Age Three: Milestone in the Development of Cognitive Flexibility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Materials and Design
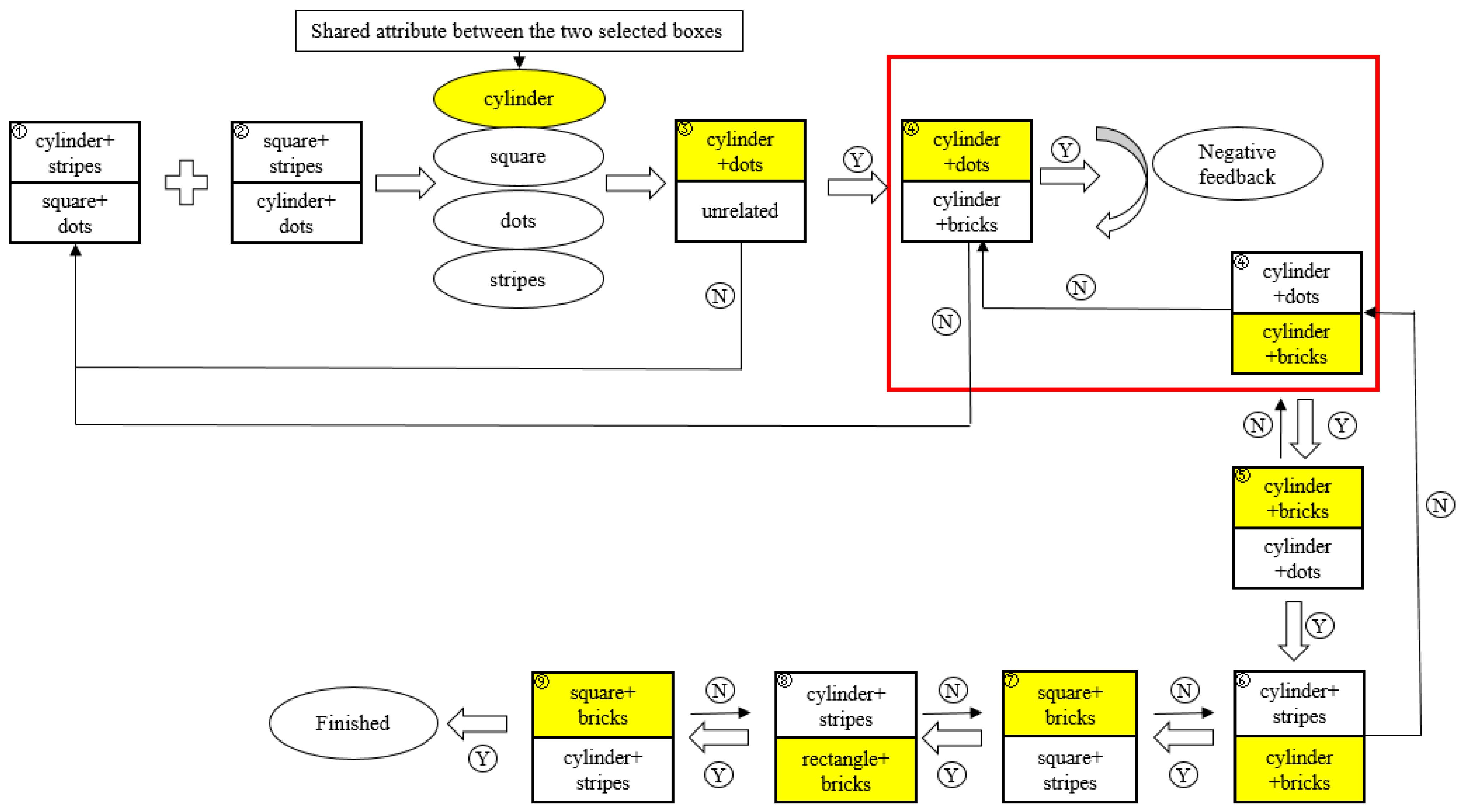
2.3. Procedure
2.3.1. Practice Session
2.3.2. Test Session
2.4. Scoring and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Howard, S.J.; Okely, A.D.; Ellis, Y.G. Evaluation of a differentiation model of preschoolers’ executive functions. Front. Psychol. 2015, 6, 285. [Google Scholar] [CrossRef]
- Moriguchi, Y.; Shinohara, I.; Todo, N.; Meng, X. Prosocial behavior is related to later executive function during early childhood: A longitudinal study. Eur. J. Dev. Psychol. 2019, 17, 352–364. [Google Scholar] [CrossRef]
- Farrant, B.M.; Fletcher, J.; Maybery, M.T. Cognitive Flexibility, Theory of Mind, and Hyperactivity/Inattention. Child Dev. Res. 2014, 2014, 741543. [Google Scholar] [CrossRef]
- Hall-McMaster, S.; Muhle-Karbe, P.S.; Myers, N.E.; Stokes, M.G. Reward Boosts Neural Coding of Task Rules to Optimize Cognitive Flexibility. J. Neurosci. 2019, 39, 8549–8561. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Feng, T. The continuous impact of cognitive flexibility on the development of emotion understanding in children aged 4 and 5 years: A longitudinal study. J. Exp. Child Psychol. 2020, 203, 105018. [Google Scholar] [CrossRef] [PubMed]
- Zajączkowska, M.; Abbot-Smith, K. “Sure I’ll help—I’ve just been sitting around doing nothing at school all day”: Cognitive flexibility and child irony interpretation. J. Exp. Child Psychol. 2020, 199, 104942. [Google Scholar] [CrossRef]
- Cragg, L.; Chevalier, N. The processes underlying flexibility in childhood. Q. J. Exp. Psychol. 2012, 65, 209–232. [Google Scholar] [CrossRef] [PubMed]
- Waring, R.; Liow, S.R.; Dodd, B.; Eadie, P. Differentiating phonological delay from phonological disorder: Executive function performance in preschoolers. Int. J. Lang. Commun. Disord. 2022, 57, 288–302. [Google Scholar] [CrossRef] [PubMed]
- Frye, D.; Zelazo, P.D.; Palfai, T. Theory of mind and rule-based reasoning. Cogn. Dev. 1995, 10, 483–527. [Google Scholar] [CrossRef]
- Moriguchi, Y. Relationship between cool and hot executive function in young children: A near-infrared spectroscopy study. Dev. Sci. 2022, 25, e13165. [Google Scholar] [CrossRef]
- Zefazo, P.D.; Frye, D.; Rapus, T. An Agem Reaed Dissociation between Knowing Rules and Using Them. Cogn. Dev. 1996, 11, 37–63. [Google Scholar]
