The Moderating Role of Cortisol and Negative Emotionality in the Effects of Classroom Size and Window View on Young Children’s Executive Functions
Abstract
:1. Introduction
1.1. Influence of Nature View and Classroom Size on Individuals’ Cognitions
1.2. Cortisol and Negative Emotionality as a Moderator in the Context of Differential Susceptibility
1.3. Research Questions and Hypotheses
2. Methods
2.1. Participants
2.2. Procedures
2.2.1. Design of VR Classrooms
2.2.2. Experimental Procedures
2.3. Measures
2.3.1. Socio-Demographic Information
2.3.2. Negative Emotionality
2.3.3. Executive Functions
2.3.4. Cortisol
2.4. Analyses
3. Results
3.1. Correlational Analysis among Main Study Variables
3.2. Moderating Effects of Children’s Baseline Cortisol
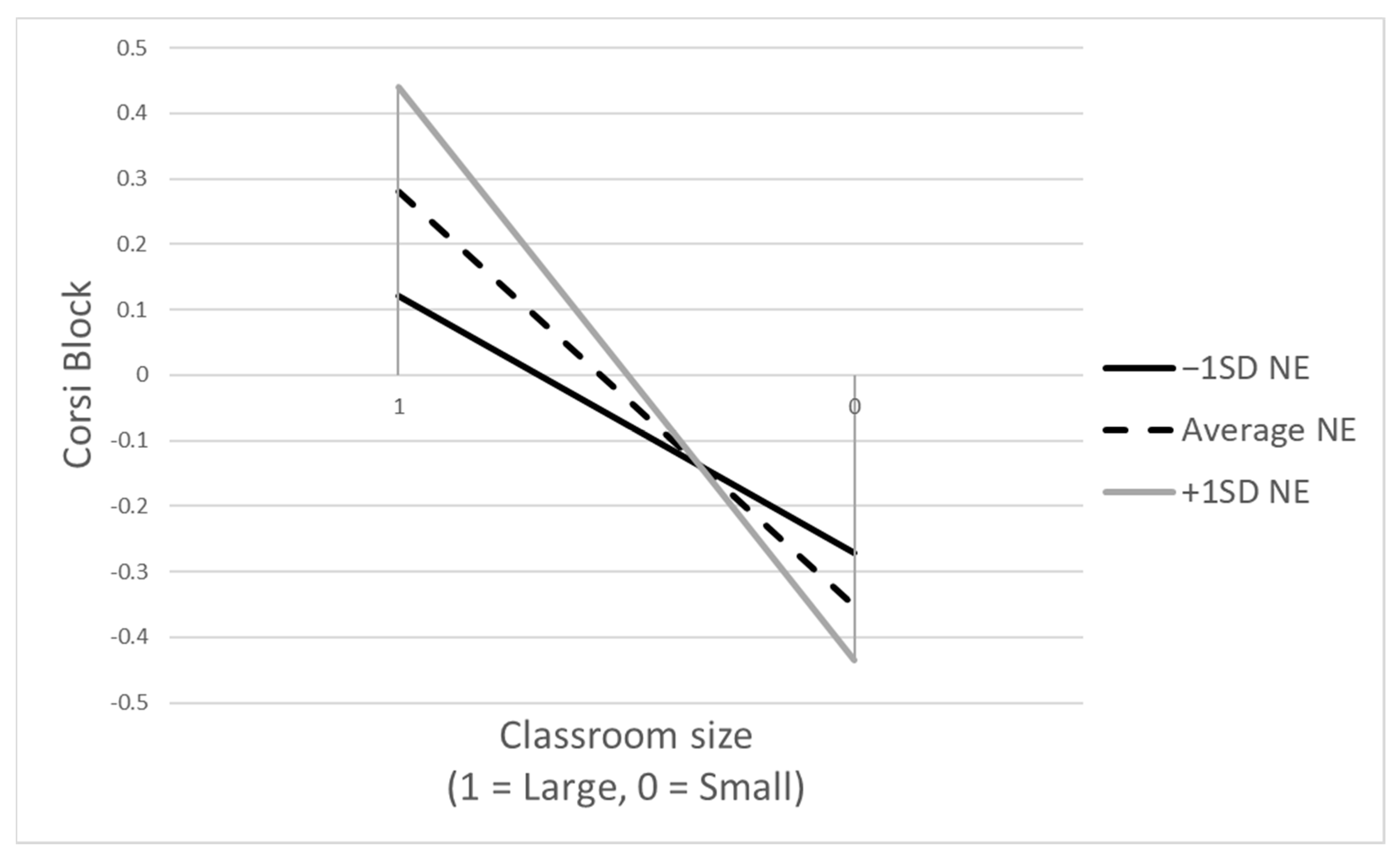
3.3. Moderating Effects of Children’s Negative Emotionality
4. Discussion
Limitations and Future Research
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ulrich, R.S. View through a window may influence recovery from surgery. Science 1984, 224, 420–421. [Google Scholar] [CrossRef] [PubMed]
- Walch, J.M.; Rabin, B.S.; Day, R.; Williams, J.N.; Choi, K.; Kang, J.D. The effect of sunlight on postoperative analgesic medication use: A prospective study of patients undergoing spinal surgery. Psychosom. Med. 2005, 67, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Aries, M.B.; Veitch, J.A.; Newsham, G.R. Windows, view, and office characteristics predict physical and psychological discomfort. J. Environ. Psychol. 2010, 30, 533–541. [Google Scholar] [CrossRef]
- Dravigne, A.; Waliczek, T.M.; Lineberger, R.D.; Zajicek, J.M. The effect of live plants and window views of green spaces on employee perceptions of job satisfaction. HortScience 2008, 43, 183–187. [Google Scholar] [CrossRef]
- AL-Ayash, A.; Kane, R.T.; Smith, D.; Green-Armytage, P. The influence of color on student emotion, heart rate, and performance in learning environments. Color Res. Appl. 2016, 41, 196–205. [Google Scholar] [CrossRef]
- Benfield, J.A.; Rainbolt, G.N.; Bell, P.A.; Donovan, G.H. Classrooms with nature views: Evidence of differing student perceptions and behaviors. Environ. Behav. 2015, 47, 140–157. [Google Scholar] [CrossRef]
- Cruz-Garza, J.G.; Darfler, M.; Rounds, J.D.; Gao, E.; Kalantari, S. EEG-based investigation of the impact of room size and window placement on cognitive performance. J. Build. Eng. 2022, 53, 104540. [Google Scholar] [CrossRef]
- Dadvand, P.; Nieuwenhuijsen, M.J.; Esnaola, M.; Forns, J.; Basagaña, X.; Alvarez-Pedrerol, M.; Rivas, I.; López-Vicente, M.; De Castro Pascual, M.; Su, J.; et al. Green spaces and cognitive development in primary schoolchildren. Proc. Natl. Acad. Sci. USA 2015, 112, 7937–7942. [Google Scholar] [CrossRef]
- Brunsek, A.; Perlman, M.; Falenchuk, O.; McMullen, E.; Fletcher, B.; Shah, P.S. The relationship between the Early Childhood Environment Rating Scale and its revised form and child outcomes: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0178512. [Google Scholar] [CrossRef]
