In Males with Adequate Dietary Needs Who Present No Sleep Disturbances, Is an Acute Intake of Zinc Magnesium Aspartate, Following Either Two Consecutive Nights of 8 or 4 h of Sleep Deprivation, Beneficial for Sleep and Morning Stroop Interference Performance?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Research Design
2.3. Measurements
2.4. Statistical Analysis
3. Results
3.1. Evening Physiological and Psychological Variables
3.2. Measures of Sleep
3.2.1. Actigraphy Variables
3.2.2. Waterhouse and Stanford Sleepiness Sleep Questionnaires
3.3. Profile of Mood State
3.4. Stroop (Colour–Word, Word–Colour Interference Test)
3.5. Pearson Correlations between Sleep Difference (h) and Mg or Zn Levels (Either mg or mg/kg Body Mass) for ZMA-NoPill for 8 h Sleep Condition
4. Discussion
4.1. Limitations
4.2. Conclusions
5. Practical Implications and Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watson, N.F.; Badr, M.S.; Belenky, G.; Bliwise, D.L.; Buxton, O.M.; Buysse, D.; Dinges, D.F.; Gangwisch, J.; Grandner, M.A.; Kushida, C.; et al. Recommended Amount of Sleep for a Healthy Adult: A Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep 2015, 38, 843–844. [Google Scholar] [CrossRef] [PubMed]
- Everson, C.A.; Crowley, W.R. Reductions in circulating anabolic hormones induced by sustained sleep deprivation in rats. American journal of physiology. Endocrinol. Metab. 2004, 286, E1060–E1070. [Google Scholar] [CrossRef]
- Edgar, D.T.; Gill, N.D.; Beaven, C.M.; Zaslona, J.L.; Driller, M.W. Sleep duration and physical performance during a 6-week military training course. J. Sleep Res. 2021, 30, e13393. [Google Scholar] [CrossRef]
- Teece, A.R.; Argus, C.K.; Gill, N.; Beaven, M.; Dunican, I.C.; Driller, M.W. Sleep and Performance during a Preseason in Elite Rugby Union Athletes. Int. J. Environ. Res. Public Health 2021, 18, 4612. [Google Scholar] [CrossRef]
- Walsh, N.P. Nutrition and Athlete Immune Health: New Perspectives on an Old Paradigm. Sports Med. 2019, 49, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Banks, S.; Dinges, D.F. Behavioral and physiological consequences of sleep restriction. J. Clin. Sleep Med. 2007, 3, 519–528. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thun, E.; Bjorvatn, B.; Flo, E.; Harris, A.; Pallesen, S. Sleep, circadian rhythms, and athletic performance. Sleep Med. Rev. 2015, 23, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Edwards, B.J.; Gallagher, C.; Pullinger, S.A.; de Mello, T.M.; Walsh, N.P. Athletic Performance; Effects of Sleep Loss. In The Encyclopedia of Sleep and Circadian Rhythms, 2nd ed.; Kushida, C.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 434–443. [Google Scholar]
- Gallagher, C.; Green, C.E.; Kenny, M.L.; Evans, J.R.; McCullagh, G.D.W.; Pullinger, S.A.; Edwards, B.J. Is implementing a post-lunch nap beneficial on evening performance, following two nights partial sleep restriction? Chronobiol. Int. 2023, 40, 1169–1186. [Google Scholar] [CrossRef]
- Walsh, N.P.; Halson, S.L.; Sargent, C.; Roach, G.D.; Nédélec, M.; Gupta, L.; Leeder, J.; Fullagar, H.H.; Coutts, A.J.; Edwards, B.J.; et al. Sleep and the athlete: Narrative review and 2021 expert consensus recommendations. Br. J. Sports Med. 2021, 55, 356–368. [Google Scholar] [CrossRef]
- Fullagar, H.H.K.; Skorski, S.; Duffield, R.; Hammes, D.; Coutts, A.J.; Meyer, T. Sleep and Athletic Performance: The Effects of Sleep Loss on Exercise Performance, and Physiological and Cognitive Responses to Exercise. Sports Med. 2015, 45, 161–186. [Google Scholar] [CrossRef]
