A Single-Session Process-Based Cognitive-Behavioral Intervention Combined with Multimodal Rehabilitation Treatment for Chronic Pain Associated with Emotional Disorders
Abstract
1. Introduction
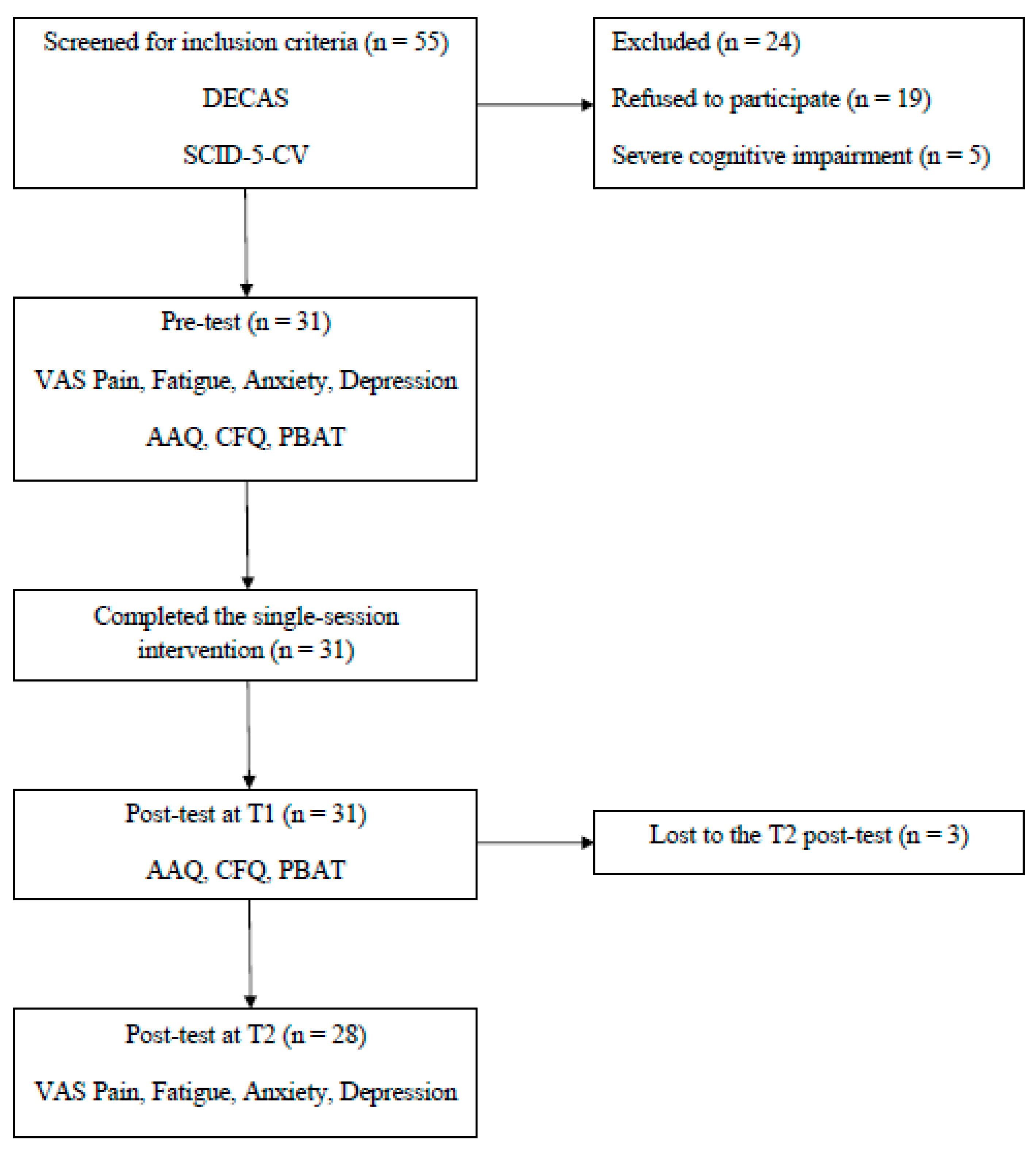
2. Materials and Methods
3. Results
3.1. Descriptive Characteristics
3.2. Baseline Measures
3.3. Intervention Outcomes
3.3.1. Results at T1
3.3.2. Results at T2
3.3.3. Results of the Linear Mixed Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anyfanti, P.; Gavriilaki, E.; Pyrpasopoulou, A.; Triantafyllou, G.; Triantafyllou, A.; Chatzimichailidou, S.; Gkaliagkousi, E.; Aslanidis, S.; Douma, S. Depression, anxiety, and quality of life in a large cohort of patients with rheumatic diseases: Common, yet undertreated. Clin. Rheumatol. 2016, 35, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Marchi, L.; Marzetti, F.; Orrù, G.; Lemmetti, S.; Miccoli, M.; Ciacchini, R.; Hitchcott, P.K.; Bazzicchi, L.; Gemignani, A.; Conversano, C. Alexithymia and Psychological Distress in Patients with Fibromyalgia and Rheumatic Disease. Front. Psychol. 2019, 10, 1735. [Google Scholar] [CrossRef] [PubMed]
- Matcham, F.; Rayner, L.; Steer, S.; Hotopf, M. The prevalence of depression in rheumatoid arthritis: A systematic review and me-ta-analysis. Rheumatology 2013, 52, 2136–2148. [Google Scholar] [CrossRef] [PubMed]
- Wróblewski, H.; Chojęta, D.; Zimna, A.; Zygmunt, E.; Kozłowska, A.; Mierzwa, M.; Wróblewska, K. Psychiatric Manifestations of Rheumatic Diseases. J. Educ. Health Sport 2022, 12, 52–60. [Google Scholar] [CrossRef]
- Wallace, Z.S.; Cook, C.; Finkelstein-Fox, L.; Fu, X.; Castelino, F.V.; Choi, H.K.; Perugino, C.; Stone, J.H.; Park, E.R.; Hall, D.L. The Association of Illness-related Uncertainty with Mental Health in Systemic Autoimmune Rheumatic Diseases. J. Rheumatol. 2022, 49, 1058–1066. [Google Scholar] [CrossRef]
- Beşirli, A.; Alptekin, J.Ö.; Kaymak, D.; Özer, Ö.A. The Relationship between Anxiety, Depression, Suicidal Ideation and Quality of Life in Patients with Rheumatoid Arthritis. Psychiatr. Q. 2020, 91, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Sarzi-Puttini, P.; Atzeni, F.; Clauw, D.J.; Perrot, S. The impact of pain on systemic rheumatic diseases. Best Pract. Res. Clin. Rheumatol. 2015, 29, 1–5. [Google Scholar] [CrossRef]
- Cohen, S.; Vase, L.; Hooten, W. Chronic pain: An update on burden, best practices, and new advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef]
- Parks, C.G.; Pettinger, M.; de Roos, A.J.; Tindle, H.A.; Walitt, B.T.; Howard, B.V. Life Events, Caregiving, and Risk of Autoimmune Rheumatic Diseases in the Women’s Health Initiative Observational Study. Arthritis Care Res. 2023, 75, 2519–2528. [Google Scholar] [CrossRef]
- Luo, G.; Boelle, P.Y.; Turbelin, C.; Costantino, F.; Kerneis, S.; Said Nahal, R.; Breban, M.; Hanslik, T. Abrupt and unexpected stressful life events are followed with increased disease activity in spondyloarthritis: A two years web-based cohort study. Jt. Bone Spine 2019, 86, 203–209. [Google Scholar] [CrossRef]
- Adnine, A.; Nadiri, K.; Soussan, I.; Coulibaly, S.; Berrada, K.; Najdi, A.; Abourazzak, F.E. Mental Health Problems Experienced by Patients with Rheumatic Diseases During COVID-19 Pandemic. Curr. Rheumatol. Rev. 2021, 17, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Guaracha-Basáñez, G.A.; Contreras-Yáñez, I.; Hernández-Molina, G.; Estrada-González, V.A.; Pacheco-Santiago, L.D.; Valverde-Hernández, S.S.; Galindo-Donaire, J.R.; Peláez-Ballestas, I.; Pascual-Ramos, V. Quality of life of patients with rheumatic diseases during the COVID-19 pandemic: The biopsychosocial path. PLoS ONE 2022, 17, e0262756. [Google Scholar] [CrossRef]
- Nasui, B.A.; Toth, A.; Popescu, C.A.; Penes, O.N.; Varlas, V.N.; Ungur, R.A.; Ciuciuc, N.; Silaghi, C.A.; Silaghi, H.; Pop, A.L. Comparative Study on Nutrition and Lifestyle of Information Technology Workers from Romania before and during COVID-19 Pandemic. Nutrients 2022, 14, 1202. [Google Scholar] [CrossRef] [PubMed]
- Nasui, B.A.; Ungur, R.A.; Nasui, G.A.; Popescu, C.A.; Hofer, A.M.; Dersidan, S.; Popa, M.; Silaghi, H.; Silaghi, C.A. Adolescents’ Lifestyle Determinants in Relation to Their Nutritional Status during COVID-19 Pandemic Distance Learning in the North-Western Part of Romania—A Cross-Sectional Study. Children 2023, 10, 922. [Google Scholar] [CrossRef]
- Brown, T.A.; Antony, M.M.; Barlow, D.H. Diagnostic comorbidity in panic disorder: Effect on treatment outcome and course of comorbid diagnoses following treatment. J. Consult. Clin. Psychol. 1995, 63, 408–418. [Google Scholar] [CrossRef]
- Groen, R.N.; Ryan, O.; Wigman, J.T.; Riese, H.; Penninx, B.W.; Giltay, E.J.; Wichers, M.; Hartman, C.A. Comorbidity between depression and anxiety: Assessing the role of bridge mental states in dynamic psychological networks. BMC Med. 2020, 18, 308. [Google Scholar] [CrossRef]
- Barlow, D.H.; Ellard, K.K.; Sauer-Zavala, S.; Bullis, J.R.; Carl, J.R. The origins of neuroticism. Perspect. Psychol. Sci. 2014, 9, 481–496. [Google Scholar] [CrossRef]
- Sauer-Zavala, S.; Wilner, J.G.; Barlow, D.H. Addressing neuroticism in psychological treatment. Personal. Disord. 2017, 8, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Barlow, D.H.; Allen, L.B.; Choate, M.L. Toward a Unified Treatment for Emotional Disorders—Republished Article. Behav. Ther. 2016, 47, 838–853. [Google Scholar] [CrossRef]
