Electroacupuncture Reduces Seizure Activity and Enhances GAD 67 and Glutamate Transporter Expression in Kainic Acid Induced Status Epilepticus in Infant Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
- SHAM: This group received the same volume of saline solution (i.p.).
- PB: This group received pentobarbital (2.5 mg/kg i.m.).
- Acup: Acupuncture of the SG-DM26 point.
- No-Acup: Placebo point puncture.
- EA: Electroacupuncture of the SG-DM26 point group.
- No-EA: Electroacupuncture of a placebo point.
- KA: Kainic acid.
- KA+PB: Pentobarbital (2.5 mg/kg i.m.).
- KA+Acup: Acupuncture of the SG-DM26 point.
- KA+No-Acup: Placebo point puncture.
- KA+Acup + PB: Treatment with acupuncture and PB (2.5 mg/kg i.m.).
- KA+No-Acup + PB: Treatment with placebo point puncture and PB (2.5 mg/kg i.m.).
- KA+EA: Electroacupuncture of the SG-DM26 point group.
- KA+No-EA: Placebo point electroacupuncture.
- KA+EA + PB: Treatment with electroacupuncture and PB (2.5 mg/kg i.m.).
- KA+No-EA + PB: Treatment with placebo point electroacupuncture and PB (2.5 mg/kg i.m).
2.2. Establishing Status Epilepticus (SE)
- -
- Phase I: No response
- -
- Phase II: Spasmodic movements, tail jolts, jolts
- -
- Phase III: Unilateral scratch movement with the lower limb and position on its side, involving either of the two lower extremities independently, and bilateral movement
- -
- Phase IV: Scratching with lower extremities involving either limb independently, while holding its position on its side
- -
- Phase V: Clonic movements of all four limbs with loss of posture and constant and irreversible state of seizure activity (Status Epilepticus); no return to normal function between seizures.
2.3. Acupuncture Shui Gou DM26 Point
2.3.1. Stimulation by Simple Acupuncture in Shui Gou DM26
2.3.2. Electrostimulation of Shui Gou DM26
2.3.3. Place Point (Non-Point)
2.4. Western Blot
2.5. Statistical Analysis
3. Results
3.1. Evaluation of Motor Behavior Associated with Convulsive Activity
- -
- KA-PB: This group maintained phase III, with interictal periods after the administration of PB until euthanasia (Figure 2).
- -
- KA-ACUP: Phase V (SE) persisted during periods of EA of the placebo point (Figure 2).
- -
- KA-EA: During the electro-stimulation period, a decrease in the convulsive activity was observed. However, at the end of the stimulation, rats showed phase IV during a period of 2 ± 0.27 min until evolution to phase V. During the second period of electrostimulation, a decrease in convulsive activity was observed again. However, at the end of the stimulation, the rats presented phase IV, establishing a maximum time of 0.35 ± 0.5 s, until they evolved to phase V. During the third period of electrostimulation, they maintained phase V, despite the application of electrostimulation (Figure 2).
- -
- KA-ACUP-PB: This group maintained phase III after the administration of PB until euthanasia (Figure 2).
- -
- KA-EA-PB: This group showed an overall decrease after the first electrostimulation seizure activity and phase I (no response) throughout treatment until euthanasia (Figure 2).
- -
- KA-No-ACUP: Phase V persisted during periods of electro-stimulation of the placebo point (Figure 2).
- -
- KA-No-EA: The convulsive activity persisted during the electro-stimulation of the placebo point, presenting during the rest periods as phase IV for a period of 0.14 ± 0.6 min and then evolving to phase V (Figure 2).
- -
- KA-No-ACUP-PB: This group maintained phase III after the administration of PB until euthanasia (Figure 2).
- -
- KA-No-EA-PB: During the first electro-stimulation period, a decrease in convulsive activity was observed; however, at the end of the same period, the rats presented with phase III for a period of 2 ± 0.36 min and evolved to phase IV. During the second period of electro-stimulation, the convulsive activity persisted, presenting as phase IV during the resting period. During the third period of electro-stimulation phase IV was maintained, despite the application of electro-stimulation throughout the procedure (Figure 2).