- Doebel, S.; Zelazo, P.D. A meta-analysis of the Dimensional Change Card Sort: Implications for developmental theories and the measurement of executive function in children. Dev. Rev. 2015, 38, 241–268. [Google Scholar] [CrossRef]
- Kalstabakken, A.W.; Desjardins, C.D.; Anderson, J.E.; Berghuis, K.J.; Hillyer, C.K.; Seiwert, M.J.; Carlson, S.M.; Zelazo, P.D.; Masten, A.S. Executive function measures in early childhood screening: Concurrent and predictive validity. Early Child. Res. Q. 2021, 57, 144–155. [Google Scholar] [CrossRef]
- Kirkham, N.Z.; Cruess, L.; Diamond, A. Helping children apply their knowledge to their behavior on a dimension-switching task. Dev. Sci. 2003, 6, 449–467. [Google Scholar] [CrossRef]
- Kloo, D.; Perner, J. Disentangling dimensions in the dimensional change card-sorting task. Dev. Sci. 2004, 8, 44–56. [Google Scholar] [CrossRef]
- Marcovitch, S.; Boseovski, J.J.; Knapp, R.J. Use it or lose it: Examining preschoolers’ difficulty in maintaining and executing a goal. Dev. Sci. 2007, 10, 559–564. [Google Scholar] [CrossRef]
- Morton, J.B.; Munakata, Y. Active versus latent representations: A neural network model of perseveration, dissociation, and decalage. Dev. Psychobiol. 2002, 40, 255–265. [Google Scholar] [CrossRef]
- Muller, U.; Dick, A.S.; Gela, K.; Overton, W.F.; Zelazo, P.D. The Role of Negative Priming in Preschoolers’ Flexible Rule Use on the Dimensional Change Card Sort Task. Child Dev. 2006, 77, 395–412. [Google Scholar] [CrossRef] [PubMed]
- Zelazo, P.D.; Muller, U.; Frye, D.; Marcovitch, S.I. The Development of Executive Function in Early Childhood. Monogr. Soc. Res. Child Dev. 2003, 68, 11–27. [Google Scholar] [CrossRef]
- Landry, O.; Al-Taie, S.; Franklin, A. 3-Year-Olds’ Perseveration on the DCCS Explained: A Meta-Analysis. J. Cogn. Dev. 2017, 18, 419–440. [Google Scholar] [CrossRef]
- Jacques, S.; Zelazo, P.D. The Flexible Item Selection Task (FIST): A Measure of Executive Function in Preschoolers. Dev. Neuropsychol. 2001, 20, 573–591. [Google Scholar] [CrossRef] [PubMed]
- Smidts, D.P.; Jacobs, R.; Anderson, V. The Object Classification Task for Children (OCTC): A Measure of Concept Generation and Mental Flexibility in Early Childhood. Dev. Neuropsychol. 2004, 26, 385–401. [Google Scholar] [CrossRef] [PubMed]