- Daïen, C.I.; Tubery, A.; Beurai-Weber, M.; du Cailar, G.; Picot, M.C.; Jaussent, A.; Roubille, F.; Cohen, J.D.; Morel, J.; Bousquet, J.; et al. Relevance and feasibility of a systematic screening of multimorbidities in patients with chronic inflammatory rheumatic diseases. Jt. Bone Spine 2019, 86, 49–54. [Google Scholar] [CrossRef]
- Golenbiewski, J.T.; Pisetsky, D.S. A Holistic Approach to Pain Management in the Rheumatic Diseases. Curr. Treat. Options Rheumatol. 2019, 5, 1–10. [Google Scholar] [CrossRef]
- Fraenkel, L.; Bathon, J.M.; England, B.R.; St Clair, E.W.; Arayssi, T.; Carandang, K.; Deane, K.D.; Genovese, M.; Huston, K.K.; Kerr, G.; et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res. 2021, 73, 924–939. [Google Scholar] [CrossRef] [PubMed]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felsonet, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2020, 72, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Fashler, S.R.; Cooper, L.K.; Oosenbrug, E.D.; Burns, L.C.; Razavi, S.; Goldberg, L.; Katz, J. Systematic Review of Multidisciplinary Chronic Pain Treatment Facilities. Pain Res. Manag. 2016, 2016, 5960987. [Google Scholar] [CrossRef] [PubMed]
- APA Division 12. Psychological Treatment of Chronic or Persistent Pain. Society of Clinical Psychology. 2012. Available online: http://www.psychologicaltreatments.org (accessed on 25 October 2023).
- Scott, A.J.; Bisby, M.A.; Heriseanu, A.I.; Salameh, Y.; Karin, E.; Fogliati, R.; Dudeney, J.; Gandy, M.; McLellan, L.F.; Wootton, B.; et al. Cognitive behavioral therapies for depression and anxiety in people with chronic disease: A systematic review and meta-analysis. Clin. Psychol. Rev. 2023, 106, 102353. [Google Scholar] [CrossRef] [PubMed]
- Geenen, R.; Newman, S.; Bossema, E.R.; Vriezekolk, J.E.; Boelen, P.A. Psychological interventions for patients with rheumatic diseases and anxiety or depression. Best Pract. Res. Clin. Rheumatol. 2012, 26, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Ehde, D.M.; Dillworth, T.M.; Turner, J.A. Cognitive-behavioral therapy for individuals with chronic pain: Efficacy, innovations, and directions for research. Am. Psychol. 2014, 69, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.C.; Chapman, J.E.; Forman, E.M.; Beck, A.T. The empirical status of cognitive-behavioral therapy: A review of meta-analyses. Clin. Psychol. Rev. 2006, 26, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Beard, C.; Millner, A.J.; Forgeard, M.J.; Fried, E.I.; Hsu, K.J.; Treadway, M.T.; Leonard, C.V.; Kertz, S.J.; Björgvinsson, T. Network analysis of depression and anxiety symptom relationships in a psychiatric sample. Psychol. Med. 2016, 46, 3359–3369. [Google Scholar] [CrossRef]
- Hofmann, S.G. Psychotherapeutic Interventions and Processes. Cogn. Behav. Pract. 2022, 29, 581–584. [Google Scholar] [CrossRef]
- Nguyen, V.V.; Zainal, N.H.; Newman, M.G. Why sleep is key: Poor sleep quality is a mechanism for the bidirectional relationship between major depressive disorder and generalized anxiety disorder across 18 years. J. Anxiety Disord. 2022, 90, 102601. [Google Scholar] [CrossRef] [PubMed]
- Sanford, B.; Ciarrochi, J.; Hofmann, S.; Chin, F.; Gates, K.; Hayes, S. Toward empirical process-based case conceptualization: An idionomic network examination of the process-based assessment tool. J. Context. Behav. Sci. 2022, 25, 10–25. [Google Scholar] [CrossRef]
- Hayes, S.C.; Hofmann, S.G.; Ciarrochi, J. A process-based approach to psychological diagnosis and treatment: The conceptual and treatment utility of an extended evolutionary meta model. Clin. Psychol. Rev. 2020, 82, 101908. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Dong, J.; Jin, S.; Zhang, H.; Zhang, Y. Acceptance and Commitment Therapy for chronic pain on functioning: A systematic review of randomized controlled trials. Neurosci. Biobehav. Rev. 2021, 131, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, E.; Wileman, V.; Galea Holmes, M.; McCracken, L.M.; Norton, S.; Moss-Morris, R.; Noonan, S.; Barcellona, M.; Critchley, D. Physical Therapy Informed by Acceptance and Commitment Therapy (PACT) Versus Usual Care Physical Therapy for Adults with Chronic Low Back Pain: A Randomized Controlled Trial. J. Pain 2020, 21, 71–81. [Google Scholar] [CrossRef]
- Malins, S.; Biswas, S.; Rathbone, J.; Vogt, W.; Pye, N.; Levene, J.; Moghaddam, N.; Russell, J. Reducing dropout in acceptance and commitment therapy, mindfulness-based cognitive therapy, and problem-solving therapy for chronic pain and cancer patients using motivational interviewing. Br. J. Clin. Psychol. 2020, 59, 424–438. [Google Scholar] [CrossRef] [PubMed]
- Twohig, M.P.; Levin, M.E. Acceptance and Commitment Therapy as a Treatment for Anxiety and Depression: A Review. Psychiatr. Clin. N. Am. 2017, 40, 751–770. [Google Scholar] [CrossRef]
- Hughes, L.S.; Clark, J.; Colclough, J.A.; Dale, E.; McMillan, D. Acceptance and Commitment Therapy (ACT) for Chronic Pain: A Systematic Review and Meta-Analyses. Clin. J. Pain 2017, 33, 552–568. [Google Scholar] [CrossRef] [PubMed]
- Wetherell, J.L.; Afari, N.; Rutledge, T.; Sorrell, J.T.; Stoddard, J.A.; Petkus, A.J.; Solomon, B.C.; Lehman, D.H.; Liu, L.; Lang, A.J.; et al. A randomized, controlled trial of acceptance and commitment therapy and cognitive-behavioral therapy for chronic pain. Pain 2011, 152, 2098–2107. [Google Scholar] [CrossRef]
- Levin, M.E.; MacLane, C.; Daflos, S.; Seeley, J.R.; Hayes, S.C.; Biglan, A.; Pistorello, J. Examining psychological inflexibility as a transdiagnostic process across psychological disorders. J. Context. Behav. Sci. 2014, 3, 155–163. [Google Scholar] [CrossRef]
