The Dynamics of the Concentration and Speciation of Arsenic in Private Drinking Water Wells in Eastern Wisconsin, USA
Abstract
1. Introduction
2. Study Area and Method
2.1. Study Area
2.2. Well Water Sampling
2.3. The Determination of Total Arsenic Concentrations and Arsenic Speciation
3. Results and Discussion
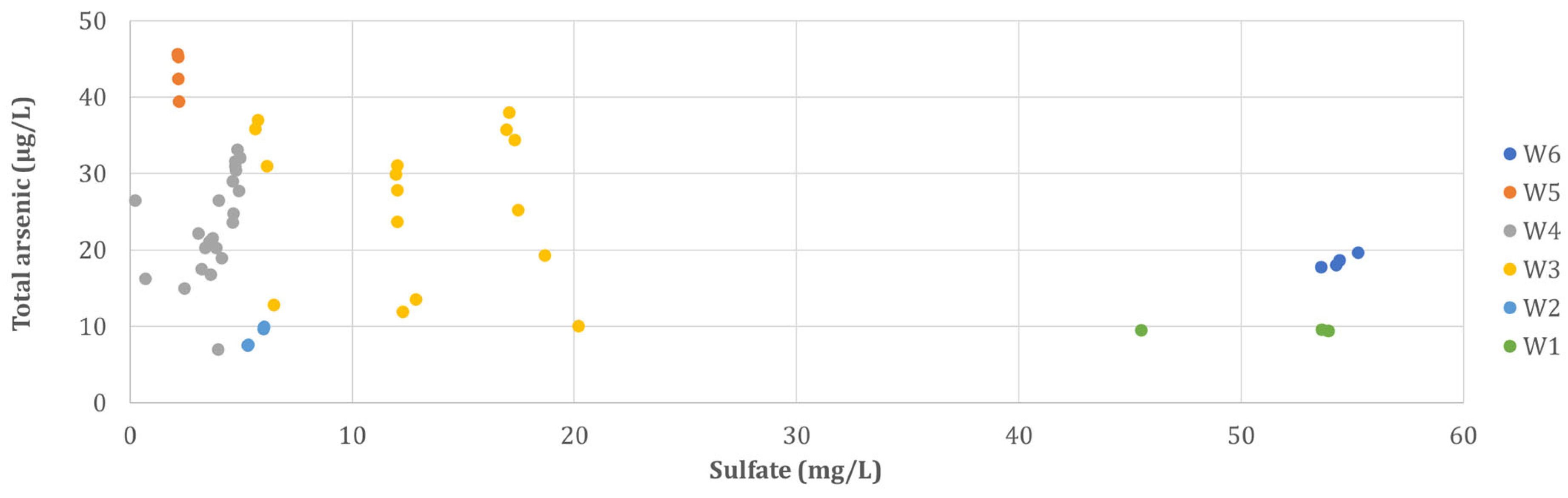
3.1. Water Chemistry and Arsenic Concentrations and Speciation
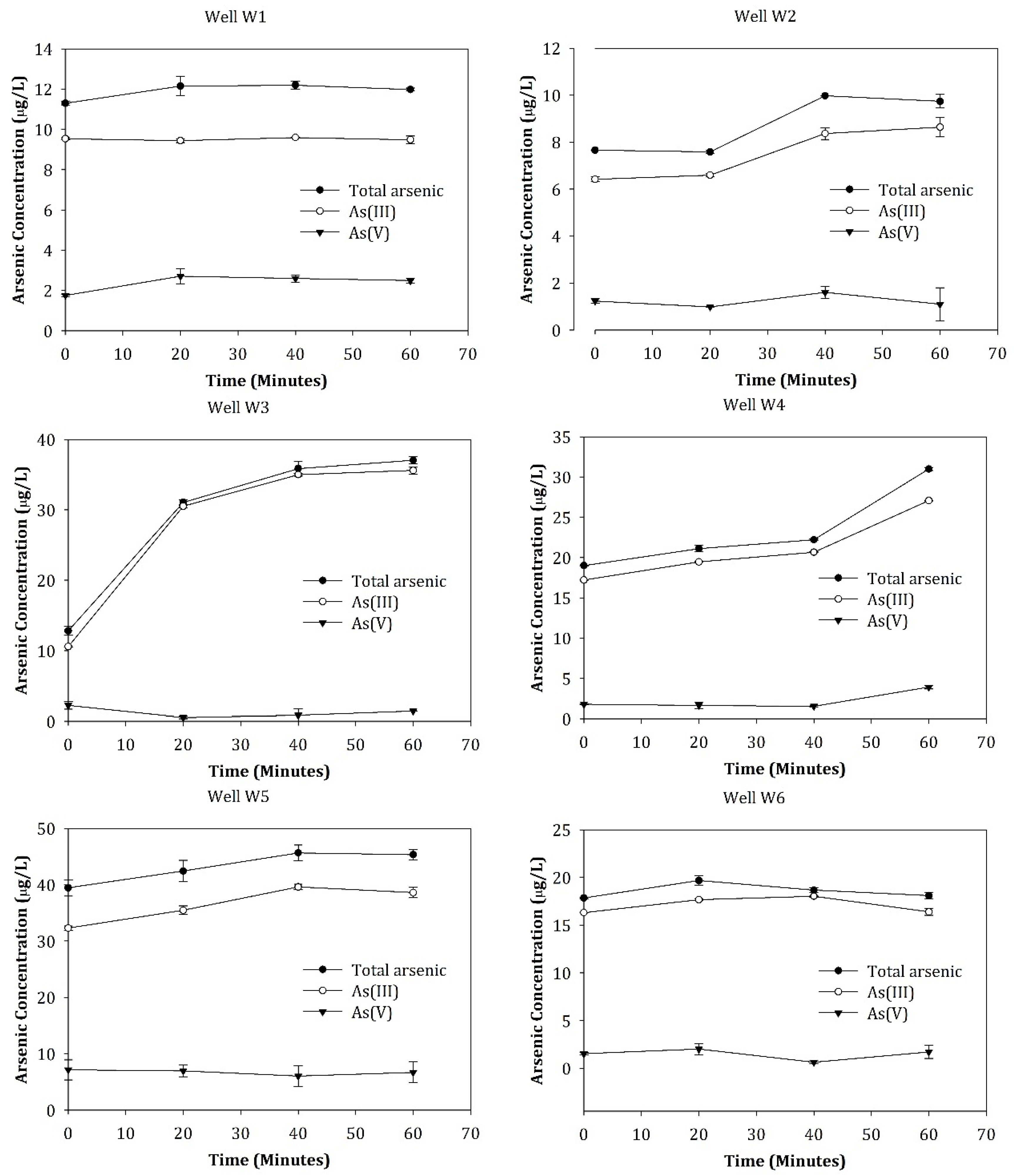
3.2. Dynamics of Arsenic Concentrations and Speciation during Well Pumping
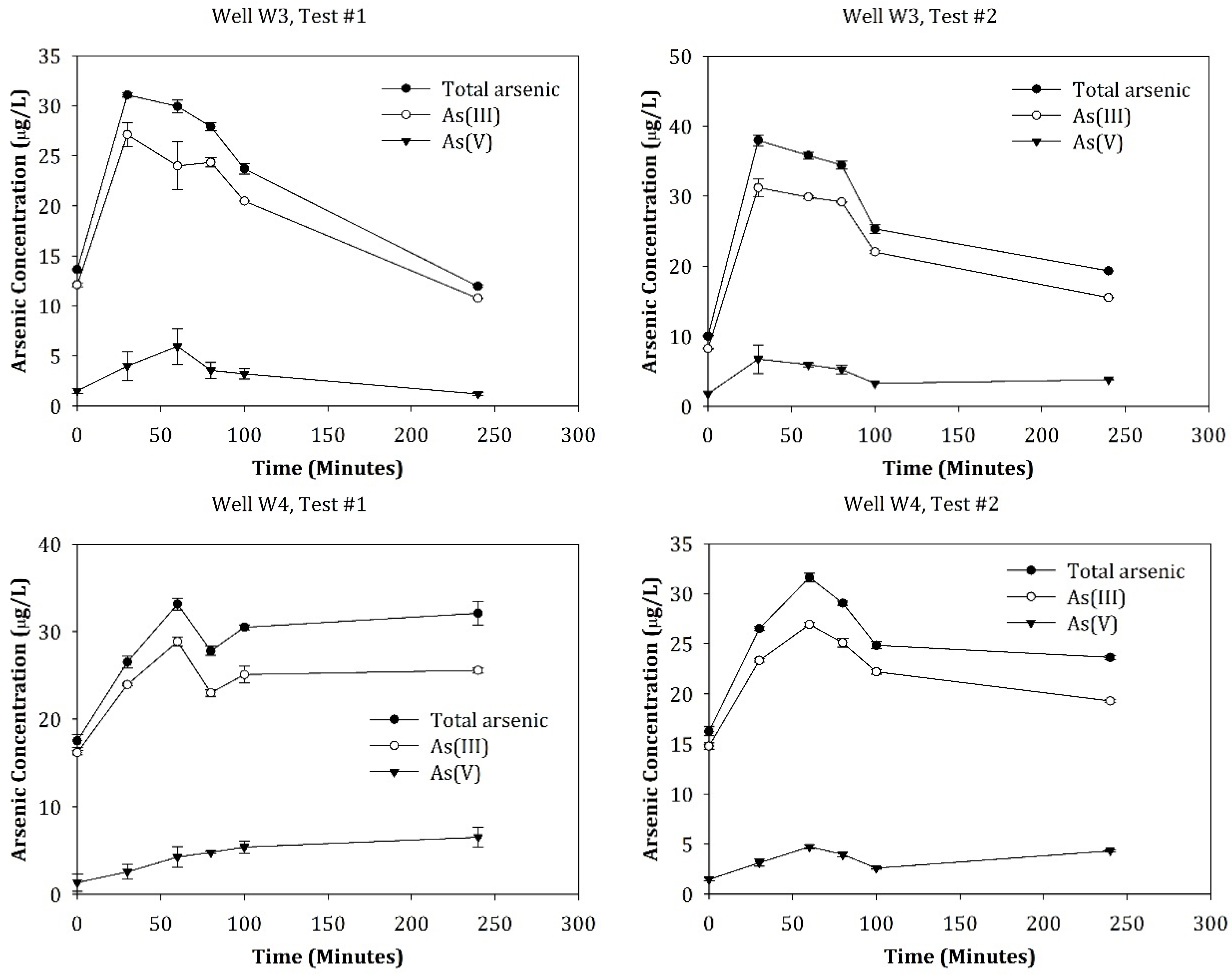
3.3. Dynamics of Arsenic Concentrations and Speciation following Intensive Well Pumping