- FitzGibbon, L.; Cragg, L.; Carroll, D.J. Primed to be inflexible: The influence of set size on cognitive flexibility during childhood. Front. Psychol. 2014, 5, 101. [Google Scholar] [CrossRef] [PubMed]
- Espy, K.A. The shape school: Assessing executive function in preschool children. Dev. Neuropsychol. 1997, 13, 495–499. [Google Scholar] [CrossRef]
- Ribeiro, F.; Cavaglia, R.; Rato, J.R. Sex differences in response inhibition in young children. Cogn. Dev. 2021, 58, 101047. [Google Scholar] [CrossRef]
- Clearfield, M.W.; Diedrich, F.J.; Smith, L.B.; Thelen, E. Young infants reach correctly in A-not-B tasks: On the development of stability and perseveration. Infant Behav. Dev. 2006, 29, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, K.; Bell, M.A. Developmental progression of looking and reaching performance on the A-not-B task. Dev. Psychol. 2010, 46, 1363–1371. [Google Scholar] [CrossRef]
- Garon, N.; Smith, I.M.; Bryson, S.E. A novel executive function battery for preschoolers: Sensitivity to age differences. Child Neuropsychol. 2013, 20, 713–736. [Google Scholar] [CrossRef]
- Johansson, M.; Marciszko, C.; Brocki, K.; Bohlin, G. Individual Differences in Early Executive Functions: A Longitudinal Study from 12 to 36 Months. Infant Child Dev. 2016, 25, 533–549. [Google Scholar] [CrossRef]
- Blakey, E.; Visser, I.; Carroll, D.J. Different Executive Functions Support Different Kinds of Cognitive Flexibility: Evidence From 2-, 3-, and 4-Year-Olds. Child Dev. 2016, 87, 513–526. [Google Scholar] [CrossRef]
- Smith, H.; Carter, A.S.; Blaser, E.; Kaldy, Z. Successful attentional set-shifting in 2-year-olds with and without Autism Spectrum Disorder. PLoS ONE 2019, 14, e0213903. [Google Scholar] [CrossRef] [PubMed]
- Gerstadt, C.L.; Hong, Y.J.; Diamond, A. The relationship between cognition and action: Performance of children 312–7 years old on a stroop- like day-night test. Cognition 1994, 53, 129–153. [Google Scholar] [CrossRef]
- Davidson, D.; Rainey, V.R.; Vanegas, S.B.; Hilvert, E. The effects of type of instruction, animacy cues, and dimensionality of objects on the shape bias in 3-to 6-year-old children. Infant Child Dev. 2018, 27, e2044. [Google Scholar] [CrossRef]
- Kimura, K.; Hunley, S.B.; Namy, L.L. Children’s use of comparison and function in novel object categorization. J. Exp. Child Psychol. 2018, 170, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, Z.; Cao, B.; Hu, L.; Zhang, Z. Children prefer pattern over shape during complex categorization. PsyCh J. 2020, 9, 819–831. [Google Scholar] [CrossRef] [PubMed]