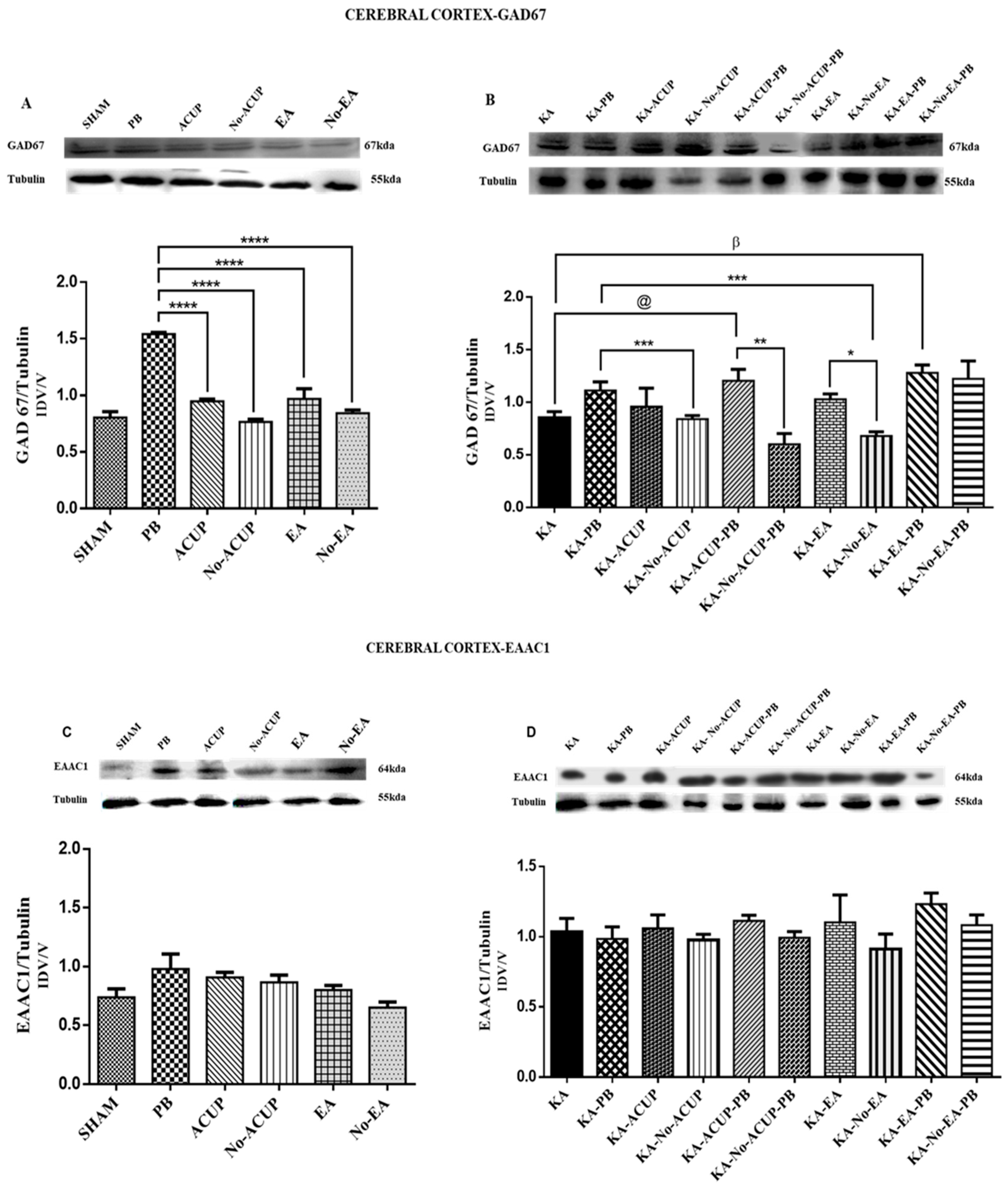
3.2. Evaluation of GAD67 Expression
3.3. Evaluation of EAAC1 Expression
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Trinka, E.; Brigo, F.; Shorvon, S. Recent advances in status epilepticus. Curr. Opin. Neurol. 2016, 29, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Hauser, W.A. The Prevalence and Incidence of Convulsive Disorders in Children; Columbia University College of Physicians and Surgeons: New York, NY, USA, 2009; pp. 1234–1239. [Google Scholar]
- Matti, S.; Dieter, S. Early seizure frequency and etiology predict long-term medical outcome in childhood-onset epilepsy. Brain 2009, 132, 989–998. [Google Scholar] [CrossRef]
- Sanjay, N.; Rakhade, F.; Jensen, E. Epileptogenesis in the immature brain: Emerging mechanisms. Nat. Rev. Neurol. 2009, 5, 380–391. [Google Scholar] [CrossRef]
- Ben-Ari, Y.; Tremblay, E.; Berger, M.; Nitecka, L. Kainic acid seizure syndrome and binding sites in developing rats. Dev. Brain Res. 1984, 2, 284–288. [Google Scholar] [CrossRef]
- Löscher, W.; Brand, C. Prevention, or modification of epileptogenesis after brain insults: Experimental approaches and translational research. Pharmacol. Rev. 2010, 62, 668–700. [Google Scholar] [CrossRef] [PubMed]
- Behrman, K.A.; Vaughan, V.; Nelson, W. Convulsiones en la Infancia. In Nelson, Tratado de Pediatría; Hill, M.G., Ed.; Interamericana: SanGerman, Puerto Rico, 2006; Chapter 2; pp. 2019–2113. ISBN 10: 8476159528. [Google Scholar]
- Chadehumbe, M.A.; Khatri, P.; Khoury, J.C.; Khoury, J.C.; Alwell, K.; Szaflarski, J.P.; Broderick, J.P.; Kissela, B.M.; Kleindorfer, D.O. Seizures are common in the acute setting of childhood stroke—A population-based study. J. Child Neurol. 2009, 24, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Max, S.A.; Jaime, A.R. Síndromes Pediátricos, Fisiopatología, Clínica Y Terapéutica. Crisis convulsivas, 2nd ed.; La prensa Médica Mexicana S.A: Mexico City, Mexico, 2003; Chapter 4; pp. 128–143. [Google Scholar]
- Goodman Gilman, A.; Hardman, J.G.; Limbird, L.W. Goodman & Gilman Las Bases Farmacológicas de la Terapéutica; Mc Graw Hill: New York, NY, USA, 2003. [Google Scholar]
- Baldry, E.P. Traditional Chinese Acupuncture. Acupuncture Trigger Points Musculoskeletal; Elsevier: Amsterdam, The Netherlands, 2005; Chapter 3; pp. 3–23. [Google Scholar]
- Ahn, A.C.; Martinsen, O.G. Electrical characterization of acupuncture points: Technical issues and challenges. J. Altern. Complement. Med. 2007, 13, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Hsiang-Tung, C. Integrative action of thalamus in the process of electroacupuncture for pain. Am. J. Chin. Med. 1974, 2, 1–39. [Google Scholar] [PubMed]