- Read, M.A.; Sugawara, A.I.; Brandt, J.A. Impact of space and color in the physical environment on preschool children’s cooperative behavior. Environ. Behav. 1999, 31, 413–428. [Google Scholar] [CrossRef]
- Abbas, M.Y.; Othman, M.; Rahman, P.Z.M.A. Pre-school children’s play behaviour influenced by classroom’s spatial definitions? Asian J. Environ.-Behav. Stud. 2016, 1, 49–65. [Google Scholar] [CrossRef]
- Belsky, J.; Pluess, M. The nature (and nurture?) of plasticity in early human development. Perspect. Psychol. Sci. 2009, 4, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Greven, C.U.; Lionetti, F.; Booth, C.; Aron, E.N.; Fox, E.; Schendan, H.E.; Pluess, M.; Bruining, H.; Acevedo, B.; Bijttebier, P.; et al. Sensory Processing Sensitivity in the context of Environmental Sensitivity: A critical review and development of research agenda. Neurosci. Biobehav. Rev. 2019, 98, 287–305. [Google Scholar] [CrossRef] [PubMed]
- Berto, R. Exposure to restorative environments helps restore attentional capacity. J. Environ. Psychol. 2005, 25, 249–259. [Google Scholar] [CrossRef]
- Bratman, G.N.; Hamilton, J.P.; Daily, G.C. The impacts of nature experience on human cognitive function and mental health. Ann. N. Y. Acad. Sci. USA 2012, 1249, 118–136. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, S. The restorative benefits of nature: Toward an integrative framework. J. Environ. Psychol. 1995, 15, 169–182. [Google Scholar] [CrossRef]
- Li, D.; Sullivan, W.C. Impact of views to school landscapes on recovery from stress and mental fatigue. Landsc. Urban Plan. 2016, 148, 149–158. [Google Scholar] [CrossRef]
- Palanica, A.; Lyons, A.; Cooper, M.; Lee, A.; Fossat, Y. A comparison of nature and urban environments on creative thinking across different levels of reality. J. Environ. Psychol. 2019, 63, 44–51. [Google Scholar] [CrossRef]
- Kaplan, R.; Kaplan, S. The Experience of Nature: A Psychological Perspective; Cambridge University Press: New York, NY, USA, 1989. [Google Scholar]
- Lindemann-Matthies, P.; Benkowitz, D.; Hellinger, F. Associations between the naturalness of window and interior classroom views, subjective well-being of primary school children and their performance in an attention and concentration test. Landsc. Urban Plan. 2021, 214, 104146. [Google Scholar] [CrossRef]
- Daly, J.; Burchett, M.; Torpy, F. Plants in the Classroom Can Improve Student Performance; National Interior Plantscape Association: Kippa-Ring, QLD, Australia, 2010; pp. 1–9. [Google Scholar]
- van den Berg, A.E.; Wesselius, J.E.; Maas, J.; Tanja-Dijkstra, K. Green walls for a restorative classroom environment: A controlled evaluation study. Environ. Behav. 2017, 49, 791–813. [Google Scholar] [CrossRef]