- Hayes, S.C.; Strosahl, K.D.; Wilson, K.G. Acceptance and Commitment Therapy: The Process and Practice of Mindful Change; Guilford Press: New York, NY, USA, 2011. [Google Scholar]
- Popa, C.O.; Rus, A.V.; Lee, W.C.; Cojocaru, C.; Schenk, A.; Văcăraș, V.; Olah, P.; Mureșan, S.; Szasz, S.; Bredicean, C. The Relation between Negative Automatic Thoughts and Psychological Inflexibility in Schizophrenia. J. Clin. Med. 2022, 11, 871. [Google Scholar] [CrossRef] [PubMed]
- Faustino, B.; Vasco, A.B.; Farinha-Fernandes, A.; Delgado, J. Psychological inflexibility as a transdiagnostic construct: Relationships between cognitive fusion, psychological well-being and symptomatology. Curr. Psychol. 2023, 42, 6056–6061. [Google Scholar] [CrossRef]
- Doorley, J.D.; Goodman, F.R.; Kelso, K.C.; Kashdan, T.B. Psychological flexibility: What we know, what we do not know, and what we think we know. Soc. Personal. Psychol. Compass 2020, 14, 1–11. [Google Scholar] [CrossRef]
- Harris, R. ACT Made Simple: An Easy-to-Read Primer on Acceptance and Commitment Therapy, 2nd ed.; New Harbinger Publications: Oakland, CA, USA, 2019. [Google Scholar]
- Ecija, C.; Catala, P.; Lopez-Gomez, I.; Bedmar, D.; Peñacoba, C. What Does the Psychological Flexibility Model Contribute to the Relationship between Depression and Disability in Chronic Pain? The Role of Cognitive Fusion and Pain Acceptance. Clin. Nurs. Res. 2022, 31, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.; Pereira, J.; Ferreira, C. How do ACT core processes underlie loneliness and psychological health? A study among people with and without physical chronic disease. Clin. Psychol. 2021, 25, 329–338. [Google Scholar] [CrossRef]
- Carvalho, S.A.; Pinto-Gouveia, J.; Gillanders, D.; Castilho, P. Pain and Depressive Symptoms: Exploring Cognitive Fusion and Self-Compassion in a Moderated Mediation Model. J. Psychol. 2019, 153, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Larsson, A.; Hooper, N.; Osborne, L.A.; Bennett, P.; McHugh, L. Using brief cognitive restructuring and cognitive defusion tech-niques to cope with negative thoughts. Behav. Modif. 2016, 40, 452–482. [Google Scholar] [CrossRef] [PubMed]
- Chawla, N.; Ostafin, B. Experiential avoidance as a functional dimensional approach to psychopathology: An empirical review. J. Clin. Psychol. 2007, 63, 871–890. [Google Scholar] [CrossRef]
- Kratz, A.L.; Ehde, D.M.; Bombardier, C.H.; Kalpakjian, C.Z.; Hanks, R.A. Pain acceptance decouples the momentary associations between pain, pain interference, and physical activity in the daily lives of people with chronic pain and spinal cord injury. J. Pain 2017, 18, 319–331. [Google Scholar] [CrossRef]
- Wright, M.A.; Wren, A.A.; Somers, T.J.; Goetz, M.C.; Fras, A.M.; Huh, B.K.; Rogers, L.L.; Keefe, F.J. Pain acceptance, hope, and optimism: Relationships to pain and adjustment in patients with chronic musculoskeletal pain. J. Pain 2011, 12, 1155–1162. [Google Scholar] [CrossRef]
- Branstetter-Rost, A.; Cushing, C.; Douleh, T. Personal values and pain tolerance: Does a values intervention add to acceptance? J. Pain 2009, 10, 887–892. [Google Scholar] [CrossRef]
- Dryden, W. Single-Session One-At-A-Time Therapy: A Personal Approach. Aust. N. Z. J. Fam. Ther. 2020, 41, 283–301. [Google Scholar] [CrossRef]
- Campbell, A. Single-Session Approaches to Therapy: Time to Review. Aust. N. Z. J. Fam. Ther. 2012, 33, 15–26. [Google Scholar] [CrossRef]
- Dochat, C.; Wooldridge, J.S.; Herbert, M.S.; Lee, M.W.; Afari, N. Single-Session Acceptance and Commitment Therapy (ACT) Interventions for Patients with Chronic Health Conditions: A Systematic Review and Meta-Analysis. J. Context. Behav. Sci. 2021, 20, 52–69. [Google Scholar] [CrossRef] [PubMed]
- Darnall, B.D.; Roy, A.; Chen, A.L.; Ziadni, M.S.; Keane, R.T.; You, D.S.; Slater, K.; Poupore-King, H.; Mackey, I.; Kao, M.C.; et al. Comparison of a Single-Session Pain Management Skills Intervention with a Single-Session Health Education Intervention and 8 Sessions of Cognitive Behavioral Therapy in Adults With Chronic Low Back Pain: A Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e2113401. [Google Scholar] [CrossRef]
- Gibbons, M.B.C.; Rothbard, A.; Farris, K.D.; Stirman, S.W.; Thompson, S.M.; Scott, K.; Crits-Christoph, P. Changes in psychotherapy utilization among consumers of services for major depressive disorder in the community mental health system. Adm. Policy Ment. Health 2011, 38, 495–503. [Google Scholar] [CrossRef]
- Hayes, S.C.; Ciarrochi, J.; Hofmann, S.G.; Chin, F.; Sahdra, B. Evolving an idionomic approach to processes of change: Towards a unified personalized science of human improvement. Behav. Res. Ther. 2022, 156, 104155. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.C.; Hofmann, S.G.; Stanton, C.E.; Carpenter, J.K.; Sanford, B.T.; Curtiss, J.E.; Ciarrochi, J. The role of the individual in the coming era of process-based therapy. Behav. Res. Ther. 2019, 117, 40–53. [Google Scholar] [CrossRef]
- Amra, B.; Ghadiry, F.; Vaezi, A.; Nematollahy, A.; Radfar, N.; Haghjoo, S.; Penzel, T.; Morin, C.M. Effect of one-shot cognitive behavioral therapy on insomnia and heart rate variability of health care workers at the time of COVID-19 pandemic: A randomized controlled trial. Sleep Breath. 2023, 27, 1411–1418. [Google Scholar] [CrossRef]
- Schleider, J.L.; Mullarkey, M.C.; Fox, K.R.; Dobias, M.L.; Shroff, A.; Hart, E.A.; Roulston, C.A. A randomized trial of online single-session interventions for adolescent depression during COVID-19. Nat. Hum. Behav. 2022, 6, 258–268. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Sava, F. Inventarul de Personalitate DECAS; ArtPress: Timişoara, Romania, 2008. [Google Scholar]