- Stroop, J.R. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935, 18, 643–662. [Google Scholar] [CrossRef]
- Scarpina, F.; Tagini, S. The Stroop Colour and Word Test. Front. Psychol. 2017, 8, 557. [Google Scholar] [CrossRef] [PubMed]
- Brilla, L.R.; Conte, V. Effects of a Novel Zinc-Magnesium Formulation on Hormones and Strength. J. Exerc. Physiol. Online 2000, 3, 26–36. [Google Scholar]
- Wilborn, C.D.; Kerksick, C.M.; Campbell, B.I.; Taylor, L.W.; Marcello, B.M.; Rasmussen, C.J.; Greenwood, M.C.; Almada, A.; Kreider, R.B. Effects of Zinc Magnesium Aspartate (ZMA) Supplementation on Training Adaptations and Markers of Anabolism and Catabolism. J. Int. Soc. Sports Nutr. 2004, 1, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Moëzzi, N.; Peeri, M.; Homaei, H. Effects of zinc, magnesium and vitamin B6 supplementation on hormones and performance in weightlifters. Ann. Biol. Res. 2013, 4, 163–168. [Google Scholar]
- Ikonte, C.J.; Mun, J.G.; Reider, C.A.; Grant, R.W.; Mitmesser, S.H. Micronutrient Inadequacy in Short Sleep: Analysis of the NHANES 2005-2016. Nutrients 2019, 11, 2335. [Google Scholar] [CrossRef]
- Deuster, P.A.; Hodgson, A.B.; Steer, S.J.; Burke, L.M.; Castell, L.M. A–Z of nutritional supplements: Dietary supplements, sports nutrition foods and ergogenic aids for health and performance: Part 46. british J. Sports Med. 2013, 47, 809–810. [Google Scholar] [CrossRef]
- Ji, X.; Grandner, M.A.; Liu, J. The relationship between micronutrient status and sleep patterns: A systematic review. Public Health Nutr. 2017, 20, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, C.; Austin, V.; Dunlop, K.; Dally, J.; Taylor, K.; Pullinger, S.; Edwards, B.J. Effects of supplementing zinc magnesium aspartate on sleep quality and submaximal weightlifting performance, following two consecutive nights of partial sleep deprivation. Nutrients 2024, 16, 251. [Google Scholar] [CrossRef]
- Koehler, K.; Parr, M.K.; Geyer, H.; Master, J.; Schanzer, W. Serum testosterone and urinary excretion of steroid hormone metabolites after administration of a high-dose zinc supplement. Eur. J. Clin. Nutr. 2007, 63, 65–70. [Google Scholar] [CrossRef]
- Thompson, B.M.; Hillebrandt, H.L.; Sculley, D.V.; Barba-Moreno, L.; Janse de Jonge, X.A.K. The acute effect of the menstrual cycle and oral contraceptive cycle on measures of body composition. Eur. J. Appl. Physiol. 2021, 121, 3051–3059. [Google Scholar] [CrossRef] [PubMed]
- McKay, A.K.A.; Stellingwerff, T.; Smith, E.S.; Martin, D.T.; Mujika, I.; Goosey-Tolfrey, V.L.; Sheppard, J.; Burke, L.M. Defining Training and Performance Caliber: A Participant Classification Framework. Int. J. Sports Physiol. Perform. 2022, 17, 317–331. [Google Scholar] [CrossRef]
- Landrum, R.E. College students’ use of caffeine and its relationship to personality. Coll. Stud. J. 1992, 26, 151–155. [Google Scholar]
- Chisholm, D.M.; Collis, M.L.; Kulak, L.L.; Davenport, W.; Gruber, N. Physical activity readiness. BC Med. J. 1975, 17, 375–378. [Google Scholar]
- Smith, C.S.; Reilly, C.; Midkiff, K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J. Appl. Psychol. 1989, 74, 728. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.nhs.uk/conditions/vitamins-and-minerals/others/#:~:text=You%20should%20be%20able%20to%20get%20all%20the%20zinc%20you,advised%20to%20by%20a%20doctor (accessed on 2 May 2024).
