Heavy Metal Accumulation in Three Varieties of Mustard Grown under Five Soil Management Practices
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Experimental Design
2.2. Cultivation Practices
2.3. Soil Sampling and Data Collection
2.4. Metal Analysis
2.5. Bioaccumulation Factor (BAF)
2.6. Statistical Analysis
3. Results and Discussions
3.1. Total Heavy Metal Concentrations in Soil and Mustard
3.2. Quantification of the BAF Values
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pikuła, D. Effect of the Degree of Soil Contamination with Cd, Zn, Cu and Zn on Its Content in the Forder Crops and Mobility in the Soil Profile. In Soil Contamination-Recent Advances and Future Perspectives; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Najeeb, U.; Ahmad, W.; Zia, M.H.; Zaffar, M.; Zhou, W. Enhancing the lead phytostabilization in wetland plant Juncus effusus L. through somaclonal manipulation and EDTA enrichment. Arab. J. Chem. 2017, 10, S3310–S3317. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, A.; MMS, C.P.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Yadav, K.K. Lead toxicity: Health hazards, influence on food chain, and sustainable remediation approaches. Int. J. Environ. Res. Public Health 2020, 17, 2179. [Google Scholar] [CrossRef]
- Gamakaranage, C.S.S.K.; Rodrigo, C.; Weerasinghe, S.; Gnanathasan, A.; Puvanaraj, V.; Fernando, H. Complications and management of acute copper sulphate poisoning; a case discussion. J. Occup. Med. Toxicol. 2011, 6, 34. [Google Scholar] [CrossRef]
- Hambidge, K.M.; Krebs, N.F. Zinc Deficiency: A Special Challenge1. J. Nutr. 2010, 137, 1101–1105. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef]
- Lamas, G.; Navas-Acien, A.; Mark, D. Heavy Metals, Cardiovascular Disease, and the Unexpected Benefits of Chelation Therapy. J. Am. Coll. Cardiol. 2016, 67, 2411–2418. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Egodawatta, P.; McGree, J.; Liu, A.; Goonetilleke, A. Human health risk assessment of heavy metals in urban stormwater. Sci. Total Environ. 2016, 557, 764–772. [Google Scholar] [CrossRef]
- Katagi, T. Bioconcentration, bioaccumulation, and metabolism of pesticides in aquatic organism. In Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 2010; Volume 204, pp. 1–321. [Google Scholar] [CrossRef]
- Sharma, J.K.; Kumar, N.; Singh, N.P.; Santal, A.R. Phytoremediation technologies and its mechanism for removal of heavy metal from contaminated soil: An approach for a sustainable environment. Front. Plant Sci. 2023, 14, 78. [Google Scholar] [CrossRef]
- Bhuiyan, M.S.U.; Min, S.R.; Jeong, W.J.; Sultana, S.; Choi, K.S.; Lee, Y.; Liu, J.R. Overexpression of atatm3 in Brassica juncea confers enhanced heavy metal tolerance and accumulation. Plant Cell Tissue Organ Cult. 2011, 107, 69–77. [Google Scholar] [CrossRef]
- Liu, D.; Jiang, W.; Liu, C.; Xin, C.; Hou, W. Uptake and accumulation of lead by roots, hypocotyls and shoots of Indian mustard [Brassica juncea (L.)]. Bioresour. Technol. 2000, 71, 273–277. [Google Scholar] [CrossRef]
- Clemente, R.; Walker, D.J.; Bernal, M.P. Uptake of heavy metals and As by Brassica juncea grown in a contaminated soil in Aznalcóllar (Spain): The effect of soil amendments. Environ. Pollut. 2005, 138, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.L. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002, 53, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Azirun, S.M.; Boyce, A.N. Enhanced accumulation of copper and lead in amaranth (Amaranthus paniculatus), Indian mustard (Brassica juncea) and sunflower (Helianthus annuus). PLoS ONE 2013, 8, e62941. [Google Scholar] [CrossRef] [PubMed]
- Lwin, C.S.; Seo, B.H.; Kim, H.U.; Owens, G.; Kim, K.R. Application of soil amendments to contaminated soils for heavy metal immobilization and improved soil quality—A critical review. Soil Sci. Plant Nutr. 2018, 64, 156–167. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, S.; Cheng, P.; Zhang, S.; Sun, Y. Effects of soil amendments on heavy metal immobilization and accumulation by maize grown in a multiple-metal-contaminated soil and their potential for safe crop production. Toxics 2020, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, R.; Liu, W.; Cheng, L.; Jiang, Q.; Zhanga, Y. Exploratory of immobilization remediation of hydroxyapatite (HAP) on lead-contaminated soils. Environ. Sci. Pollut. Res. 2019, 26, 26674–26684. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Nagpal, A.K. Soil amendments: A tool to reduce heavy metal uptake in crops for production of safe food. Rev. Environ. Sci. Bio/Technol. 2018, 17, 187–203. [Google Scholar] [CrossRef]