- McCrae, R.R.; Costa, P.T. Validation of the five-factor model of personality across instruments and observers. J. Pers. Soc. Psychol. 1987, 52, 81–90. [Google Scholar] [CrossRef]
- Barlow, D.H.; Curreri, A.J.; Woodard, L.S. Neuroticism and Disorders of Emotion: A New Synthesis. Curr. Dir. Psychol. Sci. 2021, 30, 410–417. [Google Scholar] [CrossRef]
- Sava, F.A.; Popa, R.I. Personality types based on the Big Five model. A cluster analysis over the Romanian population. Cogn. Brain Behav. 2011, 15, 359–384. [Google Scholar]
- First, M.B.; Williams, J.B.; Karg, R.S.; Spitzer, R.L. SCID-5-CV: Structured Clinical Interview for DSM-5 Disorders: Clinician Version; American Psychiatric Association Publishing: Washington, DC, USA, 2016. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM), 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Shabani, A.; Masoumian, S.; Zamirinejad, S.; Hejri, M.; Pirmorad, T.; Yaghmaeezadeh, H. Psychometric properties of Structured Clinical Interview for DSM-5 Disorders-Clinician Version (SCID-5-CV). Brain Behav. 2021, 11, e01894. [Google Scholar] [CrossRef]
- Osório, F.L.; Loureiro, S.R.; Hallak, J.E.C.; Machado-de-Sousa, J.P.; Ushirohira, J.M.; Baes, C.V.W.; Apolinario, T.D.; Donadon, M.F.; Bolsoni, L.M.; Guimarães, T.; et al. Clinical validity and intrarater and test-retest reliability of the Structured Clinical Interview for DSM-5—Clinician Version (SCID-5-CV). Psychiatry Clin. Neurosci. 2019, 73, 754–760. [Google Scholar] [CrossRef]
- Jollant, F.; Voegeli, G.; Kordsmeier, N.C.; Carbajal, J.M.; Richard-Devantoy, S.; Turecki, G.; Cáceda, R. A visual analog scale to measure psychological and physical pain: A preliminary validation of the PPP-VAS in two independent samples of depressed patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 90, 55–61. [Google Scholar] [CrossRef]
- Williamson, A.; Hoggart, B. Pain: A review of three commonly used pain rating scales. J. Clin. Nurs. 2005, 14, 798–804. [Google Scholar] [CrossRef]
- Wolfe, F. Fatigue assessments in rheumatoid arthritis: Comparative performance of visual analog scales and longer fatigue questionnaires in 7760 patients. J. Rheumatol. 2004, 31, 1896–1902. [Google Scholar]
- Huang, Z.; Kohler, I.V.; Kämpfen, F. A Single-Item Visual Analogue Scale (VAS) Measure for Assessing Depression among College Students. Community Ment. Health J. 2020, 56, 355–367. [Google Scholar] [CrossRef]
- Williams, V.S.; Morlock, R.J.; Feltner, D. Psychometric evaluation of a visual analog scale for the assessment of anxiety. Health Qual. Life Outcomes 2010, 8, 57. [Google Scholar] [CrossRef]
- Ciarrochi, J.; Sahdra, B.; Hofmann, S.G.; Hayes, S.C. Developing an item pool to assess processes of change in psychological interventions: The Process-Based Assessment Tool (PBAT). J. Context. Behav. Sci. 2022, 23, 200–213. [Google Scholar] [CrossRef]
- Bond, F.W.; Hayes, S.C.; Baer, R.A.; Carpenter, K.M.; Guenole, N.; Orcutt, H.K.; Waltz, T.; Zettle, R.D. Preliminary Psychometric Properties of the Acceptance and Action Questionnaire–II: A Revised Measure of Psychological Inflexibility and Experiential Avoidance. Behav. Ther. 2011, 42, 676–688. [Google Scholar] [CrossRef]
- Szabó, K.G.; Vargha, J.L.; Balázsi, R.; Bartalus, J.; Bogdan, V. Measuring Psychological Flexibility: Preliminary Data on the Psychometric Properties of the Romanian Version of the Acceptance and Action Questionnaire (AAQ-II). J. Cogn. Behav. Psychother. 2011, 11, 67–82. [Google Scholar]
- Gillanders, D.; Bolderston, H.; Bond, F.W.; Dempster, M.; Flaxman, P.E.; Campbell, L.; Remington, B. The development and initial validation of the Cognitive Fusion Questionnaire. Behav. Ther. 2014, 45, 83–101. [Google Scholar] [CrossRef]
- Vowles, K.E.; McCracken, L.M. Acceptance and values-based action in chronic pain: A study of treatment effectiveness and process. J. Consult. Clin. Psychol. 2008, 76, 397–407. [Google Scholar] [CrossRef]
- Dahl, J.C.; Lundgren, T.L. Living Beyond Your Pain: Using Acceptance and Commitment Therapy to Ease Chronic Pain; New Harbinger Publications: Oakland, CA, USA, 2006. [Google Scholar]
- Hayes, S.C.; Strosahl, K.; Wilson, K.G. Acceptance and Commitment Therapy: An Experiential Approach to Behavior Change; Guilford Press: New York, NY, USA, 1999. [Google Scholar]
- Kim, J.; Ryu, N.; Chibanda, D. Effectiveness of single-session therapy for adult common mental disorders: A systematic review. BMC Psychol. 2023, 11, 373. [Google Scholar] [CrossRef]
- Khera, T.; Rangasamy, V. Cognition and Pain: A Review. Front. Psychol. 2021, 12, 673962. [Google Scholar] [CrossRef]
- Benoy, C.; Knitter, B.; Knellwolf, L.; Doering, S.; Klotsche, J.; Gloster, A.T. Assessing psychological flexibility: Validation of the Open and Engaged State Questionnaire. J. Context. Behav. Sci. 2019, 12, 253–260. [Google Scholar] [CrossRef]
- Klimczak, K.S.; Schwartz, S.E.; Donahue, M.L.; Capel, L.K.; Snow, J.L.; Levin, M.E. Disentangling trait and state psychological inflexibility: A longitudinal multilevel approach. J. Context. Behav. Sci. 2023, 29, 13–22. [Google Scholar] [CrossRef]
- Sundström, F.T.; Lavefjord, A.; Buhrman, M.; McCracken, L.M. Assessing psychological flexibility and inflexibility in chronic pain using the multidimensional psychological flexibility inventory (MPFI). J. Pain 2023, 24, 770–781. [Google Scholar] [CrossRef]
- Pakenham, K.I.; Landi, G.; Cattivelli, R.; Grandi, S.; Tossani, E. Identification of psychological flexibility and inflexibility profiles during the COVID-19 pandemic. J. Clin. Psychol. 2023, 79, 2225–2250. [Google Scholar] [CrossRef]