- Boonstra, A.M.; Kooij, S.J.J.; Oosterlaan, J.; Sergeant, J.A.; Buitelaar, J.K.; Van Someren, E.J.W. Hyperactive night and day? Actigraphy studies in adult ADHD: A baseline comparison and the effect of methylphenidate. Sleep 2007, 30, 434–442. [Google Scholar] [CrossRef]
- Available online: https://urldefense.com/v3/__https://www.nutritics.com/p/home__;!!IhKztkE!fx47_Fdcc2TYhPM_WH3-eInducvJrQbeTlXvBKAc0HEIT737ACOYRlSjfmLUuyhdCoqBNGOe9pdn-jjPyaSkCqIqteU$ (accessed on 5 July 2024).
- Terry, P.C.; Lane, A.M.; Fogarty, G.J. Construct validity of the POMS-A for use with adults. Psychol. Sport Exerc. 2003, 4, 125–139. [Google Scholar] [CrossRef]
- Hoddes, E.; Zarcone, V.; Smythe, H.; Phillips, R.; Dement, W.C. Quantification of sleepiness: A new approach. Psychophysiology 1973, 10, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, J.; Reilly, T.; Atkinson, G.; Edwards, B. Jet lag: Trends and coping strategies. Lancet 2007, 369, 1117–1129. [Google Scholar] [CrossRef]
- Edwards, B.J.; Harris, C.; Jackman, Z.; Linares-Alcolea, R.; Matta, R.; Pullinger, S.A.; Doran, D.P. Is there a diurnal variation in 4-km cycling time trial performance, where a standardised approach has been employed? Biol. Rhythm 2024, 55, 184–209. [Google Scholar] [CrossRef]
- Valgimigli, S.; Padovani, R.; Budriesi, C.; Leone, M.E.; Lugli, D.; Nichelli, P. The Stroop test: A normative Italian study on a paper version for clinical use. G. Ital. Psicol. 2010, 37, 945–956. [Google Scholar] [CrossRef]
- Batterham, A.M.; Hopkins, W.G. Making meaningful inferences about magnitudes. Int. J. Sports Physiol. Perform. 2006, 1, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Siwek, M.; Dudek, D.; Paul, I.A.; Sowa-Kućma, M.; Zieba, A.; Popik, P.; Pilc, A.; Nowak, G. Zinc supplementation augments efficacy of imipramine in treatment resistant patients: A double blind, placebo-controlled study. J. Affect. Disord. 2009, 118, 187–195. [Google Scholar] [CrossRef]
- Sawada, T.; Yokoi, K. Effect of zinc supplementation on mood states in young women: A pilot study. Eur. J. Clin. Nutr. 2010, 64, 331–333. [Google Scholar] [CrossRef]
- Lai, J.; Moxey, A.; Nowak, G.; Vashum, K.; Bailey, K.; McEvoy, M. The efficacy of zinc supplementation in depression: Systematic review of randomised controlled trials. J. Affect. Disord. 2012, 136, e31–e39. [Google Scholar] [CrossRef]