- Greif, R.; Laciny, S.; Mokhtarani, M.; Doufas, A.G.; Bakhshandeh, M.; Dorfer, L.; Sessler, D.I. Transcutaneous electrical stimulation of an auricular acupuncture point decreases anesthetic requirement. Anesthesiology 2002, 96, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Ming, T.H.; Juei, T.C. Increase of locomotor activity by acupuncture on Bai-Hui in rats. Neurosci. Lett. 2001, 211, 221–225. [Google Scholar] [CrossRef]
- Chen, W.; Gu, H.W.; Ma, W.P.; Li, Q.S.; Yu, Q.; Liu, X.Q.; Dai, M.T. Multicentral randomized controlled study on effects of acupuncture at Zusanli (ST 36) and Xuanzhong (GB 39) on cerebrovascular function in the patient of ischemic stroke. Zhongguo Zhen Jiu 2006, 26, 851–853. [Google Scholar] [PubMed]
- Guo, J.; Liu, J.; Fu, W.; Ma, W.; Xu, Z.; Yuan, M.; Hu, J. The effect of electroacupuncture on spontaneous recurrent seizure and expression of GAD67 mRNA in dentate gyrus in a rat model of epilepsy. Brain Res. 2008, 1188, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Meng, P.Y.; Sun, G.J.; Liu, S.H.; Yan, H.M. Effect of electroacupuncture pretreatment on glutamate-NMDAR signal pathway in hippocampal neurons of vascular dementia rats. Zhen Ci Yan Jiu 2008, 33, 103–106. [Google Scholar] [PubMed]
- Chen, X.Y.; Zhang, Q.L.; Bai, B. Effect of electroacupuncture on mitochondrial membrane potential and apoptosis in the cerebral cortex in rats with focal cerebral ischemia/reperfusion injury. Zhen Ci Yan Jiu 2008, 33, 107–110. [Google Scholar] [PubMed]
- Wu, X.D.; Du, L.N.; Wu, G.C.; Cao, X.D. Effects of electroacupuncture on blood-brain barrier after cerebral ischemia-reperfusion in the rat. Acupunct. Electrother. Res. 2001, 26, 1–9. [Google Scholar] [CrossRef]
- Zhang, J.L.; Zhang, S.P.; Zhang, H.Q. Antiepileptic effect of electroacupuncture vs. vagus nerve stimulation in the rat thalamus. Neurosci. Lett. 2008, 441, 183–187. [Google Scholar] [CrossRef]
- Gao, X.Y.; Zhang, S.P.; Zhu, B.; Zhang, H.Q. Investigation of specificity of auricular acupuncture points in the regulation of autonomic function in anesthetized rats. Auton. Neurosci. 2008, 138, 50–56. [Google Scholar] [CrossRef]
- Zhang, T.S.; Yang, L.; Hu, R.; Liu, X.G. Effect of electroacupuncture on the contents of excitatory amino acids in cerebral tissue at different time courses in rats with cerebral ischemia and reperfusion injury. Zhen Ci Yan Jiu 2007, 32, 234–236. [Google Scholar]
- Liao, E.T.; Tang, N.Y.; Lin, Y.W.; Liang Hsieh, C. Long-term electrical stimulation at ear and electro-acupuncture at ST36-ST37 attenuated COX-2 in the CA1 of the hippocampus in kainic acid-induced epileptic seizure rats. Sci. Rep. 2017, 7, 472–480. [Google Scholar] [CrossRef]
- NOM-062-ZOO-1999. Technical Specifications for the Production, Care and Use of Laboratory Animals; NOM-062-ZOO-1999; Norma Oficial Mexicana: Mexico City, Mexico, 1999. [Google Scholar]
- Ben-Ari, Y.; Holmes, G.L. Effects of seizures on developmental processes in the immature brain. Lancet Neurol. 2006, 5, 1055–1063. [Google Scholar] [CrossRef]