- Zhou, Z.; Ergan, S. Where do we look? An eye-tracking study of architectural features in building design. In Advances in Informatics and Computing in Civil and Construction Engineering: Proceedings of the 35th CIB W78 2018 Conference: IT in Design, Construction, and Management; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Yin, J.; Zhu, S.; MacNaugton, P.; Allen, J.G.; Spengler, J.D. Physiological and cognitive performance of exposure to biophilic indoor environment. Build. Environ. 2018, 132, 255–262. [Google Scholar] [CrossRef]
- Vartanian, O.; Navarrete, G.; Chatterjee, A.; Rich, L.B.; Gonzalez-Mora, J.L.; Leder, H.; Modroño, C.; Nadal, M.; Rostrup, N.; Skov, M. Architectural design and the brain: Effects of ceiling height and perceived enclosure on beauty judgments and approach-avoidance decisions. J. Environ. Psychol. 2015, 41, 10–18. [Google Scholar] [CrossRef]
- Li, J.; Jin, Y.; Lu, S.; Wu, W.; Wang, P. Building environment information and human perceptual feedback collected through a combined virtual reality (VR) and electroencephalogram (EEG) method. Energy Build. 2020, 224, 110259. [Google Scholar] [CrossRef]
- Ehrenberg, R.G.; Brewer, D.J.; Gamoran, A.; Willms, J.D. Class size and student achievement. Psychol. Sci. Public Interest 2001, 2, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Fowler, W.J.; Walberg, H.J. School size, characteristics, and outcomes. Educ. Eval. Policy Anal. 1991, 13, 189–202. [Google Scholar] [CrossRef]
- Maxwell, L.E. Home and school density effects on elementary school children: The role of spatial density. Environ. Behav. 2003, 35, 566–578. [Google Scholar] [CrossRef]
- Burgess, J.W.; Fordyce, W.K. Effects of preschool environments on nonverbal social behavior: Toddlers’ interpersonal distances to teachers and classmates change with environmental density, classroom design, and parent–child interactions. J. Child Psychol. Psychiatry 1989, 30, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Loo, C.M. The effects of spatial density on the social behavior of children 1. J. Appl. Soc. Psychol. 1972, 2, 372–381. [Google Scholar] [CrossRef]
- Smith, P.K.; Connolly, K.J. The Ecology of Preschool Behavior; Cambridge University Press: Cambridge, UK, 1980. [Google Scholar]
- Belsky, J.; Bakermans-Kranenburg, M.J.; Van IJzendoorn, M.H. For better and for worse: Differential susceptibility to environmental influences. Curr. Dir. Psychol. Sci. 2007, 16, 300–304. [Google Scholar] [CrossRef]
- van IJzendoorn, M.H.; Bakermans-Kranenburg, M.J. Integrating temperament and attachment: The differential susceptibility paradigm. In Handbook of Temperament; Zentner, M., Shiner, R.L., Eds.; Guilford Press: New York, NY, USA, 2012; pp. 403–424. [Google Scholar]
- Ellis, B.J.; Boyce, W.T.; Belsky, J.; Bakermans-Kranenburg, M.J.; Van IJzendoorn, M.H. Differential susceptibility to the environment: An evolutionary-neurodevelopmental theory. Dev. Psychopathol. 2011, 23, 7–28. [Google Scholar] [CrossRef]