3.4. Mechanisms Underlying the Arsenic Dynamics during and following Water Pumping
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grönwall, J.; Danert, K. Regarding Groundwater and Drinking Water Access through A Human Rights Lens: Self-Supply as A Norm. Water 2020, 12, 419. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Smith, A.H.; Hopenhayn-Rich, C.; Bates, M.N.; Goeden, H.M.; Hertz-Picciotto, I.; Duggan, H.M.; Wood, R.; Kosnett, M.J.; Smith, M.T. Cancer risks from arsenic in drinking water. Environ. Health Perspect. 1992, 97, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.F. History of Arsenic as a Poison and a Medicinal Agent. In Arsenic; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 1–22. [Google Scholar] [CrossRef]
- Reid, M.S.; Hoy, K.S.; Schofield, J.R.M.; Uppal, J.S.; Lin, Y.; Lu, X.; Peng, H.; Le, X.C. Arsenic speciation analysis: A review with an emphasis on chromatographic separations. TrAC Trends Anal. Chem. 2020, 123, 115770. [Google Scholar] [CrossRef]
- Walker, M.; Seiler, R.L.; Meinert, M. Effectiveness of household reverse-osmosis systems in a Western US region with high arsenic in groundwater. Sci. Total Environ. 2008, 389, 245–252. [Google Scholar] [CrossRef] [PubMed]
- USEPA. National Primary Drinking Water Regulations: Long Term 2 Enhanced Surface Water Treatment Rule; U.S. Environmental Protection Agency: Washington, DC, USA, 2006.