- Mishra, P.; Pandey, C.M.; Singh, U.; Gupta, A.; Sahu, C.; Keshri, A. Descriptive statistics and normality tests for statistical data. Ann. Card. Anaesth. 2019, 22, 67–72. [Google Scholar] [CrossRef]
- Sturgeon, J.A.; Finan, P.H.; Zautra, A.J. Affective disturbance in rheumatoid arthritis: Psychological and disease-related pathways. Nat. Rev. Rheumatol. 2016, 12, 532–542. [Google Scholar] [CrossRef]
- Usubini, A.G.; Varallo, G.; Giusti, E.M.; Cattivelli, R.; Granese, V.; Consoli, S.; Bastoni, I.; Volpi, C.; Castelnuovo, G. The mediating role of psychological inflexibility in the relationship between anxiety, depression, and emotional eating in adult individuals with obesity. Front. Psychol. 2022, 13, 861341. [Google Scholar] [CrossRef]
- Crocker, L.D.; Heller, W.; Warren, S.L.; O’Hare, A.J.; Infantolino, Z.P.; Miller, G.A. Relationships among cognition, emotion, and motivation: Implications for intervention and neuroplasticity in psychopathology. Front. Hum. Neurosci. 2013, 7, 261. [Google Scholar] [CrossRef]
- Beck, A.T.; Haigh, E.A. Advances in cognitive theory and therapy: The generic cognitive model. Annu. Rev. Clin. Psychol. 2014, 10, 1–24. [Google Scholar] [CrossRef]
- Leonard, M.T.; Chatkoff, D.K.; Maier, K.J. Couples’ Relationship Satisfaction and Its Association with Depression and Spouse Responses within the Context of Chronic Pain Adjustment. Pain. Manag. Nurs. 2019, 19, 400–407. [Google Scholar] [CrossRef]
- Van Damme, S.; Becker, S.; Van der Linden, D. Tired of pain? Toward a better understanding of fatigue in chronic pain. Pain 2018, 159, 7–10. [Google Scholar] [CrossRef]
- Dindo, L.; Zimmerman, M.B.; Hadlandsmyth, K.; StMarie, B.; Embree, J.; Marchman, J.; Tripp-Reimer, T.; Rakel, B. Acceptance and Commitment Therapy for Prevention of Chronic Postsurgical Pain and Opioid Use in At-Risk Veterans: A Pilot Randomized Controlled Study. J. Pain 2018, 19, 1211–1221. [Google Scholar] [CrossRef]
- Åkerblom, S.; Perrin, S.; Rivano Fischer, M.; McCracken, L.M. The Mediating Role of Acceptance in Multidisciplinary Cognitive-Behavioral Therapy for Chronic Pain. J. Pain 2015, 16, 606–615. [Google Scholar] [CrossRef]
- Simister, H.D.; Tkachuk, G.A.; Shay, B.L.; Vincent, N.; Pear, J.J.; Skrabek, R.Q. Randomized controlled trial of online acceptance and commitment therapy for fibromyalgia. J. Pain 2018, 19, 741–753. [Google Scholar] [CrossRef]
- Alda, M.; Luciano, J.V.; Andrés, E.; Serrano-Blanco, A.; Rodero, B.; del Hoyo, Y.L.; Roca, M.; Moreno, S.; Magallón, R.; García-Campayo, J. Effectiveness of cognitive behaviour therapy for the treatment of catastrophisation in patients with fibromyalgia: A randomised controlled trial. Arthritis Res. Ther. 2011, 13, R173. [Google Scholar] [CrossRef] [PubMed]
- Darnall, B.D.; Sturgeon, J.A.; Kao, M.C.; Hah, J.M.; Mackey, S.C. From catastrophizing to recovery: A pilot study of a single-session treatment for pain catastrophizing. J. Pain Res. 2014, 7, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Gilliam, W.P.; Schumann, M.E.; Cunningham, J.L.; Evans, M.M.; Luedtke, C.A.; Morrison, E.J.; Sperry, J.A.; Vowles, K.E. Pain catastrophizing as a treatment process variable in cognitive behavioural therapy for adults with chronic pain. Eur. J. Pain 2021, 25, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, A.; Kazemi-Zahrani, H. The Effectiveness of Group Acceptance and Commitment Therapy on Pain Intensity, Pain Catastrophizing and Pain-Associated Anxiety in Patients with Chronic Pain. Asian Soc. Sci. 2015, 11, 112. [Google Scholar] [CrossRef]
- Nijs, J.; D’Hondt, E.; Clarys, P.; Deliens, T.; Polli, A.; Malfliet, A.; Coppieters, I.; Willaert, W.; Tumkaya Yilmaz, S.; Elma, Ö.; et al. Lifestyle and Chronic Pain across the Lifespan: An Inconvenient Truth? PM&R 2020, 12, 410–419. [Google Scholar] [CrossRef]
- Znidarsic, J.; Kirksey, K.N.; Dombrowski, S.M.; Tang, A.; Lopez, R.; Blonsky, H.; Todorov, I.; Schneeberger, D.; Doyle, J.; Libertiniet, L.; et al. “Living Well with Chronic Pain”: Integrative Pain Management via Shared Medical Appointments. Pain Med. 2021, 22, 181–190. [Google Scholar] [CrossRef]
- Will, K.K.; Johnson, M.L.; Lamb, G. Team-Based Care and Patient Satisfaction in the Hospital Setting: A Systematic Review. J. Patient Cent. Res. Rev. 2019, 6, 158–171. [Google Scholar] [CrossRef]
- Moore, A.J.; Holden, M.A.; Foster, N.E.; Jinks, C. Therapeutic alliance facilitates adherence to physiotherapy-led exercise and physical activity for older adults with knee pain: A longitudinal qualitative study. J. Physiother. 2020, 66, 45–53. [Google Scholar] [CrossRef]
- Vowles, K.E.; Sowden, G.; Hickman, J.; Ashworth, J. An analysis of within-treatment change trajectories in valued activity in relation to treatment outcomes following interdisciplinary Acceptance and Commitment Therapy for adults with chronic pain. Behav. Res. Ther. 2019, 115, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.P.; Vowles, K.E.; Johnson, L.E.; Gertz, K.J. Living Well with Pain: Development and Preliminary Evaluation of the Valued Living Scale. Pain Med. 2015, 16, 2109–2120. [Google Scholar] [CrossRef] [PubMed]
- McCracken, L.M.; Gutiérrez-Martínez, O. Processes of change in psychological flexibility in an interdisciplinary group-based treatment for chronic pain based on Acceptance and Commitment Therapy. Behav. Res. Ther. 2011, 49, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Kanzler, K.E.; Robinson, P.J.; McGeary, D.D.; Mintz, J.; Kilpela, L.S.; Finley, E.P.; McGeary, C.; Lopez, E.J.; Velligan, D.; Munante, M.; et al. Addressing chronic pain with Focused Acceptance and Commitment Therapy in integrated primary care: Findings from a mixed methods pilot randomized controlled trial. BMC Prim. Care 2022, 23, 77. [Google Scholar] [CrossRef] [PubMed]