- Barrios, C.S.; Bufferd, S.J.; Klein, D.N.; Dougherty, L.R. The interaction between parenting and children’s cortisol reactivity at age 3 predicts increases in children’s internalizing and externalizing symptoms at age 6. Dev. Psychopathol. 2017, 29, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Kalomiris, A.E.; Phelps, R.A.; Kiel, E.J. The relation between specific parenting behaviors and toddlers’ early anxious behaviors is moderated by toddler cortisol reactivity. J. Abnorm. Child Psychol. 2019, 47, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Nofech-Mozes, J.; Pereira, J.; Gonzalez, A.; Atkinson, L. Cortisol secretion moderates the association between mother-infant attachment at 17 months and child behavior at age 5 years. Dev. Psychobiol. 2019, 61, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Obradović, J.; Portilla, X.A.; Ballard, P.J. Biological Sensitivity to Family Income: Differential Effects on Early Executive Functioning. Child Dev. 2016, 87, 374–384. [Google Scholar] [CrossRef]
- Shakiba, N.; Gao, M.; Conradt, E.; Terrell, S.; Lester, B.M. Parent–child relationship quality and adolescent health: Testing the differential susceptibility and diathesis-stress hypotheses in African American youths. Child Dev. 2022, 93, 269–287. [Google Scholar] [CrossRef] [PubMed]
- Obradović, J.; Bush, N.R.; Stamperdahl, J.; Adler, N.E.; Boyce, W.T. Biological sensitivity to context: The interactive effects of stress reactivity and family adversity on socioemotional behavior and school readiness. Child Dev. 2010, 81, 270–289. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.; Blair, C.; Ursache, A.; Willoughby, M.T.; Granger, D.A.; Family Life Project Key Investigators. Early childcare, executive functioning, and the moderating role of early stress physiology. Dev. Psychol. 2014, 50, 1250–1261. [Google Scholar] [CrossRef]
- Del Puerto-Golzarri, N.; Pascual-Sagastizabal, E.; Muñoz, J.M.; Carreras, M.R.; Ruiz-Ortiz, R.M.; Azurmendi, A. Differential susceptibility to parenting influences on reactive and proactive aggression: The role of testosterone and cortisol in children. Psychoneuroendocrinology 2023, 155, 106341. [Google Scholar] [CrossRef]
- Fong, M.C.; Measelle, J.; Conradt, E.; Ablow, J.C. Links between early baseline cortisol, attachment classification, and problem behaviors: A test of differential susceptibility versus diathesis-stress. Infant Behav. Dev. 2017, 46, 158–168. [Google Scholar] [CrossRef]
- Pascual-Sagastizabal, E.; del Puerto-Golzarri, N.; Azurmendi, A. Differential susceptibility or diathesis-stress: Testing the moderating role of temperament and cortisol levels between fathers’ parenting and children’s aggressive behavior. Brain Sci. 2021, 11, 1088. [Google Scholar] [CrossRef]
- Vaillancourt, T.; Brittain, H.; Haltigan, J.D.; Ostrov, J.M.; Muir, C. Cortisol moderates the relation between physical peer victimization and physical aggression in preschoolers attending high-quality child care: Evidence of differential susceptibility across informants. Merrill-Palmer Q. 2018, 64, 101–134. [Google Scholar] [CrossRef]