- D’Cruz, A.-M.; Ragozzino, M.E.; Mosconi, M.W.; Shrestha, S.; Cook, E.H.; Sweeney, J.A. Reduced behavioral flexibility in autism spectrum disorders. Neuropsychology 2013, 27, 152–160. [Google Scholar] [CrossRef]
- Schmitt, L.M.; Sweeney, J.A.; Erickson, C.A.; Shaffer, R. Brief Report: Feasibility of the Probabilistic Reversal Learning Task as an Outcome Measure in an Intervention Trial for Individuals with Autism Spectrum Disorder. J. Autism Dev. Disord. 2022, 52, 4191–4199. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.M.; Moses, L.J. Individual Differences in Inhibitory Control and Children’s Theory of Mind. Child Dev. 2001, 72, 1032–1053. [Google Scholar] [CrossRef] [PubMed]
- Duggan, E.C.; Garcia-Barrera, M.A. Executive functioning and intelligence. In Handbook of Intelligence; Springer: New York, NY, USA, 2015; pp. 435–458. [Google Scholar]
- Carroll, D.J.; Blakey, E.; FitzGibbon, L. Cognitive Flexibility in Young Children: Beyond Perseveration. Child Dev. Perspect. 2016, 10, 211–215. [Google Scholar] [CrossRef]
- Halford, G.S.; Bunch, K.; McCredden, J. Problem decomposability as a factor in complexity of the dimensional change card sort task. Cogn. Dev. 2007, 22, 384–391. [Google Scholar] [CrossRef]
- Rennie, D.A.C.; Bull, R.; Diamond, A. Executive Functioning in Preschoolers: Reducing the Inhibitory Demands of the Dimensional Change Card Sort Task. Dev. Neuropsychol. 2004, 26, 423–443. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, N.; Sheffield, T.D.; Nelson, J.M.; Clark, C.A.C.; Wiebe, S.A.; Espy, K.A. Underpinnings of the Costs of Flexibility in Preschool Children: The Roles of Inhibition and Working Memory. Dev. Neuropsychol. 2012, 37, 99–118. [Google Scholar] [CrossRef]
- Carpenter, M.; Nagell, K.; Tomasello, M.; Butterworth, G.; Moore, C. Social Cognition, Joint Attention, and Communicative Competence from 9 to 15 Months of Age. Monogr. Soc. Res. Child Dev. 1998, i-174. [Google Scholar] [CrossRef]
- Butterfuss, R.; Kendeou, P. The Role of Executive Functions in Reading Comprehension. Educ. Psychol. Rev. 2018, 30, 801–826. [Google Scholar] [CrossRef]