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Arsenic; U.S. Department of Health and Human Services, Public Health Service: Washington, DC, USA, 2007.
- Byron, W.R.; Bierbower, G.W.; Brouwer, J.B.; Hansen, W.H. Pathologic changes in rats and dogs from two-year feeding of sodium arsenite or sodium arsenate. Toxicol. Appl. Pharm. 1967, 10, 132–147. [Google Scholar] [CrossRef]
- Tamio, M.; Nobuko, S.; Mayumi, A.; Sadao, U.; Yukio, S. Chemical form-dependent induction of hepatic zincthionein by arsenic administration and effect of co-administered selenium in mice. Toxicol. Lett. 1987, 39, 63–70. [Google Scholar] [CrossRef]
- Willhite, C.C.; Ferm, V.H. Prenatal and Developmental Toxicology of Arsenicals. In Nutritional and Toxicological Aspects of Food Safety; Friedman, M., Ed.; Springer: Boston, MA, USA, 1984; pp. 205–228. [Google Scholar] [CrossRef]
- Raab, A.; Feldmann, J. Arsenic. In Encyclopedia of Analytical Science, 2nd ed.; Worsfold, P., Townshend, A., Poole, C., Eds.; Elsevier: Oxford, UK, 2005; pp. 132–141. [Google Scholar] [CrossRef]
- Vahter, M.; Concha, G. Role of Metabolism in Arsenic Toxicity. Pharmacol. Toxicol. 2001, 89, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Petrick, J.S.; Ayala-Fierro, F.; Cullen, W.R.; Carter, D.E.; Vasken Aposhian, H. Monomethylarsonous Acid (MMAIII) Is More Toxic Than Arsenite in Chang Human Hepatocytes. Toxicol. Appl. Pharm. 2000, 163, 203–207. [Google Scholar] [CrossRef]
- Ayotte, J.D.; Medalie, L.; Qi, S.L.; Backer, L.C.; Nolan, B.T. Estimating the High-Arsenic Domestic-Well Population in the Conterminous United States. Environ. Sci. Technol. 2017, 51, 12443–12454. [Google Scholar] [CrossRef]
- Thornburg, K.; Sahai, N. Arsenic occurrence, mobility, and retardation in sandstone and dolomite formations of the Fox River Valley, eastern Wisconsin. Environ. Sci. Technol. 2004, 38, 5087–5094. [Google Scholar] [CrossRef] [PubMed]
- Riewe, T. Wisconsin: Geologic Solution for Private Wells in Outagamie and Winnebago Counties; U.S. Environmental Protection Agency: Washington, DC, USA, 2015.
- Benson, D. Test for arsenic, Ozaukee well owners told. Milwaukee Journal Sentinel, 15 July 2009. [Google Scholar]
- WDNR. Arsenic in Drinking Water: Drinking Water Wells Tested with Arsenic Levels Greater than 10 ppb; Wisconsin Department of Natural Resources: Madison, WI, USA, 2017.
- Kassulke, N.; Chern, L. Groundwater, Wisconsin’s Buried Treasury; Wisconsin Department of Natural Resources: Madison, WI, USA, 2006.