- Wang, J.; Um, P.; Dickerman, B.A.; Liu, J. Zinc, Magnesium, Selenium and Depression: A Review of the Evidence, Potential Mechanisms and Implications. Nutrients 2018, 10, 584. [Google Scholar] [CrossRef] [PubMed]
- Munnilari, M.; Bommasamudram, T.; Easow, J.; Tod, D.; Varamenti, E.; Edwards, B.J.; Ravindrakumar, A.; Gallagher, C.; Pullinger, S. Diurnal variation in variables related to cognitive performance: A systematic review. Sleep Breath. 2023, 28, 495–510. [Google Scholar] [CrossRef]
- Williams, H.L.; Lubin, A.; Goodnow, J.J. Impaired performance with acute sleep loss. Psychol. Monogr. Gen. Appl. 1959, 73, 1–26. [Google Scholar] [CrossRef]
- Doran, S.M.; Van Dongen, H.P.; Dinges, D.F. Sustained attention performance during sleep deprivation: Evidence of state instability. Arch. Ital. Biol. 2001, 139, 253–267. [Google Scholar] [PubMed]
- Killgore, W.D.S. Effects of sleep deprivation on cognition. Prog. Brain Res. 2010, 185, 105–129. [Google Scholar] [CrossRef]
- Lingenfelser, T.; Kaschel, R.; Weber, A.; Zaiser-Kaschel, H.; Jakober, B.; Küper, J. Young hospital doctors after night duty: Their task-specific cognitive status and emotional condition. Med. Educ. 1994, 28, 566–572. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.E.; Waters, W.F. Decreased attentional responsivity during sleep deprivation: Orienting response latency, amplitude, and habituation. Sleep 1997, 20, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Binks, P.G.; Waters, W.F.; Hurry, M. Short-term total sleep deprivations does not selectively impair higher cortical functioning. Sleep 1999, 22, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Patricia Sagaspe, P.; Sanchez-Ortuno, M.; Charles, A.; Taillard, J.; Valtat, C.; Bioulac, B.; Philip, P. Effects of sleep deprivation on Colour-Word, Emotional, and Specific Stroop interference and on self-reported anxiety. Brain Cogn. 2006, 60, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Brotherton, E.J.; Moseley, S.E.; Langan-Evans, C.; Pullinger, S.A.; Robertson, C.M.; Burniston, J.G.; Edwards, B.J. Effects of two nights partial sleep deprivation on an evening submaximal weightlifting performance; are 1 h powernaps useful on the day of competition? Chronobiol. Int. 2019, 36, 407–426. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.H.; Tsai, P.S.; Fang, S.C.; Liu, J.F. Effect of kiwifruit consumption on sleep quality in adults with sleep problems. Asia Pac. J. Clin. Nutr. 2011, 20, 169–174. [Google Scholar] [PubMed]