- Zhang, X.; Widaman, K.; Belsky, J. Beyond orchids and dandelions: Susceptibility to environmental influences is not bimodal. Dev. Psychopathol. 2023, 35, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Holmboe, K.; Nemoda, Z.; Fearon, R.M.; Sasvari-Szekely, M.; Johnson, M.H. Dopamine D4 receptor and serotonin transporter gene effects on the longitudinal development of infant temperament. Genes Brain Behav. 2011, 10, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Belsky, J.; Newman, D.A.; Widaman, K.F.; Rodkin, P.; Pluess, M.; Fraley, R.C.; Berry, D.; Helm, J.L.; Roisman, G.I. Differential susceptibility to effects of maternal sensitivity? A study of candidate plasticity genes. Dev. Psychopathol. 2015, 27, 725–746. [Google Scholar] [CrossRef] [PubMed]
- Kochanska, G.; Kim, S.; Boldt, L.J.; Yoon, J.E. Children’s callous-unemotional traits moderate links between their positive relationships with parents at preschool age and externalizing behavior problems at early school age. J. Child Psychol. Psychiatry Allied Discip. 2013, 54, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Raver, C.C.; Blair, C.; Willoughby, M. Poverty as a predictor of 4-year-olds’ executive function: New perspectives on models of differential susceptibility. Dev. Psychol. 2013, 49, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Stright, A.D.; Gallagher, K.; Kelley, K. Infant temperament moderates relations between maternal parenting in early childhood and children’s adjustment in first grade. Child Dev. 2008, 79, 186–200. [Google Scholar] [CrossRef] [PubMed]
- van Aken, C.; Junger, M.; Verhoeven, M.; Van Aken, M.A.G.; Dekovic, M. The interactive effects of temperament and maternal parenting on toddlers’ externalizing behaviours. Infant Child Dev. 2007, 16, 553–572. [Google Scholar] [CrossRef]
- Arnsten, A.F.T. Stress signaling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 2009, 10, 410–422. [Google Scholar] [CrossRef]
- Cha, K. The influence of classroom size and window view on young children’s executive functions and physiological responses, based on VR technology. Behav. Sci. 2023, 13, 936. [Google Scholar] [CrossRef]
- OECD. Encouraging Quality in Early Childhood Education and Care (ECEC). In Starting Strong III: A Quality Toolbox for Early Childhood Education and Care; OECD, Ed.; OECD PublishCo: Washington, DC, USA, 2013; pp. 23–42. [Google Scholar]
- Putnam, S.P.; Rothbart, M.K. Development of short and very short forms of the Children’s Behavior Questionnaire. J. Personal. Assess. 2006, 87, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.M. Developmentally sensitive measures of executive function in preschool. Dev. Neuropsychol. 2005, 28, 595–616. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Stevenson, H.W. Cross-linguistic differences in digit span of preschool children. J. Exp. Child Psychol. 1988, 46, 150–158. [Google Scholar] [CrossRef]
- Zelazo, P.D. The Dimensional Change Card Sort (DCCS): A method of assessing executive function in children. Nat. Protoc. 2006, 1, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Lewis, C. Korean preschoolers’ executive function skills: A comparison with US children. Early Child. Res. Q. 2018, 42, 144–152. [Google Scholar]