- Schreiber, M.E.; Simo, J.A.; Freiberg, P.G. Stratigraphic and geochemical controls on naturally occurring arsenic in groundwater, eastern Wisconsin, USA. Hydrogeol. J. 2000, 8, 161–176. [Google Scholar] [CrossRef]
- Luczaj, J.A.; McIntire, M.J.; Olson Hunt, M.J. Geochemical Characterization of Trace MVT Mineralization in Paleozoic Sedimentary Rocks of Northeastern Wisconsin, USA. Geosciences 2016, 6, 29. [Google Scholar] [CrossRef]
- Burkel, R.S.; Stoll, R.C. Naturally occurring arsenic in sandstone aquifer water supply wells of north-eastern Wisconsin. Ground Water Monit. Remediat. 1999, 19, 114–121. [Google Scholar] [CrossRef]
- Root, T.L.; Gotkowitz, M.B.; Bahr, J.M.; Attig, J.W. Arsenic Geochemistry and Hydrostratigraphy in Midwestern U.S. Glacial Deposits. Ground Water 2010, 48, 903–912. [Google Scholar] [CrossRef]
- Gotkowitz, M.B.; Schreiber, M.E.; Simo, J.A. Effects of water use on arsenic release to well water in a confined aquifer. Ground Water 2004, 42, 568–575. [Google Scholar] [CrossRef]
- Schreiber, M.E.; Gotkowitz, M.B.; Simo, J.A.; Freiberg, P.G. Mechanisms of Arsenic Release to Ground Water from Naturally Occurring Sources, Eastern Wisconsin. In Arsenic in Ground Water: Geochemistry and Occurrence; Welch, A.H., Stollenwerk, K.G., Eds.; Springer: Boston, MA, USA, 2003; pp. 259–280. [Google Scholar] [CrossRef]
- Simo, T. Geologic Constraints on Arsenic in Groundwater with Applications to Groundwater Modeling; University of Wisconsin Aquatic Sciences Center: Madison, WI, USA, 1994. [Google Scholar]
- Nessman, M. How to Collect a Water Sample; Wisconsin Department of Natural Resources: Madison, WI, USA, 2010.
- Clean Water Testing, LLC. How to Take a Water Sample; Clean Water Testing, LLC: Appleton, WI, USA, 2015. [Google Scholar]
- Flanagan, S.V.; Spayd, S.E.; Procopio, N.A.; Chillrud, S.N.; Ross, J.; Braman, S.; Zheng, Y. Arsenic in private well water part 2 of 3: Who benefits the most from traditional testing promotion? Sci. Total Environ. 2016, 562, 1010–1018. [Google Scholar] [CrossRef]
- USEPA. Method 200.8: Determination of Trace Elements in Waters and Wastes by Inductively Coupled Plasma-Mass Spectrometry; U.S. Environmental Protection Agency: Washington, DC, USA, 1994.
- Wang, L.; Giammar, D.E. Effects of pH, dissolved oxygen, and aqueous ferrous iron on the adsorption of arsenic to lepidocrocite. J. Colloid Interface Sci. 2015, 448, 331–338. [Google Scholar] [CrossRef]
- Wilkie, J.A.; Hering, J.G. Rapid oxidation of geothermal arsenic(III) in streamwaters of the eastern Sierra Nevada. Environ. Sci. Technol. 1998, 32, 657–662. [Google Scholar] [CrossRef]
- Heller, S.A. Seasonal Geochemistry of Springs from Dolomite Aquifers of Southwestern Wisconsin; Wisconsin Geological and Natural History Survey: Madison, WI, USA, 1992.
- Hem, J.D. Study and Interpretation of the Chemical Characteristics of Natural Water; U.S. Geological Survey: Reston, VA, USA, 1985.
- Shafer, M.; Overdier, J.T.; Kerr, S.C. Arsenic Species (III,V) Distribution In Wisconsin Groundwaters: Field Measurements and Prediction Using Multivariate Analysis of Geochemical Data; University of Wisconsin Aquatic Sciences Center: Madison, WI, USA, 2007. [Google Scholar]
- West, N.; Schreiber, M.; Gotkowitz, M. Arsenic release from chlorine-promoted alteration of a sulfide cement horizon: Evidence from batch studies on the St. Peter Sandstone, Wisconsin, USA. Appl. Geochem. 2012, 27, 2215–2224. [Google Scholar] [CrossRef]
- Bissen, M.; Frimmel, F.H. Arsenic—A Review. Part II: Oxidation of Arsenic and Its Removal in Water Treatment. Acta Hydrochim. Hydrobiol. 2003, 31, 97–107. [Google Scholar] [CrossRef]
- New York State Department of Health. Individual Water Supply Wells—Fact Sheet #2. 2018. Available online: https://www.health.ny.gov/environmental/water/drinking/regulations/fact_sheets/fs2_water_storage.htm (accessed on 1 March 2024).