- Ntoumanis, N.; Ng, J.Y.Y.; Prestwich, A.; Quested, E.; Hancox, J.E.; Thøgersen-Ntoumani, C.; Deci, E.L.; Ryan, R.M.; Lonsdale, C.; Williams, G.C. A meta-analysis of self-determination theory-informed intervention studies in the health domain: Effects on motivation, health behavior, physical, and psychological health. Health Psychol. Rev. 2021, 15, 214–244. [Google Scholar] [CrossRef] [PubMed]
- Sheeran, P.; Wright, C.E.; Avishai, A.; Villegas, M.E.; Rothman, A.J.; Klein, W.M.P. Does increasing autonomous motivation or perceived competence lead to health behavior change? A meta-analysis. Health Psychol. 2021, 40, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.M.; Huck, G.; Iwanaga, K.; Chan, F.; Wu, J.R.; Finnicum, C.A.; Brinck, E.A.; Estala-Gutierrez, V.Y. Towards an inte-gration of the health promotion models of self-determination theory and theory of planned behavior among people with chronic pain. Rehabil. Psychol. 2018, 63, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Uysal, A.; Ascigil, E.; Turunc, G. Spousal autonomy support, need satisfaction, and well-being in individuals with chronic pain: A longitudinal study. J. Behav. Med. 2017, 40, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Vriezekolk, J.E.; Geenen, R.; van den Ende, C.H.M.; Slot, H.; van Lankveld, W.G.J.M.; van Helmond, T. Behavior change, acceptance, and coping flexibility in highly distressed patients with rheumatic diseases: Feasibility of a cognitive-behavioral therapy in multimodal rehabilitation. Patient Educ. Couns. 2012, 87, 171–177. [Google Scholar] [CrossRef]
- Hajihasani, A.; Rouhani, M.; Salavati, M.; Hedayati, R.; Kahlaee, A.H. The influence of cognitive behavioral therapy on pain, quality of life, and depression in patients receiving physical therapy for chronic low back pain: A systematic review. PM&R 2019, 11, 167–176. [Google Scholar] [CrossRef]
- Ma, T.W.; Yuen, A.S.; Yang, Z. The efficacy of acceptance and commitment therapy for chronic pain: A systematic review and meta-analysis. Clin. J. Pain 2023, 39, 147–157. [Google Scholar] [CrossRef]
- Burns, J.W.; Jense, M.P.; Thorn, B.; Lillis, T.A.; Carmody, J.; Newman, A.K.; Keefe, F. Cognitive therapy, mindfulness-based stress reduction, and behavior therapy for the treatment of chronic pain: Randomized controlled trial. Pain 2022, 163, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Fernández, M.; López-López, A.; Losada, A.; González, J.L.; Wetherell, J.L. Acceptance and Commitment Therapy and Selective Optimization with Compensation for Institutionalized Older People with Chronic Pain. Pain Med. 2016, 17, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Ólason, M.; Andrason, R.H.; Jónsdóttir, I.H.; Kristbergsdóttir, H.; Jensen, M.P. Cognitive Behavioral Therapy for Depression and Anxiety in an Interdisciplinary Rehabilitation Program for Chronic Pain: A Randomized Controlled Trial with a 3-Year Fol-low-up. Int. J. Behav. Med. 2018, 25, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.P.; Poulis, N.; Moreton, B.J.; Walsh, D.A.; Lincoln, N.B. Evaluation of a group acceptance commitment therapy intervention for people with knee or hip osteoarthritis: A pilot randomized controlled trial. Disabil. Rehabil. 2017, 39, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Trompetter, H.R.; Bohlmeijer, E.T.; Veehof, M.M.; Schreurs, K.M. Internet-based guided self-help intervention for chronic pain based on Acceptance and Commitment Therapy: A randomized controlled trial. J. Behav. Med. 2015, 38, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Luciano, J.V.; Guallar, J.A.; Aguado, J.; López-Del-Hoyo, Y.; Olivan, B.; Magallón, R.; Alda, M.; Serrano-Blanco, A.; Gili, M.; Garcia-Campayo, J. Effectiveness of group acceptance and commitment therapy for fibromyalgia: A 6-month randomized con-trolled trial (EFFIGACT study). Pain 2013, 155, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Garnefski, N.; Kraaij, V.; Benoist, M.; Bout, Z.; Karels, E.; Smit, A. Effect of a Cognitive Behavioral Self-Help Intervention on Depression, Anxiety, and Coping Self-Efficacy in People with Rheumatic Disease. Arthritis Care Res. 2013, 65, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Bonilla-Jaime, H.; Sánchez-Salcedo, J.A.; Estevez-Cabrera, M.M.; Molina-Jiménez, T.; Cortes-Altamirano, J.L.; Alfaro-Rodríguez, A. Depression and Pain: Use of Antidepressants. Curr. Neuropharmacol. 2022, 20, 384–402. [Google Scholar] [CrossRef]
- Ferreira, G.E.; McLachlan, A.J.; Lin, C.W.C.; Zadro, J.R.; Abdel-Shaheed, C.; O’Keeffe, M.; Maher, C.G. Efficacy and safety of antidepressants for the treatment of back pain and osteoarthritis: Systematic review and meta-analysis. BMJ 2021, 372, m4825. [Google Scholar] [CrossRef]
- Marris, D.; Theophanous, K.; Cabezon, P.; Dunlap, Z.; Donaldson, M. The impact of combining pain education strategies with physical therapy interventions for patients with chronic pain: A systematic review and meta-analysis of randomized controlled trials. Physiother. Theory Pract. 2021, 37, 461–472. [Google Scholar] [CrossRef]
- Kinney, M.; Seider, J.; Beaty, A.F.; Coughlin, K.; Dyal, M.; Clewley, D. The impact of therapeutic alliance in physical therapy for chronic musculoskeletal pain: A systematic review of the literature. Physiother. Theory Pract. 2020, 36, 886–898. [Google Scholar] [CrossRef]