- Davis, E.P.; Bruce, J.; Gunnar, M.R. The anterior attention network: Associations with temperament and neuroendocrine activity in 6-year-old children. Dev. Psychobiol. 2002, 40, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Aiken, L.S.; West, S.G. Multiple Regression: Testing and Interpreting Interactions; Sage: Newbury Park, CA, USA, 1991. [Google Scholar]
- Cohen, J.; Cohen, P. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences, 2nd ed.; Erlbaum: Hillsdale, NJ, USA, 1983. [Google Scholar]
- Phillips, D.A.; Fox, N.A.; Gunnar, M.R. Same place, different experiences: Bringing individual differences to research in child care. Child Dev. Perspect. 2011, 5, 44–49. [Google Scholar] [CrossRef]
- Lupien, S.J.; Maheu, F.; Tu, M.; Fiocco, A.; Schramek, T.E. The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain Cogn. 2007, 65, 209–237. [Google Scholar] [CrossRef]
- Lupien, S.J.; Wilkinson, C.W.; Brière, S.; Ménard, C.; Ng Ying Kin, N.M.; Nair, N.P. The modulatory effects of corticosteroids on cognition: Studies in young human populations. Psychoneuroendocrinology 2002, 27, 401–416. [Google Scholar] [CrossRef]
- Belsky, J.; Zhang, X.; Sayler, K. Differential susceptibility 2.0: Are the same children affected by different experiences and exposures? Dev. Psychopathol. 2022, 34, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, E.B.; Granger, D.A.; Susman, E.J.; Gunnar, M.R.; Laird, B. Assessing salivary cortisol in studies of child development. Child Dev. 1998, 69, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Stansbury, K.; Gunnar, M.R. Adrenocortical activity and emotion regulation. Monogr. Soc. Res. Child Dev. 1994, 59, 108–134. [Google Scholar] [CrossRef] [PubMed]
- Shoal, G.D.; Giancola, P.R.; Kirillova, G.P. Salivary cortisol, personality, and aggressive behavior in adolescent boys: A 5-year longitudinal study. J. Am. Acad. Child Adolesc. Psychiatry 2003, 42, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Ortega, R.; López, V.; Carrasco, X.; Escobar, M.J.; García, A.M.; Parra, M.A.; Aboitiz, F. Neurocognitive mechanisms underlying working memory encoding and retrieval in Attention-Deficit/Hyperactivity Disorder. Sci. Rep. 2020, 10, 7771. [Google Scholar] [CrossRef]
- Panichello, M.F.; Buschman, T.J. Shared mechanisms underlie the control of working memory and attention. Nature 2021, 592, 601–605. [Google Scholar] [CrossRef]
- Stevenson, M.P.; Schilhab, T.; Bentsen, P. Attention Restoration Theory II: A systematic review to clarify attention processes affected by exposure to natural environments. J. Toxicol. Environ. Health Part B Crit. Rev. 2018, 21, 227–268. [Google Scholar] [CrossRef]
- Owen, A.M.; McMillan, K.M.; Laird, A.R.; Bullmore, E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 2005, 25, 46–59. [Google Scholar] [CrossRef]
- Wager, T.D.; Jonides, J.; Reading, S. Neuroimaging studies of shifting attention: A meta-analysis. NeuroImage 2004, 22, 1679–1693. [Google Scholar] [CrossRef]