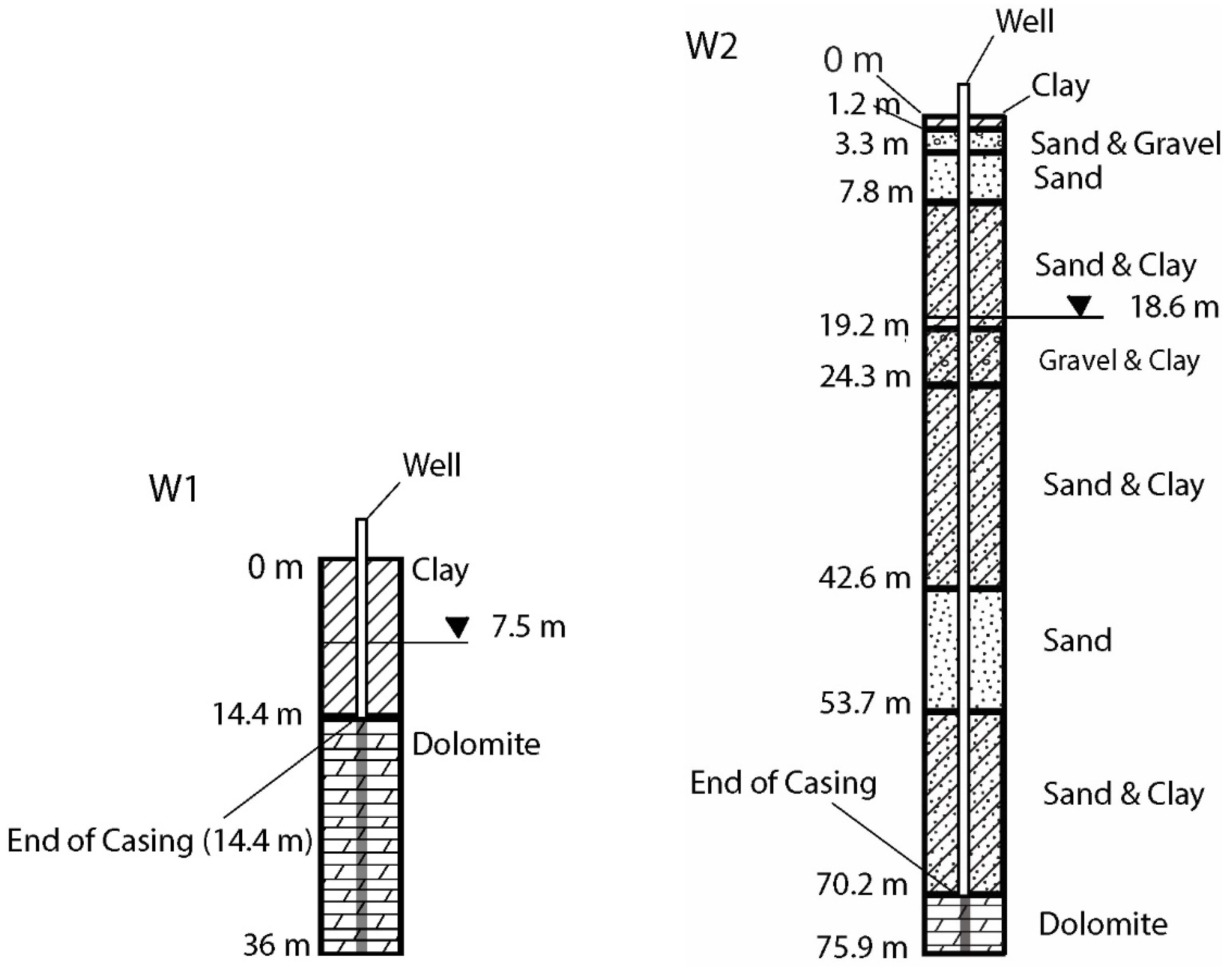
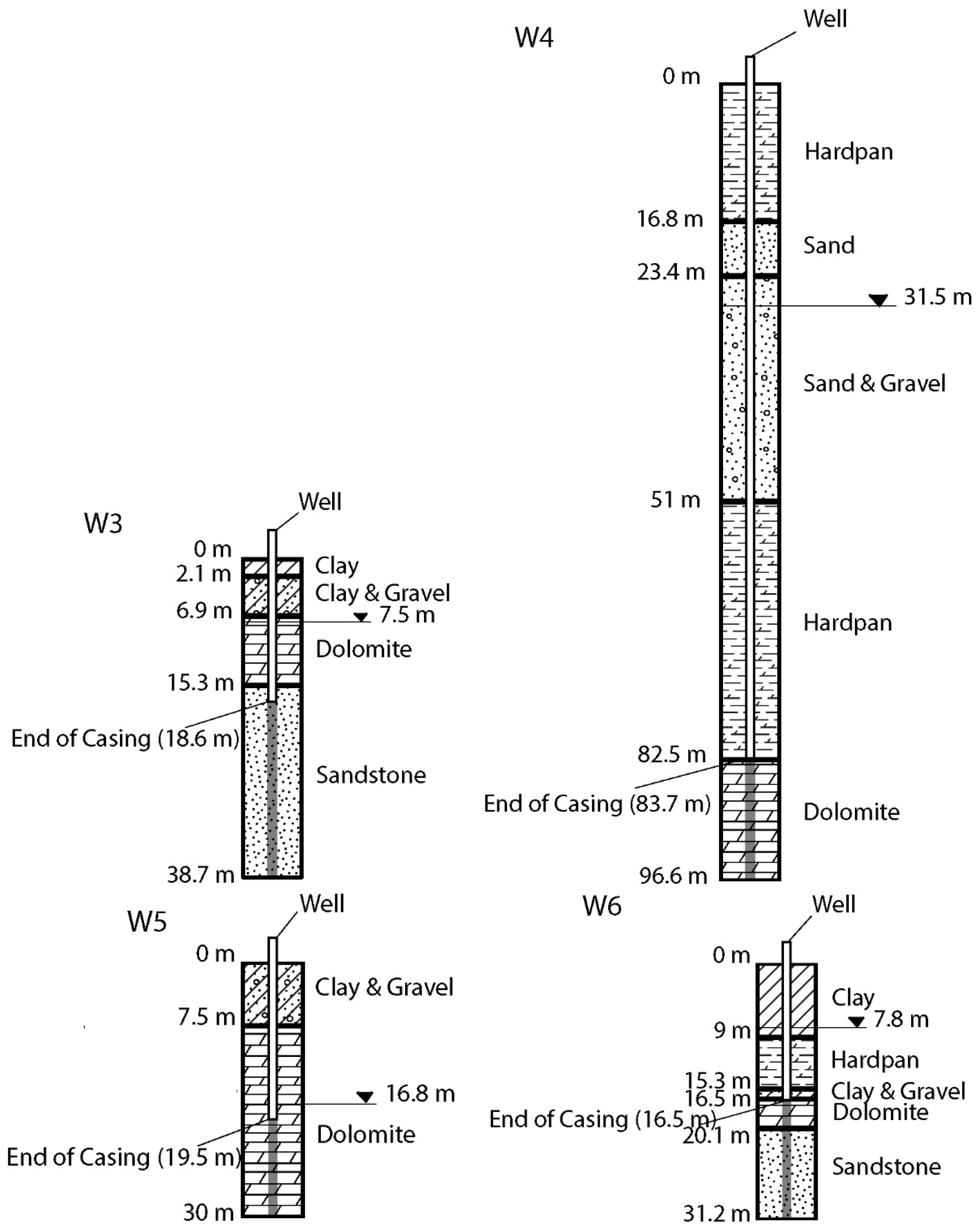
| Well ID | County | Aquifer | Well Screen Depth (m) |
|---|---|---|---|
| W1 | Ozaukee | Dolomite | 14.4–36 |
| W2 | Washington | Dolomite | 70.2–75.9 |
| W3 | Dodge | Dolomite + Sandstone | 18.6–38.7 |
| W4 | Jefferson | Dolomite | 83.7–96.6 |
| W5 | Outagamie | Dolomite | 19.5–30 |
| W6 | Winnebago | Dolomite + Sandstone | 16.5–31.2 |
| W7 | Waukesha | Dolomite | 35.1–79.5 |
| W8 | Waukesha | Dolomite | 21.6–43.5 |
| W9 | Waukesha | Dolomite | 23.1–55.5 |
| W10 | Winnebago | Dolomite + Sandstone | 12.6–42.6 |
| W11 | Winnebago | Dolomite + Sandstone | 12.9–24 |
| Well Number | Arsenic Results from First Sampling Event | Arsenic Results from Second Sampling Event | ||||
|---|---|---|---|---|---|---|