- Koepp, A.E.; Gershoff, E.T.; Castelli, D.M.; Bryan, A.E. Preschoolers’ executive functions following indoor and outdoor free play. Trends Neurosci. Educ. 2022, 28, 100182. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shields, G.S.; Zhang, Y.; Wu, H.; Chen, H.; Romer, A.L. Child executive function and future externalizing and internalizing problems: A meta-analysis of prospective longitudinal studies. Clin. Psychol. Rev. 2022, 97, 102194. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Y.-C.; Scalise, N.R. Numeracy skills mediate the relation between executive function and mathematics achievement in early childhood. Cogn. Dev. 2022, 62, 101154. [Google Scholar] [CrossRef]
- Miller, E.K.; Cohen, J.D. An Integrative Theory of Prefrontal Cortex Function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef]
- Xie, S.; Gong, C.; Lu, J.; Zhang, H.; Wu, D.; Chi, X.; Li, H.; Chang, C. An fNIRS Study of Applicability of the Unity–Diversity Model of Executive Functions in Preschoolers. Brain Sci. 2022, 12, 1722. [Google Scholar] [CrossRef]
- Xie, S.; Wu, D.; Yang, J.; Luo, J.; Chang, C.; Li, H. An fNIRS examination of executive function in bilingual young children. Int. J. Biling. 2021, 25, 516–530. [Google Scholar] [CrossRef]
- Yates, T.S.; Ellis, C.T.; Turk-Browne, N.B. Emergence and organization of adult brain function throughout child development. NeuroImage 2021, 226, 117606. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, Y.; Hiraki, K. Neural origin of cognitive shifting in young children. Proc. Natl. Acad. Sci. USA 2009, 106, 6017–6021. [Google Scholar] [CrossRef]
- Moriguchi, Y.; Phillips, S. Evaluating the Distinction between Cool and Hot Executive Function during Childhood. Brain Sci. 2023, 13, 313. [Google Scholar] [CrossRef]
- Chen, B.; Linke, A.; Olson, L.; Ibarra, C.; Kinnear, M.; Fishman, I. Resting state functional networks in 1-to-3-year-old typically developing children. Dev. Cogn. Neurosci. 2021, 51, 100991. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, J.; Chen, Y.; Salzwedel, A.; Cornea, E.; Gilmore, J.H.; Gao, W. Developmental heatmaps of brain functional connectivity from newborns to 6-year-olds. Dev. Cogn. Neurosci. 2021, 50, 100976. [Google Scholar] [CrossRef]
- Hendry, A.; Jones, E.J.; Charman, T. Executive function in the first three years of life: Precursors, predictors and patterns. Dev. Rev. 2016, 42, 1–33. [Google Scholar] [CrossRef]