- Kaptchuk, T.J.; Hemond, C.C.; Miller, F.G. Placebos in chronic pain: Evidence, theory, ethics, and use in clinical practice. BMJ 2020, 370, m1668. [Google Scholar] [CrossRef]
- Joypaul, S.; Kelly, F.S.; King, M.A. Turning pain into gain: Evaluation of a multidisciplinary chronic pain management pro-gram in primary care. Pain Med. 2019, 20, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Casey, M.; Smart, K.; Segurado, R.; Doody, C. Multidisciplinary-based Rehabilitation (MBR) Compared with Active Physical Interventions for Pain and Disability in Adults With Chronic Pain A Systematic Review and Meta-analysis. Clin. J. Pain 2020, 36, 874–886. [Google Scholar] [CrossRef]
- Sumner, L.A.; IsHak, W.W.; Dang, J.; Vanle, B.; Mahtani, N.; Danovitch, I. Psychological interventions in inpatient medical settings: A brief review. Int. J. Healthc. Med. Sci. 2018, 4, 73–83. [Google Scholar]
- Davis, J.P.; Prindle, J.J.; Eddie, D.; Pedersen, E.R.; Dumas, T.M.; Christie, N.C. Addressing the opioid epidemic with be-havioral interventions for adolescents and young adults: A quasi-experimental design. J. Consult. Clin. Psychol. 2019, 87, 941–951. [Google Scholar] [CrossRef]
- Vassilopoulou, E.; Efthymiou, D.; Papatriantafyllou, E.; Markopoulou, M.; Sakellariou, E.M.; Popescu, A.C. Long Term Metabolic and Inflammatory Effects of Second-Generation Antipsychotics: A Study in Mentally Disordered Offenders. J. Pers. Med. 2021, 11, 1189. [Google Scholar] [CrossRef]
- Tagliaferri, S.D.; Miller, C.T.; Owen, P.J.; Mitchell, U.H.; Brisby, H.; Fitzgibbon, B.; Masse-Alarie, H.; Van Oosterwijck, J.; Belavy, D.L. Domains of chronic low back pain and assessing treatment effectiveness: A clinical perspective. Pain Pract. 2020, 20, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Jack, K.; McLean, S.M.; Moffett, J.K.; Gardiner, E. Barriers to treatment adherence in physiotherapy outpatient clinics: A sys-tematic review. Man. Ther. 2010, 15, 220–228. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Jiang, J.; Xu, X.; Wu, J.; Xu, Y.; Lin, X.; Hall, J.; Xu, H.; Xu, J.; et al. The effect of cognitive behavioral therapy on depression, anxiety, and stress in patients with COVID-19: A randomized controlled trial. Front. Psychiatry 2020, 11, 580827. [Google Scholar] [CrossRef] [PubMed]
| SSI CBT | Standard CBT | |
|---|---|---|
| Advantages | Increased feasibility for the healthcare/ in-patient setting; Improved accessibility; Compact and comprehensive intervention; The provision of all protocol steps to each beneficiary; Efficient use of resources; Larger number of beneficiaries in a shorter amount of time. | Detailed approach to each protocol step; Improved therapeutic rapport and continuity of the intervention; More time for results consolidation; Facilitation of follow-up and progress assessment. |
| Potential Disadvantages | Fewer time dedicated to each protocol step, including relapse prevention. | Lower compliance and motivation. |
| Time (Average length) | Between 40–50 min and 8 h | 4–12 h |
| Cost | Low | High |
| Limitations | Difficulties related to longitudinal and follow-up assessments. | Accessibility barriers and time constraints. |
| Attrition | Low | High |
| Required qualifications | Formal certification in Cognitive-Behavioral Therapy | Formal certification in Cognitive-Behavioral Therapy |
| Topic | Description |
|---|---|
| 1. Dealing with pain | The tendency to avoid difficult internal experiences, including pain, as well as the consequences of this strategy were discussed. The acceptance alternative was introduced as a healthier way to cope with pain. |
| 2. Present moment awareness | Fewer time dedicated to each protocol step, including relapse prevention. This part was more practical, involving the use of a brief body scan mindfulness exercise. This practice focused on breathing and the acknowledgement of physical sensations. Also, the “expansion” technique was implemented, encouraging participants to create space for the pain, by delimitating it from other bodily parts and focusing their attention to various characteristics of this sensation. |
| 3. Practising cognitive defusion | The idea that people could get entangled in their thoughts, especially when confronted with difficult experiences was outlined. The concept of cognitive defusion was then explained to participants through practical examples. Specifically, the “Leaves on a stream” technique was applied to promote an observer stance and facilitate a functional attitude towards pain-related thoughts. |
| 4. Towards a values-based life | A discussion related to the role of personal values for developing a sense of meaning was initiated. For this purpose, a metaphor based on the “Batteries exercise” was presented, pointing to potential discrepancies between deeply held values and the behaviors in which participants may involve. Relying on this framework, a brief action plan based on the identified personal values focusing on the upcoming two weeks was developed for each participant. |
| Age (M, SD) | 58.9 (12.03) |
| Gender (N,%) | |
| Female | 29 (94) |
| Male | 2 (6) |
| Marital status | |
| Married | 25 (81) |
| Single | 1 (3) |
| Widowed | 5 (16) |
| Occupational status | |
| Employed | 4 (13) |
| Unemployed | 8 (26) |
| Retired | 19 (61) |
| Main diagnosis | |
| Ankylosing spondylitis | 1 (3) |
| Chronic post-surgical pain | 2 (7) |
| Coxarthrosis/Gonarthrosis | 2 (7) |
| Osteoarthritis | 9 (29) |
| Psoriatic arthritis | 1 (3) |
| Rheumatoid arthritis | 10 (32) |
| Spondylosis (cervical/lumbar) Psychodiagnosis | 6 (19) |
