg, P.; Larsson, J.; Thörne, J.; Eneroth, M.; Apelqvist, J. Complexity of factors related to outcome of neuropathic and neuroischaemic/ischaemic diabetic foot ulcers: A cohort study. Diabetologia 2009, 52, 398–407. [Google Scholar] [CrossRef] [Green Version]
- Apelqvist, J.; Bakker, K.; van Houtum, W.H.; Schaper, N.C. International Working Group on the Diabetic Foot (IWGDF) Editorial Board. The development of global consensus guidelines on the management of the diabetic foot. Diabetes Metab. Res. Rev. 2008, 24, S116–S118. [Google Scholar] [CrossRef]
- Lipsky, B.A. Evidence-based antibiotic therapy of diabetic foot infections. FEMS Immunol. Med. Microbiol. 1999, 26, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Demirdal, T.; Emir, B. Meta-analysis of risk factors for amputation in diabetic foot infections. Diabetes Metab. Res. Rev. 2019, 35, e3165. [Google Scholar] [CrossRef]
- Coulombe, P.A. Wound epithelialization: Accelerating the pace of discovery. J. Investig. Dermatol. 2003, 121, 219–230. [Google Scholar] [CrossRef] [Green Version]
- Sweeney, I.R.; Miraftab, M.; Collyer, G. A critical review of modern and emerging absorbent dressings used to treat exuding wounds. Int. Wound J. 2012, 9, 601–612. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Purna, S.K.; Babu, M. Collagen based dressings—A review. Burns 2000, 26, 54–62. [Google Scholar]
- Queen, D.; Orsted, H.; Sanada, H.; Sussman, G. A dressing history. Int. Wound J. 2004, 1, 59–77. [Google Scholar] [CrossRef] [PubMed]
- Falabella, A.F. Debridement and wound bed preparation. Dermatol. Ther. 2006, 19, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, M.; Rahimi, R.; Zhou, J.; Jiang, H.; Yoon, C.K.; Maddipatla, D.; Narakathu, B.B.; Jain, V.; Oscai, M.M.; Morken, T.J.; et al. Integrated sensing and delivery of oxygen for next-generation smart wound dressings. Microsyst. Nanoeng. 2020, 6, 46. [Google Scholar] [CrossRef]
- Rogina-Car, B.; Rogina, J.; Govorčin Bajsić, E.; Budimid, A. Propolis–Eco-friendly natural antibacterial finish for nonwoven fabrics for medical application. J. Ind. Text. 2020, 49, 1100–1119. [Google Scholar] [CrossRef]
- D’Alessandro, S.; Magnavacca, A.; Perego, F.; Fumagalli, M.; Sangiovanni, E.; Prato, M.; Dell’Agli, M.; Basilico, N. Effect of hypoxia on gene expression in cell populations involved in wound healing. Biomed Res. Int. 2019, 2019, 2626374. [Google Scholar] [CrossRef] [Green Version]
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K.; Kahveci, M.U. Alginate: Current use and future perspectives in pharmaceutical and biomedical applications. Int. J. Polym. Sci. 2016, 2016, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Thomas, S. Alginate dressings in surgery and wound management—Part 1. J. Wound Care 2000, 9, 56–60. [Google Scholar] [CrossRef]
- Hoigne, D.; Hug, U. Amputationsverletzungen am Fingerendglied: Regeneration mittels folienverband. Schweiz Med. Forum 2014, 14, 356–360. [Google Scholar] [CrossRef] [Green Version]
- Moshakis, V.; Fordyce, M.J.; Griffiths, J.D.; McKinna, J.A. Tegadern versus gauze dressing in breast surgery. Br. J. Clin. Pract. 1984, 38, 149–152. [Google Scholar] [PubMed]
- Zehrer, C.L.; Holm, D.; Solfest, S.E.; Walters, S.A. A comparison of the in vitro moisture vapour transmission rate and in vivo fluid-handling capacity of six adhesive foam dressings to a newly reformulated adhesive foam dressing. Int. Wound J. 2014, 11, 681–690. [Google Scholar] [CrossRef]
- Leong, S.; Lo, Z.J. Use of disposable negative pressure wound therapy on split-thickness skin graft recipient sites for peripheral arterial disease foot wounds: A case report. Int. Wound J. 2020, 17, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Leavitt, T.; Bayer, L.R.; Orgill, D.P. Effect of negative pressure wound therapy on wound healing. Curr. Probl. Surg. 2014, 51, 301–331. [Google Scholar] [CrossRef] [Green Version]
- Bartlett, R.H. Skin substitutes. J. Trauma Inj. Infect. Crit. Care 1981, 21, 731. [Google Scholar] [CrossRef]
- Ramshaw, J.A.; Werkmeister, J.A.; Glattauer, V. Collagen-based biomaterials. Biotechnol. Genet. Eng. Rev. 1996, 13, 335–382. [Google Scholar] [CrossRef] [Green Version]
- Doillon, C.J.; Silver, F.H. Collagen-based wound dressing: Effects of hyaluronic acid and fibronectin on wound healing. Biomaterials 1986, 7, 3–8. [Google Scholar] [CrossRef]