- Segal, O.; Elkana, O. The ventrolateral prefrontal cortex is part of the modular working memory system: A functional neuroanatomical perspective. Front. Neuroanat. 2023, 17, 1076095. [Google Scholar] [CrossRef]
- Baddeley, A.D. Short-term and working memory. In The Oxford Handbook of Memory; Tulving, E., Craik, F.I.M., Eds.; Oxford University Press: Oxford, UK, 2000; pp. 77–92. [Google Scholar]
- Baddeley, A.D.; Hitch, G. Working memory. In The Psychology of Learning and Motivation: Advances in Research and Theory; Bower, G.H., Ed.; Academic Press: New York, NY, USA, 1974; Volume 8, pp. 47–89. [Google Scholar]
- Wager, T.D.; Smith, E.E. Neuroimaging studies of working memory: A meta-analysis. Cogn. Affect. Behav. Neurosci. 2003, 3, 255–274. [Google Scholar] [CrossRef] [PubMed]
- Zandstra, A.R.E.; Ormel, J.; Nederhof, E.; Hoekstra, P.J.; Hartman, C.A. The Role of Basal Cortisol in Predicting Change in Mental Health Problems Across the Transition to Middle School. J. Adolesc. Health 2015, 56, 489–495. [Google Scholar] [CrossRef] [PubMed]


| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Neg Emo | − | − | − | − | − | − | − | − | − | − | − | − |
| 2. Pre-DS | −0.01 | − | − | − | − | − | − | − | − | − | − | − |
| 3. Post-DS | 0.05 | 0.53 *** | − | − | − | − | − | − | − | − | − | − |
| 4. Pre-CB | 0.16 | 0.08 | 0.11 | − | − | − | − | − | − | − | − | − |
| 5. Post-CB | 0.01 | 0.12 | 0.18 * | 0.49 *** | − | − | − | − | − | − | − | − |
| 6. Pre-DCCS | −0.08 | 0.09 | 0.03 | 0.19 * | 0.18 * | − | − | − | − | − | − | − |
| 7. Post-DCCS | −0.18 | 0.06 | 0.00 | 0.17 * | 0.08 | 0.43 *** | − | − | − | − | − | − |
| 8. Pre-cortisol | 0.13 | 0.15 | 0.11 | 0.02 | 0.01 | 0.11 | 0.00 | − | − | − | − | |
| 9. Post-cortisol | 0.05 | 0.14 | 0.07 | −0.01 | −0.01 | 0.10 | 0.02 | 0.76 *** | − | − | − | − |
| 10. Father Edu | −0.08 | 0.09 | −0.03 | 0.04 | 0.01 | 0.04 | 0.05 | 0.07 | 0.10 | − | − | − |
| 11. Mother Edu | 0.04 | 0.16 | 0.13 | −0.01 | 0.02 | −0.02 | 0.01 | 0.11 | 0.08 | 0.42 *** | − | − |
| 12. Income | −0.11 | 0.17 * | 0.16+ | −0.01 | 0.11 | −0.01 | −0.00 | 0.25 ** | 0.14 | 0.26 ** | 0.29 *** | − |
| 13. Child’s sex | 0.00 | 0.00 | 0.01 | −0.02 | 0.12 | −0.06 | 0.11 | 0.11 | 0.03 | 0.04 | 0.09 | 0.20 * |
| Room Size | Window View | ||||
|---|---|---|---|---|---|
| Large (n = 34) | Small (n = 35) | Nature (n = 34) | Built (n = 38) | ||
| M (SD) | M (SD) | M (SD) | M (SD) | ||
| Digit Span | Pre | 3.67 (0.68) | 3.57 (0.88) | 3.50 (1.05) | 3.26 (1.08) |
| Post | 3.79 (0.97) | 3.65 (0.93) | 3.76 (1.13) | 3.50 (0.86) | |
| Corsi block | Pre | 3.12 (1.45) | 3.66 (1.13) | 3.18 (1.35) | 2.79 (1.21) |
| Post | 3.29 (1.40) | 3.14 (1.42) | 3.14 (1.37) | 2.71 (1.50) | |
| DCCS | Pre | 28.85 (3.70) | 29.57 (4.05) | 28.26 (4.26) | 28.08 (3.67) |
| Post | 30.68 (3.51) | 29.26 (3.57) | 29.76 (3.54) | 29.32 (3.97) | |
| Cortisol | Pre | 7.19 (3.55) | 6.47 (2.77) | 6.49 (2.83) | 5.83 (2.48) |