- Saito, H.; Cherasse, Y.; Suzuki, R.; Mitarai, M.; Ueda, F.; Urade, Y. Zinc-rich oysters as well as zinc-yeast- and astaxanthin-enriched food improved sleep efficiency and sleep onset in a randomized controlled trial of healthy individuals. Mol. Nutr. Food Res. 2017, 61, 1600882. [Google Scholar] [CrossRef]
- Prasad, A.S. Zinc deficiency in women, infants and children. J. Am. Coll. Nutr. 1996, 15, 113–120. [Google Scholar] [CrossRef]
- Antonio, J.; Stout, J.R. Supplements for Endurance Athletes; Human Kinetics: Champaign, IL, USA, 2002. [Google Scholar]
- Cherasse, Y.; Urade, Y. Dietary Zinc Acts as a Sleep Modulator. Int. J. Mol. Sci. 2017, 18, 2334. [Google Scholar] [CrossRef]
- Arab, A.; Rafie, N.; Amani, R.; Shirani, F. The Role of Magnesium in Sleep Health: A Systematic Review of Available Literature. Biol. Trace Elem. Res. 2023, 201, 121–128. [Google Scholar] [CrossRef]
- Hu, J.; Jia, J.; Zhang, Y.; Miao, R.; Huo, X.; Ma, F. Effects of Vitamin D3 Supplementation on Cognition and Blood Lipids: A 12-Month Randomised, Double-Blind, Placebo-controlled Trial. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Pluss, M.A.; Bennett, K.J.M.; Novak, A.R.; Panchuk, D.; Coutts, A.J.; Fransen, J. Esports: The Chess of the 21st Century. Front. Psychol. 2019, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- Fekedulegn, D.; Andrew, M.E.; Shi, M.; Violanti, J.M.; Knox, S.; Innes, K.E. Actigraphy-Based Assessment of Sleep Parameters. Ann. Work Expo. Health 2020, 64, 350–367. [Google Scholar] [CrossRef] [PubMed]


| 4 h Sleep Group (n = 36) | 8 h Sleep Group (n = 33) | Control and Recommended Values | |
|---|---|---|---|
| Physical Characteristics | 36 males | 33 males | 18 males and 21 females |
| Age (yr) | 22.3 ± 1.8 | 23.1 ± 3.6 | 37.8 ± 9.5 # |
| Height (cm) | 176.2 ± 6.0 | 176.3 ± 7.1 | Not given # |
| Mass (kg) | 78.9 ± 10.6 | 78.9 ± 9.4 | Not given # |
| BMI (kg/m2) | 25.4 ± 2.4 | 25.3 ± 2.9 | 18.5-24.9 * |
| Baseline Actimetry | Control values | ||
| Fragmentation (Au) | 29.4 ± 13.3 | 29.2 ± 13.1 | 28.0 ± 1.0 # |
| Sleep efficiency (%) | 82.0 ± 6.9 | 82.2 ± 7.1 | 86.0 ± 1.0 # |
| Actual sleep time (h:mm) | 6:45 ± 00:45 | 6:42 ± 00:41 | 6:53 ± 00:06 # |
| Habitual retiring time (h:mm) | 23:01 ± 00:32 | 23:17 ± 00:36 | 24:33 ± 00:07 # |