- Schneider, N.; Greenstreet, E.; Deoni, S.C.L. Connecting inside out: Development of the social brain in infants and toddlers with a focus on myelination as a marker of brain maturation. Child Dev. 2022, 93, 359–371. [Google Scholar] [CrossRef]
- Short, S.J.; Willoughby, M.T.; Camerota, M.; Stephens, R.L.; Steiner, R.J.; Styner, M.; Gilmore, J.H. Individual differences in neonatal white matter are associated with executive function at 3 years of age. Brain Struct. Funct. 2019, 224, 3159–3169. [Google Scholar] [CrossRef]
- Tau, G.Z.; Peterson, B.S. Normal Development of Brain Circuits. Neuropsychopharmacology 2010, 35, 147–168. [Google Scholar] [CrossRef] [PubMed]
- Fiske, A.; Holmboe, K. Neural substrates of early executive function development. Dev. Rev. 2019, 52, 42–62. [Google Scholar] [CrossRef] [PubMed]
- DeLoache, J.S.; Sugarman, S.; Brown, A.L. The Development of Error Correction Strategies in Young Children’s Manipulative Play. Child Dev. 1985, 56, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Piotti, P.; Szabó, D.; Bognár, Z.; Egerer, A.; Hulsbosch, P.; Carson, R.S.; Kubinyi, E. Effect of age on discrimination learning, reversal learning, and cognitive bias in family dogs. Learn. Behav. 2018, 46, 537–553. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. The functions of the orbitofrontal cortex. Brain Cogn. 2004, 55, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Marulis, L.M.; Palincsar, A.S.; Berhenke, A.L.; Whitebread, D. Assessing metacognitive knowledge in 3–5 year olds: The development of a metacognitive knowledge interview (McKI). Metacognit. Learn. 2016, 11, 339–368. [Google Scholar] [CrossRef]
- Urban, K.; Urban, M. Influence of Fluid Intelligence on Accuracy of Metacognitive Monitoring in Preschool Children Fades with the Calibration Feedback. Stud. Psychol. 2018, 60, 123–136. [Google Scholar] [CrossRef]
- Ansari, A.; Pianta, R.C.; Whittaker, J.V.; Vitiello, V.E.; Ruzek, E.A. Starting Early: The Benefits of Attending Early Childhood Education Programs at Age 3. Am. Educ. Res. J. 2019, 56, 1495–1523. [Google Scholar] [CrossRef]
- Gupta, N.D.; Simonsen, M. Non-cognitive child outcomes and universal high quality child care. J. Public Econ. 2010, 94, 30–43. [Google Scholar] [CrossRef]