| GAD 1 | 16 (52) |
| MDD 2 | 3 (10) |
| GAD 1 + MDD 2 | 12 (38) |
| Pain intensity (M, SD) | 8.12 (1.78) |
| Fatigue | 8.09 (2) |
| Anxiety | 6.54 (2.89) |
| Depression | 6.35 (2.52) |
| Dimension | Pain | Fatigue | Anxiety | Depression | Experiential Avoidance | Cognitive Fusion |
|---|---|---|---|---|---|---|
| Hurt Connect | 0.40 * | −0.07 | 0.07 | −0.04 | 0.24 | 0.31 |
| Experience Range Emotions | −0.31 | 0.06 | −0.17 | −0.17 | −0.39 * | −0.36 * |
| Thinking Got In Way | 0.22 | 0.09 | 0.42 * | 0.13 | 0.33 | 0.30 |
| Important Challenge | −0.08 | 0.49 ** | 0.01 | 0.23 | −0.10 | −0.10 |
| Stuck Unable Change | −0.08 | −0.37 * | −0.08 | −0.21 | −0.11 | −0.10 |
| Thinking Helped Life | −0.29 | 0.18 | −0.13 | 0.04 | −0.41 * | −0.35 * |
| Struggle Connect Moments | −0.23 | 0.27 | −0.20 | −0.06 | −0.35 * | −0.30 |
| Connect To People | −0.12 | 0.41 * | −0.10 | −0.06 | −0.29 | −0.33 |
| Personal Import | −0.09 | 0.41 * | −0.09 | 0.13 | −0.22 | −0.16 |
| Hurt Health | 0.17 | −0.01 | 0.11 | 0.11 | 0.45 ** | 0.60 ** |
| Pain | - | 0.34 | 0.40 * | 0.24 | 0.29 | 0.43 * |
| Fatigue | - | 0.23 | 0.41 * | 0.07 | 0.02 | |
| State Anxiety | - | 0.14 | 0.37 * | 0.28 | ||
| State Depression | - | 0.16 | 0.18 | |||
| Experiential Avoidance | - | 0.60 ** |
| Outcome Measure T1 | Mean (SD) | t (30) | p Value | Effect Size | ||
|---|---|---|---|---|---|---|
| Pre-Test | Post-Test T1 | (Cohen’s d) | Hedges’ g | |||
| Cognitive Fusion | 28.19 (17.77) | 17.77 (6.58) | 9.96 | 0.001 | 1.78 (1.21; 2.35) | 1.74 (1.18; 2.29) |
| Experiential avoidance | 28.06 (6.86) | 19.84 (6.38) | 7.79 | 0.001 | 1.4 (0.89; 1.89) | 1.36 (0.87; 1.84) |
| Able To Change Behavior (PBAT_1) | 64.84 (22.19) | 81.61 (20.67) | −4.37 | 0.001 | −0.78 (−1.18; −0.37) | −0.76 (−1.15; −0.36) |
| Helped Health (PBAT_6) | 60.00 (24.76) | 79.68 (19.40) | −4.32 | 0.001 | −0.77 (−1.17; −0.36) | −0.75 (−1.14; −0.36) |
| Complying (PBAT_9) | 61.61 (27.21) | 41.94 (22.12) | 3.86 | 0.001 | 0.69 (0.29; 1.08) | 0.67 (0.28; 1.05) |
| Stuck To Working Strategies (PBAT_10) | 45.81 (29.18) | 28.39 (25.04) | 3.27 | 0.003 | 0.58 (0.2; 0.96) | 0.57 (0.19; 0.94) |
| Hurt Health (PBAT_17) | 45.16 (25.41) | 25.48 (22.03) | 4.05 | 0.001 | 0.72 (0.32; 1.12) | 0.71 (0.31; 1.09) |
| Outcome Measure T1 | N (%) | |
|---|---|---|
| Mild Improvement (≥25%) | Moderate Improvement (≥50%) | |
| Cognitive Fusion | 16 (52) | 9 (29) |
| Experiential avoidance | 15 (49) | 5 (16) |
| Able To Change Behavior (PBAT_1) | 8 (26) | 4 (13) |
| Helped Health (PBAT_6) | 9 (29) | 8 (26) |
| Complying (PBAT_9) | 5 (16) | 8 (26) |
| Stuck To Working Strategies (PBAT_10) | 6 (19) | 12 (39) |
| Hurt Health (PBAT_17) | 3 (10) | 13 (42) |
| Outcome Measure | Mean (SD) | Before Imputation Analysis | After Imputation Analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Test | Post-Test | Effect Size | Effect Size | |||||||
| t (26) | p Value | (Cohen’s d) | Hedges’ g | t (30) | p Value | (Cohen’s d) | Hedges’ g | |||
| Pain | 8.26 (1.65) | 5.70 (2.52) | 6.43 | 0.001 | 1.23 (0.72; 1.73) | 1.2 (0.7; 1.68) | 6.21 | 0.001 | 1.11 (0.65; 1.56) | 1.08 (0.64; 1.52) |
| Fatigue | 7.96 (2.06) | 7.33 (2.03) | 1.55 | 0.174 | - | - | 1.97 | 0.057 | - | - |
| Anxiety | 7.11 (2.57) | 4.96 (2.68) | 4.45 | 0.001 | 0.85 (0.4; 1.29) | 0.83 (0.39; 1.25) | 3.08 | 0.004 | 0.55 (0.17; 0.93) | 0.54 (0.16; 0.9) |
| Depression | 6.26 (2.56) | 4.15 (2.69) | 3.43 | 0.002 | 0.66 (0.23; 1.04) | 0.64 (0.23; 1.04) | 3.96 | 0.001 | 0.72 (0.31; 1.12) | 0.7 (0.31; 1.08) |
| Estimate | 95% CI | SE | |
|---|---|---|---|
| Cognitive Fusion | 10.41 | 8.28; 12.55 | 1.04 |
| Experiential avoidance | 8.22 | 6.07; 10.38 | 1.05 |
| Able To Change Behavior (PBAT_1) | −16.77 | −24.6; −8.94 | 3.83 |
| Helped Health (PBAT_6) | −19.67 | −28.98; −10.37 | 4.55 |
| Complying (PBAT_9) | 19.67 | 9.28; 30.07 | 5.09 |
| Stuck To Working Strategies (PBAT_10) | 17.41 | 6.54; 28.29 | 5.32 |
| Hurt Health (PBAT_17) | 19.67 | 9.76; 29.58 | 4.85 |
| Pain | 2.38 | 1.6; 3.17 | 0.38 |
| Anxiety | 1.58 | 0.53; 2.62 | 0.51 |
| Depression | 2.25 | 1.05; 3.36 | 0.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cojocaru, C.-M.; Popa, C.O.; Schenk, A.; Jakab, Z.; Suciu, B.A.; Olah, P.; Popoviciu, H.; Szasz, S. A Single-Session Process-Based Cognitive-Behavioral Intervention Combined with Multimodal Rehabilitation Treatment for Chronic Pain Associated with Emotional Disorders. Behav. Sci. 2024, 14, 327. https://doi.org/10.3390/bs14040327
Cojocaru C-M, Popa CO, Schenk A, Jakab Z, Suciu BA, Olah P, Popoviciu H, Szasz S. A Single-Session Process-Based Cognitive-Behavioral Intervention Combined with Multimodal Rehabilitation Treatment for Chronic Pain Associated with Emotional Disorders. Behavioral Sciences. 2024; 14(4):327. https://doi.org/10.3390/bs14040327
Chicago/Turabian StyleCojocaru, Cristiana-Manuela, Cosmin Octavian Popa, Alina Schenk, Zsolt Jakab, Bogdan Andrei Suciu, Peter Olah, Horațiu Popoviciu, and Simona Szasz. 2024. "A Single-Session Process-Based Cognitive-Behavioral Intervention Combined with Multimodal Rehabilitation Treatment for Chronic Pain Associated with Emotional Disorders" Behavioral Sciences 14, no. 4: 327. https://doi.org/10.3390/bs14040327
APA StyleCojocaru, C.-M., Popa, C. O., Schenk, A., Jakab, Z., Suciu, B. A., Olah, P., Popoviciu, H., & Szasz, S. (2024). A Single-Session Process-Based Cognitive-Behavioral Intervention Combined with Multimodal Rehabilitation Treatment for Chronic Pain Associated with Emotional Disorders. Behavioral Sciences, 14(4), 327. https://doi.org/10.3390/bs14040327