| Habitual wake time (h:mm) | 7:09 ± 0:33 | 7:25 ± 0:36 | 8:24 ± 0:09 # |
| Sleep onset latency (h:mm) | 0:12 ± 00:09 | 0:11 ± 00:10 | 0:10 ± 00:01 # |
| Time in bed (h:mm) | 8:16 ± 00:31 | 8:08 ± 00:29 | 8:01 ± 0:07 # |
| Baseline Food Intake | RDA | ||
| Daily calories (kcal) | 2544 ± 692 | 2507 ± 648 | 2500 * |
| Fats (g) | 116 ± 78 | 117 ± 73 | 65 * |
| Protein (g) | 163 ± 60 | 149 ± 56 | 56 * |
| Carbohydrates (g) | 212 ± 93 | 215 ± 91 | 130 * |
| Zinc (mg) | 13.7 ± 7.1 | 12.9 ± 6.4 | 11 * |
| Magnesium (mg) | 450 ± 171 | 417 ± 124 | 400 * |
| Vitamin B6 (mg) | 2.9 ± 1.2 | 3.0 ± 1.0 | 8 * |
| Variable | Sleep 4 or 8 h | NoPill | PLAC | ZMA | Significance of between Effect for ‘Sleep Time’ | Significance of Main Effect for ‘Pill’ | Significance of Interaction |
|---|---|---|---|---|---|---|---|
| Actigraphy | |||||||
| Sleep onset latency (decimal min) | 4 | 8.6 ± 8.3 | 7.3 ± 8.0 | 8.4 ± 11.2 | F1.0, 67.0, 7.085, p = 0.010 | F1.7, 114.1, 0.731, p = 0.463 | F1.7, 114.1, 0.018, p = 0.971 |
| 8 | 13.9 ± 13.3 | 12.6 ± 10.8 | 14.1 ± 9.3 | ||||
| Sleep efficiency (%) | 4 | 83.8 ± 6.5 | 84.6 ± 7.3 | 85.0 ± 7.0 | F1.0, 67.0, 2.415, p = 0.125 | F1.7, 116.2, 0.639, p = 0.503 | F1.7, 116.2, 1.070, p = 0.337 |
| 8 | 82.1 ± 7.3 | 82.5 ± 7.6 | 81.4 ± 7.7 | ||||
| Actual sleep time (decimal h) | 4 | 3.4 ± 0.2 | 3.5 ± 0.3 | 3.5 ± 0.3 | F1.0, 67.0, 585.594, p < 0.001 | F1.6, 108.9, 23.378, p < 0.001 | F1.6, 108.9, 24.157, p < 0.001 |
| 8 | 6.5 ± 0.9 | 6.5 ± 1.0 | 5.6 ± 0.3 | ||||
| Fragmentation index (Au) | 4 | 22.9 ± 10.6 | 27.2 ± 11.8 | 26.5 ± 12.5 | F1.0, 67.0, 2.783, p = 0.100 | F1.7, 113.4, 0.513, p = 0.570 | F1.7, 112.6, 2.758, p = 0.079 |
| 8 | 30.5 ± 11.4 | 29.0 ± 10.8 | 28.3 ± 11.8 | ||||
| Subjective Sleep Q’ | |||||||
| Ease to sleep? | 4 | 0.5 ± 2.9 | 1.1 ± 2.7 | 0.3 ± 2.6 | F1.0, 67.0, 6.197, p = 0.015 | F1.3, 81.7, 0.155, p = 0.745 | F1.3, 81.7, 5.249, p = 0.019 |
| 8 | −0.2 ± 2.0 | −1.2 ± 2.4 | −0.2 ± 2.0 | ||||
| Time to sleep? | 4 | 2.7 ± 2.4 | 2.6 ± 2.6 | 2.5 ± 2.8 | F1.0, 67.0, 24.401, p < 0.001 | F1.3, 91.6, 0.259, p = 0.679 | F1.3, 91.6, 0.161, p = 0.761 |
| 8 | 0.1 ± 2.8 | −0.1 ± 2.4 | 0.1 ± 2.8 | ||||
| How well did you sleep? | 4 | 0.6 ± 2.5 | 0.6 ± 2.4 | 0.9 ± 2.1 | F1.0, 67.0, 5.765, p = 0.019 | F1.4, 95.1, 0.114, p = 0.822 | F1.4, 95.1, 0.152, p = 0.784 |