- Ishihara, M.; Nakanishi, K.; Ono, K.; Sato, M.; Kikuchi, M.; Saito, Y.; Yura, H.; Matsui, T.; Hattori, H.; Uenoyama, M.; et al. Photocrosslinkable chitosan as a dressing for wound occlusion and accelerator in healing process. Biomaterials 2002, 23, 833–840. [Google Scholar] [CrossRef]
- Khalil, H.; Cullen, M.; Chambers, H.; Carroll, M.; Walker, J. Elements affecting wound healing time: An evidence based analysis. Wound Repair. Regen. 2015, 23, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, D.; Dey, N.; Bhardwaj, N.; Mandal, B.B. Emerging and innovative approaches for wound healing and skin regeneration: Current status and advances. Biomaterials 2019, 216, 119267. [Google Scholar] [CrossRef]
- Afsharian, Y.P.; Rahimnejad, M. Bioactive electrospun scaffolds for wound healing applications: A comprehensive review. Polym. Test. 2020, 93, 106952. [Google Scholar] [CrossRef]
- Stoica, A.E.; Chircov, C.; Grumezescu, A.M. Nanomaterials for wound dressings: An up-to-date overview. Molecules 2020, 10, 2699. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Li, P.Y.; Cheng, H. Smart materials or tissue engeneering: Applications. R. Soc. Chem. 2017, 2017, 258–289. [Google Scholar]
- Wilhelm, K.; Putz, R.; Hierner, R.; Giunta, R.E. Lappenplastiken in der Handchirurgie; Urban & Schwarzenberg: München, Germany, 1997; 82–83p. [Google Scholar]
- Foucher, G.; Braun, J.B. A new island flap transfer from the dorsum of the index to the thumb. Plast. Reconstr. Surg. 1979, 63, 344–349. [Google Scholar] [CrossRef]
- Homann, H.H.; Tilkorn, D.; Goertz, O.; Lehnhardt, M. Die Behandlung des Weichteilschadens an der Hand. Trauma Berufskrankh. 2007, 9, 220–224. [Google Scholar] [CrossRef]
- Sauerbier, M.; Arsalan-Werner, A.; Neubrech, F. Retrograd gestielte Lappenplastiken der dorsalen Metakarpalarterien (DMCA) [The distally based dorsal metacarpal artery flap (DMCA)]. Oper. Orthop. Traumatol. 2020, 32, 501–508. [Google Scholar] [CrossRef]
- Yang, G.F.; Chen, P.J.; Gao, Y.Z.; Liu, X.Y.; Li, J.; Jiang, S.X.; He, S.P. Forearm free skin flap transplantation: A report of 56 cases. 1981. Br. J. Plast. Surg. 1997, 50, 162–165. [Google Scholar] [CrossRef]
- McGregor, I.A.; Jackson, I.T. The groin flap. Br. J. Plast. Surg. 1972, 25, 3–16. [Google Scholar] [CrossRef]
- Song, Y.G.; Chen, G.Z.; Song, Y.L. The free thigh flap: A new free flap concept based on the septocutaneous artery. Br. J. Plast Surg. 1984, 37, 149–159. [Google Scholar] [CrossRef]
- Yoshimura, M.; Shimada, T.; Imura, S.; Shimamura, K.; Yamauchi, S. The venous skin graft method for repairing skin defects of the fingers. Plast. Reconstr. Surg. 1987, 79, 243–250. [Google Scholar] [CrossRef]
- Grey, J.E.; Harding, K. Ärztliche Wundversorgung; Elsevier: München, Germany, 2008; 92–93p. [Google Scholar]
- Akbari, A.; Moodi, H.; Ghiasi, F.; Sagheb, H.M.; Rashidi, H. Effects of vacuum-compression therapy on healing of diabetic foot ulcers: Randomized controlled trial. J. Rehabil. Res. Dev. 2007, 44, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Küntscher, M.V.; Erdmann, D.; Homann, H.H.; Steinau, H.U.; Levin, S.L.; Germann, G. The concept of fillet flaps: Classification, indications, and analysis of their clinical value. Plast. Reconstr. Surg. 2001, 108, 885–896. [Google Scholar] [CrossRef]
- Ducic, I.; Hung, V.; Dellon, A.L. Innervated free flaps for foot reconstruction: A review. J. Reconstr. Microsurg. 2006, 22, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.P.; Kim, E.K. Sole reconstruction using anterolateral thigh perforator free flaps. Plast. Reconstr. Surg. 2007, 119, 186–193. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demmer, W.; Sorg, H.; Steiert, A.; Hauser, J.; Tilkorn, D.J. Wound Healing and Therapy in Soft Tissue Defects of the Hand and Foot from a Surgical Point of View. Med. Sci. 2021, 9, 71. https://doi.org/10.3390/medsci9040071
Demmer W, Sorg H, Steiert A, Hauser J, Tilkorn DJ. Wound Healing and Therapy in Soft Tissue Defects of the Hand and Foot from a Surgical Point of View. Medical Sciences. 2021; 9(4):71. https://doi.org/10.3390/medsci9040071
Chicago/Turabian StyleDemmer, Wolfram, Heiko Sorg, Andreas Steiert, Jörg Hauser, and Daniel Johannes Tilkorn. 2021. "Wound Healing and Therapy in Soft Tissue Defects of the Hand and Foot from a Surgical Point of View" Medical Sciences 9, no. 4: 71. https://doi.org/10.3390/medsci9040071
APA StyleDemmer, W., Sorg, H., Steiert, A., Hauser, J., & Tilkorn, D. J. (2021). Wound Healing and Therapy in Soft Tissue Defects of the Hand and Foot from a Surgical Point of View. Medical Sciences, 9(4), 71. https://doi.org/10.3390/medsci9040071