| Total Arsenic (µg/L) | As(III) (%) | As(V) (%) | Total Arsenic (µg/L) | As(III) (%) | As(V) (%) | |
| W1 | 9.00 ± 0.23 | 76.2 | 23.8 | 11.29 ± 0.11 | 84.4 | 15.6 |
| W2 | 6.80 ± 0.29 | 76.9 | 23.1 | 7.66 ± 0.00 | 83.8 | 16.2 |
| W3 | 14.25 ± 0.51 | 73.5 | 26.5 | 12.84 ± 0.63 | 82.5 | 17.5 |
| W4 | 21.13 ± 0.47 | 88.8 | 11.2 | 19.01 ± 0.07 | 90.8 | 9.2 |
| W5 | 39.77 ± 1.64 | 82.4 | 17.6 | 39.46 ± 1.39 | 81.9 | 18.1 |
| W6 | 17.80 ± 0.64 | 81.9 | 18.1 | 17.84 ± 0.10 | 91.4 | 8.6 |
| W7 | 9.85 ± 1.01 | 71.1 | 28.9 | NA | NA | NA |
| W8 | 8.05 ± 0.14 | 79.0 | 21.1 | NA | NA | NA |
| W9 | 12.74 ± 0.24 | 45.6 | 54.4 | NA | NA | NA |
| W10 | 764.83 ± 15.88 | 85.7 | 14.3 | NA | NA | NA |
| W11 | 51.17 ± 1.46 | 80.3 | 19.7 | NA | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plank, E.; Wang, Y.; Xu, S. The Dynamics of the Concentration and Speciation of Arsenic in Private Drinking Water Wells in Eastern Wisconsin, USA. Environments 2024, 11, 75. https://doi.org/10.3390/environments11040075
Plank E, Wang Y, Xu S. The Dynamics of the Concentration and Speciation of Arsenic in Private Drinking Water Wells in Eastern Wisconsin, USA. Environments. 2024; 11(4):75. https://doi.org/10.3390/environments11040075
Chicago/Turabian StylePlank, Evvan, Yin Wang, and Shangping Xu. 2024. "The Dynamics of the Concentration and Speciation of Arsenic in Private Drinking Water Wells in Eastern Wisconsin, USA" Environments 11, no. 4: 75. https://doi.org/10.3390/environments11040075
APA StylePlank, E., Wang, Y., & Xu, S. (2024). The Dynamics of the Concentration and Speciation of Arsenic in Private Drinking Water Wells in Eastern Wisconsin, USA. Environments, 11(4), 75. https://doi.org/10.3390/environments11040075