| 8 | −0.4 ± 1.9 | −0.3 ± 1.8 | −0.4 ± 1.9 | ||||
| What was your waking time? | 4 | −2.8 ± 1.8 | −2.6 ± 1.8 | −2.9 ± 1.8 | F1.0, 67.0, 27.448, p < 0.001 | F1.4, 94.8, 0.975, p = 0.353 | F1.4, 94.8, 3.777, p = 0.042 |
| 8 | −0.8 ± 1.9 | −0.8 ± 1.9 | −0.5 ± 1.6 | ||||
| Alertness 30-min after waking? | 4 | −1.8 ± 2.4 | −1.7 ± 2.2 | −2.0 ± 2.3 | F1.0, 67.0, 18.642, p < 0.001 | F2.0, 133.7, 0.784, p = 0.454 | F2.0, 133.7, 0.594, p = 0.546 |
| 8 | 0.0 ± 1.4 | −0.1 ± 1.8 | −0.2 ± 1.5 | ||||
| Stanford Sleep Q’ | |||||||
| Degree of sleepiness | 4 | 4.2 ± 1.3 | 4.1 ± 1.3 | 4.1 ± 1.3 | F1.0, 67.0, 40.736, p < 0.001 | F2.0, 134.0, 0.415, p = 0.661 | F2.0, 134.0, 0.754, p = 0.473 |
| 8 | 2.7 ± 0.7 | 2.8 ± 0.9 | 2.9 ± 0.9 |
| Variable | Sleep 4 or 8 h | NoPill | PLAC | ZMA | Significance of between Effect for ‘Sleep Time’ | Significance of Main Effect for ‘Pill’ | Significance of Interaction |
|---|---|---|---|---|---|---|---|
| POMS: | |||||||
| Vigour | 4 | 3.0 ± 2.6 | 3.3 ± 3.0 | 3.5 ± 3.2 | F1.0, 67.0, 13.096; p < 0.001 | F1.8, 117.5 1.349; p = 0.262 | F1.8, 117.5 0.698; p = 0.482 |
| 8 | 5.1 ± 3.1 | 5.9 ± 3.3 | 5.3 ± 2.7 | ||||
| Anger | 4 | 1.7 ± 1.7 | 1.9 ± 2.6 | 1.0 ± 2.2 | F1.0, 67.0, 3.148; p = 0.081 | F2.0, 134.0, 0.200; p = 0.819 | F2.0, 134.0, 4.114; p = 0.018 |
| 8 | 0.8 ± 1.7 | 0.6 ± 1.5 | 1.2 ± 2.3 | ||||
| Tension | 4 | 0.8 ± 1.3 | 0.6 ± 0.7 | 0.5 ± 0.9 | F1.0, 67.0, 6.805; p = 0.011 | F1.7, 112.4, 1.832; p = 0.171 | F1.7, 112.4, 0.999; p = 0.359 |
| 8 | 0.3 ± 0.8 | 0.4 ± 0.8 | 0.1 ± 0.2 | ||||
| Calm | 4 | 5.8 ± 3.1 | 6.7 ± 3.9 | 5.6 ± 3.9 | F1.0, 67.0, 1.087; p = 0.301 | F2.0, 134.0, 0.656; p = 0.521 | F2.0, 134.0, 2.93; p = 0.057 |
| 8 | 7.3 ± 3.6 | 6.3 ± 3.2 | 6.6 ± 2.7 | ||||
| Happy | 4 | 4.1 ± 3.0 | 4.2 ± 3.3 | 4.0 ± 4.1 | F1.0, 67.0, 6.709; p = 0.012 | F2.0, 133.7, 1.212; p = 0.301 | F2.0, 133.7, 0.530; p = 0.590 |
| 8 | 5.6 ± 2.8 | 6.3 ± 2.9 | 5.3 ± 3.0 | ||||
| Confusion | 4 | 2.1 ± 2.9 | 2.0 ± 2.1 | 1.6 ± 1.5 | F1.0, 67.0, 8.891; p = 0.004 | F1.8, 118.7, 0.604; p = 0.529 | F1.8, 118.7, 0.156; p = 0.831 |
| 8 | 0.9 ± 2.0 | 0.9 ± 1.5 | 0.8 ± 1.6 | ||||
| Depressed | 4 | 1.7 ± 1.8 | 1.0 ± 1.4 | 1.5 ± 1.6 | F 1.0, 67.0, 5.342; p = 0.024 | F1.9 129.7, 4.272; p = 0.017 | F1.9, 129.7, 0.562; p = 0.566 |
| 8 | 0.8 ± 1.7 | 0.4 ± 1.1 | 0.8 ± 1.6 | ||||
| Fatigue | 4 | 8.7 ± 3.7 | 8.2 ± 5.2 | 8.7 ± 3.6 | F1.0, 67.0, 30.384; p < 0.001 | F1.6, 108.1, 2.577; p = 0.092 | F1.6, 108.1, 0.547; p = 0.543 |