- Preskitt, J.; Johnson, H.; Becker, D.; Ernest, J.; Fifolt, M.; Adams, J.; Strichik, T.; Ross, J.; Sen, B. The persistence of reading and math proficiency: The benefits of Alabama’s pre-kindergarten program endure in elementary and middle school. Int. J. Child Care Educ. Policy 2020, 14, 1–12. [Google Scholar] [CrossRef]
- Bassok, D.; Gibbs, C.R.; Latham, S. Preschool and Children’s Outcomes in Elementary School: Have Patterns Changed Nationwide Between 1998 and 2010? Child Dev. 2019, 90, 1875–1897. [Google Scholar] [CrossRef]
- Loeb, S.; Bridges, M.; Fuller, B.; Rumberger, R.; Bassok, D. How Much is Too Much? The Influence of Preschool Centers on Children’s Social and Cognitive Development; National Bureau of Economic Research: Cambridge, MA, USA, 2005. [Google Scholar] [CrossRef]
- Zachrisson, H.D.; Owen, M.T.; Nordahl, K.B.; Ribeiro, L.; Dearing, E. Too Early for Early Education? Effects on Parenting for Mothers and Fathers. J. Marriage Fam. 2021, 83, 683–698. [Google Scholar] [CrossRef]


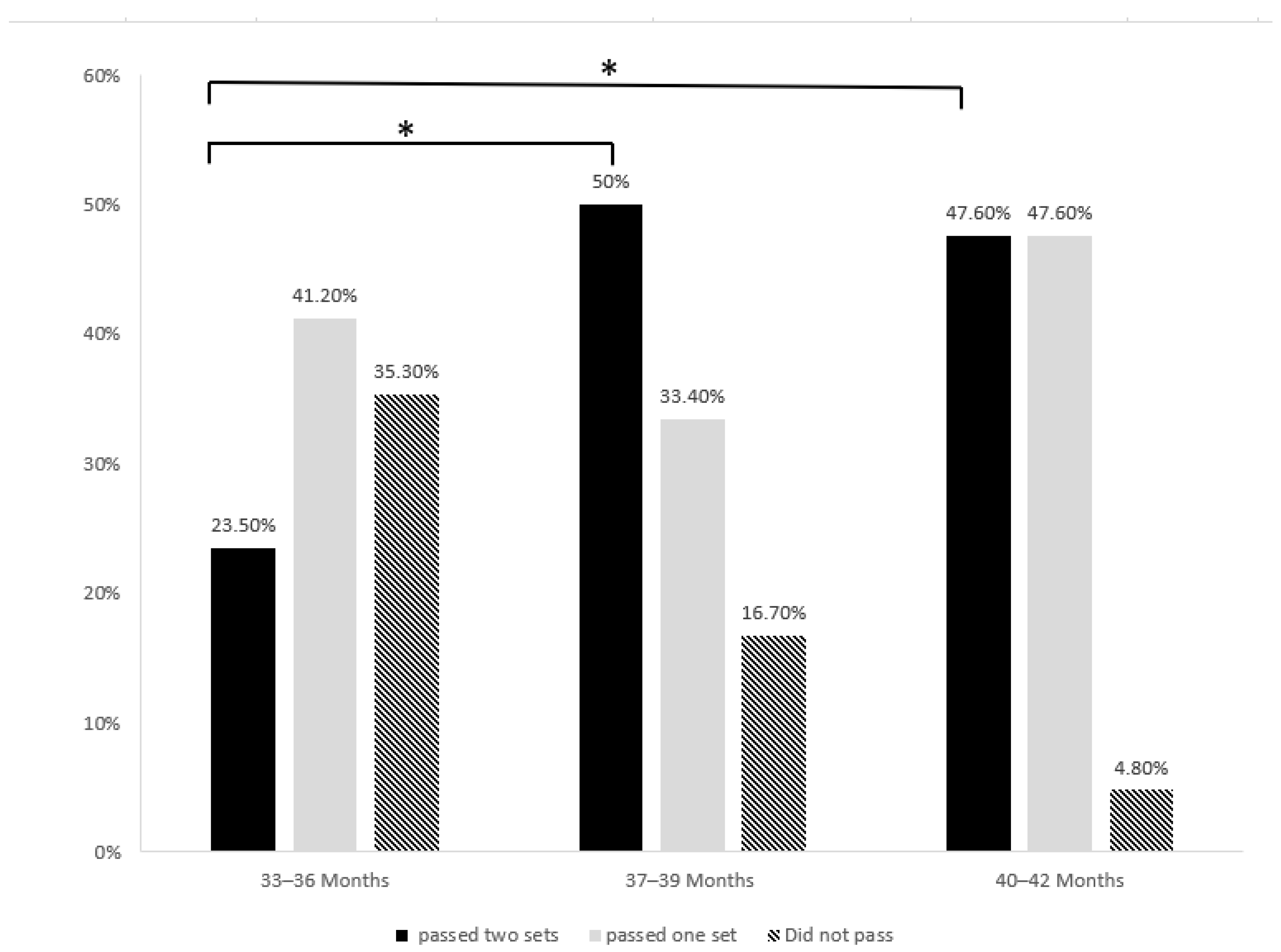
| Age (Month) | M | SD | F | p | |
|---|---|---|---|---|---|
| Step 5 (Rule switching) | 33–36 | 2.29 | 2.392 | 9.200 | <0.001 ** |
| 37–39 | 0.67 | 0.840 | |||
| 40–42 | 0.38 | 0.590 | |||
| Step 7 (Rule generalization) | 33–36 | 0.94 | 1.144 | 3.591 | 0.034 * |
| 37–39 | 0.33 | 0.594 | |||
| 40–42 | 0.29 | 0.644 | |||
| The total number of trial-and-errors of Steps 5–9 | 33–36 | 6.06 | 5.202 | 5.118 | 0.009 * |
| 37–39 | 3.22 | 2.315 | |||
| 40–42 | 2.86 | 1.526 |
| Age (Month) | M | SD | χ2 | p | |
|---|---|---|---|---|---|
| Step 5 (Rule switching) | 33–36 | 1.00 | 0.791 | 7.596 | 0.022 * |
| 37–39 | 1.44 | 0.705 | |||
| 40–42 | 1.67 | 0.483 | |||
| Step 7 (Rule generalization) | 33–36 | 1.29 | 0.772 | 5.328 | 0.070 |
| 37–39 | 1.67 | 0.594 | |||
| 40–42 | 1.76 | 0.539 | |||
| The total score of Steps 5–9 | 33–36 | 6.47 | 2.125 | 3.421 | 0.181 |
| 37–39 | 7.50 | 1.917 | |||
| 40–42 | 7.67 | 0.913 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, C.; Cai, H.; Li, F. Age Three: Milestone in the Development of Cognitive Flexibility. Behav. Sci. 2024, 14, 578. https://doi.org/10.3390/bs14070578
Wan C, Cai H, Li F. Age Three: Milestone in the Development of Cognitive Flexibility. Behavioral Sciences. 2024; 14(7):578. https://doi.org/10.3390/bs14070578
Chicago/Turabian StyleWan, Chufan, Hui Cai, and Fuhong Li. 2024. "Age Three: Milestone in the Development of Cognitive Flexibility" Behavioral Sciences 14, no. 7: 578. https://doi.org/10.3390/bs14070578