- Cherubini, E.; De Feo, M.R.; Mecarelli, O.; Ricci, G.F. Behavioral and electrographic patterns induced by systemic administration of kainic acid in developing rats. Dev. Brain Res. 1983, 1, 69–77. [Google Scholar] [CrossRef]
- Albala, B.J.; Moshé, S.L.; Okada, R. Kainic-acid-induced seizures: A developmental study. Dev. Brain Res. 1984, 13, 139–148. [Google Scholar] [CrossRef]
- Dzhala, V.I.; Talos, D.M.; Sdrulla, D.A.; Mathews, G.C.; Benke, T.A.; Delpire, E.; Jensen, F.E.; Staley, K.J. NKCC1 transporter facilitates seizures in the developing brain. Nat. Med. 2005, 11, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Zayachkivsky, M.J.; Lehmkuhle, J.H.; Fisher, J.J.; Ekstrand, F.E. Dudek. Recording EEG in immature rats with a novel miniature telemetry system. J. Neurophysiol. 2013, 109, 900–911. [Google Scholar] [CrossRef]
- Sayin, U.; Hutchinson, E.; Meyerand, M.E.; Sutula, T. Age-dependent long-term structural and functional effects of early-life seizures: Evidence for a hippocampal critical period influencing plasticity in adulthood. Neuroscience 2015, 288, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. Skull Diagram of a Sprague Dawley Rat. The Rat Brain in Stereotaxic Coordinates; Academic Press: San Diego, CA, USA, 1997; p. 12. [Google Scholar]
- Takeshige, C. Differentiation between acupuncture and nonacupuncture points by association with analgesia inhibitory system. Acupunct. Electrother. Res. 1985, 10, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Chu, N.N.; Liang, J.; Li, Y.J.; Zhang, R.; Han, J.S.; Cui, C.L. Electroacupuncture of 2 Hz has a rewarding effect: Evidence from a conditioned place preference study in rats. Evid. Based Complement. Altern. Med. 2011, 2011, 730514. [Google Scholar] [CrossRef]
- Longhurst, J.C.; Tjen-A-Looi, S.C. Evidence-based blood pressure reducing actions of electroacupuncture: Mechanisms and clinical application. Sheng Li Xue Bao 2017, 69, 587–597. [Google Scholar]
- Napadow, V.; Makris, N.; Liu, J.; Kettner, N.W.; Kwong, K.K.; Hui, K.K. Effects of electroacupuncture versus manual acupuncture on the human brain as measured by fMRI. Hum. Brain Mapp. 2005, 24, 193–205. [Google Scholar] [CrossRef]
- Vase, L.; Baram, S.; Takakura, N.; Takayama, M.; Yajima, H.; Kawase, A.; Schuster, L.; Kaptchuk, T.; Schou, S.S.; Jensen, T.; et al. Can acupuncture treatment be double-blinded? An evaluation of double-blind acupuncture treatment of postoperative pain. PLoS ONE 2015, 10, e0119612. [Google Scholar] [CrossRef]
- Yang, W.O.; Huang, Y.L.; Da, C.D.; Cheng, J.S. Electroacupuncture reduces rat’s neuronal ischemic injury and enhances the expression of basic fibroblast growth factor. Acupunct. Electrother. Res. 1999, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Prism version 7.00 for Windows, GraphPad Software, La Jolla, San Diego, CA, USA. Available online: www.graphpad.com (accessed on 26 June 2019).