| 8 | 5.9 ± 1.8 | 4.4 ± 2.2 | 5.1 ± 2.5 | ||||
| Stroop | |||||||
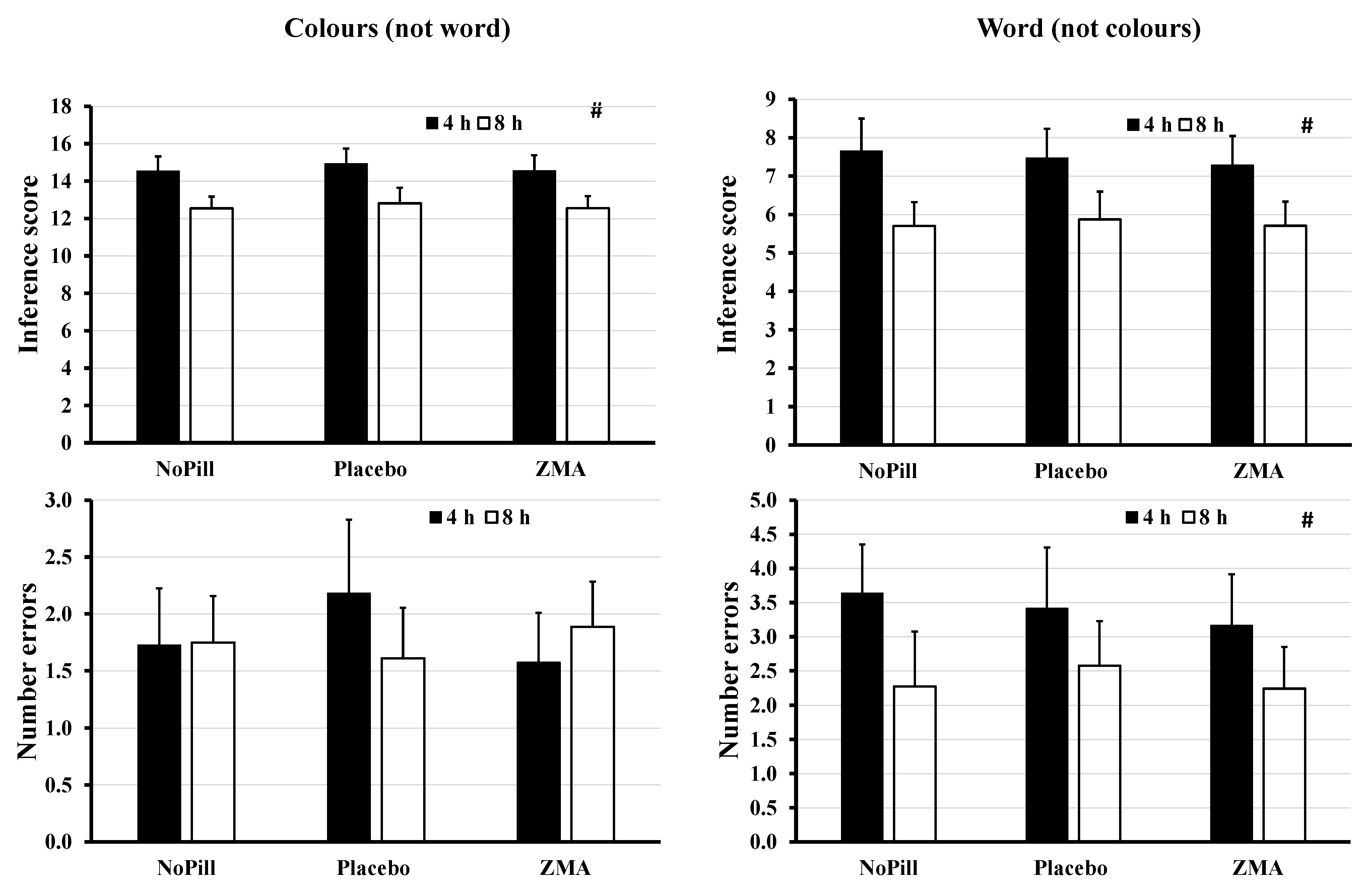
| Colours number | 4 | 57.2 ± 9.1 | 55.6 ± 9.8 | 57.5 ± 10.9 | F1.0, 67.0, 17.165; p < 0.001 | F2.0, 134.0, 0.911; p = 0.405 | F2.0, 134.0, 0.342; p = 0.711 |
| 8 | 68.0 ± 12.3 | 67.6 ± 15.7 | 68.0 ± 12.6 | ||||
| Colour error | 4 | 1.7 ± 1.5 | 2.2 ± 1.9 | 1.6 ± 1.3 | F1.0, 67.0, 0.085; p = 0.771 | F1.7, 111.1, 0.505; p = 0.570 | F1.7, 111.1, 2.963; p = 0.065 |
| 8 | 1.8 ± 1.3 | 1.6 ± 1.4 | 1.9 ± 1.2 | ||||
| Word number | 4 | 133.1 ± 62.8 | 122.4 ± 57.2 | 137.7 ± 60.3 | F1.0, 67.0, 7.069; p = 0.010 | F1.8, 117.7, 1.390; p = 0.253 | F1.8, 117.7, 0.940; p = 0.383 |
| 8 | 184.8 ± 90.9 | 184.0 ± 93.0 | 184.9 ± 91.0 | ||||
| Word error | 4 | 3.6 ± 2.5 | 3.4 ± 2.0 | 3.2 ± 1.9 | F1.0, 67.0, 4.711; p = 0.034 | F1.8, 122.5, 1.250; p = 0.288 | F1.0, 67.0, 0.997; p = 0.366 |
| 8 | 2.3 ± 2.1 | 2.6 ± 2.6 | 2.2 ± 2.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edwards, B.J.; Adam, R.L.; Gallagher, C.; Germaine, M.; Hulton, A.T.; Pullinger, S.A.; Chester, N.J. In Males with Adequate Dietary Needs Who Present No Sleep Disturbances, Is an Acute Intake of Zinc Magnesium Aspartate, Following Either Two Consecutive Nights of 8 or 4 h of Sleep Deprivation, Beneficial for Sleep and Morning Stroop Interference Performance? Behav. Sci. 2024, 14, 622. https://doi.org/10.3390/bs14070622
Edwards BJ, Adam RL, Gallagher C, Germaine M, Hulton AT, Pullinger SA, Chester NJ. In Males with Adequate Dietary Needs Who Present No Sleep Disturbances, Is an Acute Intake of Zinc Magnesium Aspartate, Following Either Two Consecutive Nights of 8 or 4 h of Sleep Deprivation, Beneficial for Sleep and Morning Stroop Interference Performance? Behavioral Sciences. 2024; 14(7):622. https://doi.org/10.3390/bs14070622
Chicago/Turabian StyleEdwards, Ben J., Ryan L. Adam, Chloe Gallagher, Mark Germaine, Andrew T. Hulton, Samuel A. Pullinger, and Neil J. Chester. 2024. "In Males with Adequate Dietary Needs Who Present No Sleep Disturbances, Is an Acute Intake of Zinc Magnesium Aspartate, Following Either Two Consecutive Nights of 8 or 4 h of Sleep Deprivation, Beneficial for Sleep and Morning Stroop Interference Performance?" Behavioral Sciences 14, no. 7: 622. https://doi.org/10.3390/bs14070622