| Post | 6.21 (2.45) | 5.77 (2.01) | 5.81 (2.32) | 5.55 (3.01) | |
| Negative Emotionality | - | 3.84 (0.65) | 3.92 (0.68) | 3.74 (0.66) | 3.93 (0.69) |
| Classroom Size | Window View | |||||
|---|---|---|---|---|---|---|
| Outcome Variable | Digit Span (Post) | Corsi Block (Post) | DCCS (Post) | Digit Span (Post) | Corsi Block (Post) | DCCS (Post) |
| Model A β (SE) | Model B β (SE) | Model C β (SE) | Model D β (SE) | Model E β (SE) | Model F β (SE) | |
| EF score (pre) | 0.47 *** (0.14) | 0.54 *** (0.12) | 0.34 ** (0.10) | 0.56 *** (0.08) | 0.57 *** (0.11) | 0.48 *** (0.10) |
| Cortisol (pre) | −0.04 (0.05) | −0.04 (0.08) | 0.02 (0.21) | −0.03 (0.05) | 0.00 (0.08) | 0.17 (0.21) |
| VR condition | −0.26 (0.52) | −0.49 (0.75) | 3.35 † (1.95) | 0.15 (0.46) | 0.26 (0.77) | 2.57 (2.01) |
| (Pre-cortisol) × (VR condition) | 0.05 (0.07) | 0.14 (0.10) | −0.23 (0.27) | 0.14 * (0.07) | −0.01 (0.11) | −0.36 (0.30) |
| R2 | 0.16 | 0.26 | 0.20 | 0.45 | 0.27 | 0.26 |
| Adjusted R2 | 0.10 | 0.22 | 0.15 | 0.42 | 0.23 | 0.21 |
| F | 3.07 | 5.77 | 4.08 | 13.83 | 6.33 | 5.92 |
| (df) | (64) | (64) | (64) | (67) | (67) | (67) |
| p | <0.05 | <0.001 | <0.01 | <0.001 | <0.001 | <0.001 |
| Classroom Size | Window View | |||||
|---|---|---|---|---|---|---|
| Outcome Variable | Digit Span (Post) | Corsi Block (Post) | DCCS (Post) | Digit Span (Post) | Corsi Block (Post) | DCCS (Post) |
| Model A β (SE) | Model B β (SE) | Model C β (SE) | Model D β (SE) | Model E β (SE) | Model F β (SE) | |
| EF score (Pre) | 0.46 ** | 0.52 *** | 0.35 ** | 0.59 *** | 0.56 *** | 0.48 *** |
| NE | 0.05 | −0.52 † | −0.10 | 0.20 | 0.11 | 0.25 |
| VR condition | 0.09 | 0.49 | 1.67 * | 0.14 | 0.18 | 0.26 |
| (NE) × (VR condition) | 0.08 | 0.70 † (p = 0.086) | −0.63 | −0.26 | −0.37 | −0.90 |
| R2 | 0.16 | 0.27 | 0.2 | 0.43 | 0.29 | 0.26 |
| Adjusted R2 | 0.11 | 0.23 | 0.15 | 0.39 | 0.25 | 0.21 |
| F | 3.08 | 5.97 | 4.08 | 12.37 | 6.8 | 5.85 |
| (df) | −64 | −64 | −64 | −67 | −67 | −67 |
| p | <0.05 | <0.001 | <0.01 | <0.001 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cha, K. The Moderating Role of Cortisol and Negative Emotionality in the Effects of Classroom Size and Window View on Young Children’s Executive Functions. Behav. Sci. 2024, 14, 18. https://doi.org/10.3390/bs14010018
Cha K. The Moderating Role of Cortisol and Negative Emotionality in the Effects of Classroom Size and Window View on Young Children’s Executive Functions. Behavioral Sciences. 2024; 14(1):18. https://doi.org/10.3390/bs14010018
Chicago/Turabian StyleCha, Kijoo. 2024. "The Moderating Role of Cortisol and Negative Emotionality in the Effects of Classroom Size and Window View on Young Children’s Executive Functions" Behavioral Sciences 14, no. 1: 18. https://doi.org/10.3390/bs14010018
APA StyleCha, K. (2024). The Moderating Role of Cortisol and Negative Emotionality in the Effects of Classroom Size and Window View on Young Children’s Executive Functions. Behavioral Sciences, 14(1), 18. https://doi.org/10.3390/bs14010018