- Cheng, L.L.; Ding, M.X.; Xiong, C.; Zhou, M.Y.; Qiu, Z.Y.; Wang, Q. Effects of electroacupuncture of different frequencies on the release profile of endogenous opioid peptides in the central nerve system of goats. Evid.-Based Complement. Alternat. Med. 2012, 2012, 476457. [Google Scholar] [CrossRef] [PubMed]
- Yang, J. Treatment of status epilepticus with acupuncture. J. Tradit. Chin. Med. 1990, 10, 101–102. [Google Scholar] [PubMed]
- Chang, C.Y.; Chang, C.; Chu, H.L. Peripheral afferent pathway for acupuncture analgesia. Science 2000, 16, 210–217. [Google Scholar]
- Fisher, S.R. Therapeutic devices for epilepsy. Ann. Neurol. 2012, 71, 157–168. [Google Scholar] [CrossRef] [PubMed]
- DeGiorgio, C.M.; Soss, J.; Cook, I.A.; Markovic, D.; Gornbein, J.; Murray, D.; Heck, C.N. Randomized controlled trial of trigeminal nerve stimulation for drug-resistant epilepsy. Neurology 2013, 80, 786–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.J.; Wang, J.J.; Lin, Y.P.; Liu, W.Y.; Wang, L.H. Clinical observation on treatment of epilepsy general tonic-clonic attack with catgut implantation at acupoint plus antiepileptic Western Medicine of small dose. Zhongguo Zhen Jiu 2001, 21, 271–273. [Google Scholar]
- Ma, R.; Zhang, X.; Liu, Y.; Li, X.; Yang, C.; Xiong, J. Clinical observation on treatment of tonoclonic attack of infantile epilepsy with acupuncture plus Xi Feng capsule. J. Tradit. Chin. Med. 2001, 42, 276–278. [Google Scholar]
- Wu, Y.; Zou, L.P.; Han, T.L. Randomized controlled trial of traditional Chinese medicine (acupuncture and Tuina) in cerebral palsy: Part 1—any increase in seizure in integrated acupuncture and rehabilitation group versus rehabilitation group? J. Altern. Complement. Med. 2008, 14, 1005–1009. [Google Scholar] [CrossRef]
- Chao, D.; Shen, X.; Xia, Y. From acupuncture to interaction between opioid receptors and Na+ channels: A potential pathway to inhibit epileptic hyperexcitability. Evid. Based Complement. Altern. Med. 2013, 2013, 17. [Google Scholar] [CrossRef]
- Shu, J.; Liu, R.Y.; Huang, X.F. The effects of ear-point stimulation on the contents of somatostatin and amino acid neurotransmitter in the brain of rat whit experimental seizure. Acupunct. Electrother. Res. 2004, 29, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Mraovitch, S.; Calando, Y. Interactions between limbic, thalamo-striatal-cortical, and central autonomic pathways during epileptic seizure progression. J. Comp. Neurol. 1999, 4111, 145–161. [Google Scholar] [CrossRef]
- Kaptchuk, T. Acupuncture: Theory, efficacy, and practice. Ann. Intern. Med. 2002, 136, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Panzer, R.B.; Chrisman, C.L. An auricular acupuncture treatment for idiopathic canine epilepsy: A preliminary report. Am. J. Chin. Med. 2004, 22, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Huntsman, M.M.; Porcello, D.M.; Homanics, G.E.; DeLorey, T.M.; Huguenard, J.R. Reciprocal inhibitory connections and network synchrony in the mammalian thalamus. Science 1999, 283, 541–543. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.; Sanchez-Vives, M.V.; McCormick, D.A. Functional dynamics of GABAergic inhibition in the thalamus. Science 1997, 278, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Kim, Y.J.; Lee, H.J.; Huh, Y.; Chung, J.H.; Seo, J.C.; Kim, C.J. Acupuncture increases cell proliferation in dentate gyrus after transient global ischemia in gerbils. Neurosci. Lett. 2001, 297, 21–24. [Google Scholar] [CrossRef]
- Pinault, D.; Smith, Y.; Deschenes, M. Dendrodendritic and axoaxonic synapses in the thalamic reticular nucleus of the adult rat. J. Neurosci. 1997, 17, 3215–3233. [Google Scholar] [CrossRef]
- Dedeurwaerdere, S.; Vonck, K.; Van Hese, P.; Wadman, W.; Boon, P. The acute and chronic effect of vagus nerve stimulation in genetic absence epilepsy rats from Strasbourg (GAERS). Epilepsia 2005, 46, 94–97. [Google Scholar] [CrossRef]
- Chen, Y.H.; Ivanic, B.; Chuang, C.M.; Lu, D.Y.; Lin, J.G. Electroacupuncture reduces cocaine-induced seizures and mortality in mice. Evid.-Based Complement. Alternat. Med. 2013, 2013, 134610. [Google Scholar] [CrossRef]
- Jia, Y.J.; Deng, J.H.; Zhang, W.Z.; Sun, Z.L.; Yang, J.; Yu, Y.; Wang, X.M. The Role of Group II Metabotropic Glutamate Receptors in the Striatum in Electroacupuncture Treatment of Parkinsonian Rats. CNS Neurosci. Ther. 2016, 23, 23–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klide, A.M.; Farnbach, G.C.; Gallagher, S.M. Acupuncture therapy for the treatment of intractable, idiopathic epilepsy in dogs. Acupunct. Electrother. Res. 2003, 12, 71–74. [Google Scholar] [CrossRef]
- Haker, E.; Egekvist, H.; Bjerring, P. Effect of sensory stimulation (acupuncture) on sympathetic and parasympathetic activities in healthy subjects. J. Auton. Nerv. Syst. 2000, 79, 52–59. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, L.P.; Du, J.B.; Wong, V. Electro-acupuncture protects against hypoxic-ischemic brain-damaged immature rat via hydrogen sulfide as a possible mediator. Neurosci. Lett. 2010, 485, 74–78. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vega-García, A.; Neri-Gómez, T.; Buzoianu-Anguiano, V.; Guerra-Araiza, C.; Segura-Uribe, J.; Feria-Romero, I.; Orozco-Suarez, S. Electroacupuncture Reduces Seizure Activity and Enhances GAD 67 and Glutamate Transporter Expression in Kainic Acid Induced Status Epilepticus in Infant Rats. Behav. Sci. 2019, 9, 68. https://doi.org/10.3390/bs9070068
Vega-García A, Neri-Gómez T, Buzoianu-Anguiano V, Guerra-Araiza C, Segura-Uribe J, Feria-Romero I, Orozco-Suarez S. Electroacupuncture Reduces Seizure Activity and Enhances GAD 67 and Glutamate Transporter Expression in Kainic Acid Induced Status Epilepticus in Infant Rats. Behavioral Sciences. 2019; 9(7):68. https://doi.org/10.3390/bs9070068
Chicago/Turabian StyleVega-García, Angelica, Teresa Neri-Gómez, Vinnitsa Buzoianu-Anguiano, Christian Guerra-Araiza, Julia Segura-Uribe, Iris Feria-Romero, and Sandra Orozco-Suarez. 2019. "Electroacupuncture Reduces Seizure Activity and Enhances GAD 67 and Glutamate Transporter Expression in Kainic Acid Induced Status Epilepticus in Infant Rats" Behavioral Sciences 9, no. 7: 68. https://doi.org/10.3390/bs9070068
APA StyleVega-García, A., Neri-Gómez, T., Buzoianu-Anguiano, V., Guerra-Araiza, C., Segura-Uribe, J., Feria-Romero, I., & Orozco-Suarez, S. (2019). Electroacupuncture Reduces Seizure Activity and Enhances GAD 67 and Glutamate Transporter Expression in Kainic Acid Induced Status Epilepticus in Infant Rats. Behavioral Sciences, 9(7), 68. https://doi.org/10.3390/bs9070068