- Liu, L.; Chen, H.; Cai, P.; Liang, W.; Huang, Q. Immobilization and phytotoxicity of Cd in contaminated soil amended with chicken manure compost. J. Hazard. Mater. 2009, 163, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Alamgir, M.; Kibria, M.G.; Islam, M. Effects of farmyard manure on cadmium and lead accumulation in Amaranth (Amaranthus oleracea L.). J. Soil Sci. Environ. Manag. 2011, 2, 237–240. [Google Scholar]
- Adriano, D.C. Trace Elements in Terrestrial Environments: Biogeochemistry, Bioavailability, and Risks of Metals, 2nd ed.; Springer: New York, NY, USA, 2001. [Google Scholar]
- Desaules, A. Critical evaluation of soil contamination assessment methods for trace metals. Sci. Total Environ. 2012, 426, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, U. Enhancing phytoextraction: The effect of chemical soil manipulation on mobility, plant accumulation, and leaching of heavy metals. J. Environ. Qual. 2003, 32, 1939–1954. [Google Scholar] [CrossRef]
- Pfeufer, E.; Bessin, R.; Wright, S.; Strang, J. Vegetable Production Guide for Commercial Growers; College of Agriculture, Food and Environment Cooperative Extension Service, University of Kentucky: Lexington, KY, USA, 2018; pp. 44–48. [Google Scholar]
- Antonious, G.F.; Kochhar, T.S.; Coolong, T. Yield, quality, and concentration of seven heavy metals in cabbage and broccoli grown in sewage sludge and chicken manure amended soil. J. Environ. Sci. Health Part A 2012, 47, 1955–1965. [Google Scholar] [CrossRef] [PubMed]
- Matejovic, I.; Durackova, A. Comparison of microwave digestion, wet and dry mineralization, and solubilization of plant samples for determination of calcium, magnesium, potassium, phosphorus, sodium, iron, zinc, copper, and manganese. Commun. Soil Sci. Plant Anal. 1994, 25, 1277–1288. [Google Scholar] [CrossRef]
- Lee, J.; Park, Y.S.; Lee, D.Y. Fast and green microwave-assisted digestion with diluted nitric acid and hydrogen peroxide and subsequent determination of elemental composition in brown and white rice by ICP-MS and ICP-OES. LWT 2023, 173, 11435. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Method 6010b Inductively Coupled Plasma Atomic Emission Spectrometry. Revision 2; 1996. Available online: https://www.epa.gov/sites/default/files/documents/6010b.pdf (accessed on 20 February 2024).
- Ekere, N.R.; Ugbor, M.C.J.; Ihedioha, J.N.; Ukwueze, N.N.; Abugu, H.O. Ecological and potential health risk assessment of heavy metals in soils and food crops grown in abandoned urban open waste dumpsite. J. Environ. Health Sci. Eng. 2020, 18, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 25 February 2024).
- ALINORM 01/12A; Food Additives and Contaminants—Joint Codex Alimentarius Commission, FAO/WHO Food Standards Program. FAO: Rome, Italy; WHO: Geneva, Switzerland, 2014; p. 1289.
- Tatu, G.L.A.; Vladut, N.V.; Voicea, I.; Vanghele, N.A.; Pruteanu, M.A. Removal of heavy metals from contaminated soil using phytoremediation. In Proceedings of the MATEC Web of Conferences, Petrosani, Romania, 3 October 2019; EDP Sciences: Les Ulis, France, 2020; Volume 305, p. 00061. [Google Scholar] [CrossRef][Green Version]
- Johnson, A.; Gunawardana, B.; Singhal, N. Amendments for enhancing copper uptake by Brassica juncea and Lolium perenne from solution. Int. J. Phytoremediation 2009, 11, 215–234. [Google Scholar] [CrossRef]
- Radulescu, C.; Stihi, C.; Popescu, I.V.; Dulama, I.D.; Chelarescu, E.D.; Chilian, A. Heavy metal accumulation and translocation in different parts of Brassica oleracea L. Rom. J. Phys. 2013, 58, 1337–1354. [Google Scholar]
- Satpathy, D.; Reddy, M.V.; Dhal, S.P. Risk assessment of heavy metals contamination in paddy soil, plants, and grains (Oryza sativa L.) at the East Coast of India. BioMed Res. Int. 2014, 2014, 545473. [Google Scholar] [CrossRef]
- Nepal, A.; Antonious, G.F.; Gyawali, B.R.; Webster, T.C.; Bebe, F. Assessing the Bioaccumulation of Heavy Metals in Cabbage Grown under Five Soil Amendments. Pollutants 2024, 4, 58–71. [Google Scholar] [CrossRef]
- Swain, A.; Singh, S.K.; Mohapatra, K.K.; Patra, A. Sewage sludge amendment affects spinach yield, heavy metal bioaccumulation, and soil pollution indexes. Arab. J. Geosci. 2021, 14, 717. [Google Scholar] [CrossRef]
- Amin, H.; Arain, B.A.; Jahangir, T.M.; Abbasi, M.S.; Amin, F. Accumulation and distribution of lead (Pb) in plant tissues of guar (Cyamopsis tetragonoloba L.) and sesame (Sesamum indicum L.): Profitable phytoremediation with biofuel crops. Geol. Ecol. Landsc. 2018, 2, 51–60. [Google Scholar]
- Vasile, G.-G.; Tenea, A.-G.; Dinu, C.; Iordache, A.M.M.; Gheorghe, S.; Mureseanu, M.; Pascu, L.F. Bioavailability, Accumulation and Distribution of Toxic Metals (As, Cd, Ni and Pb) and Their Impact on Sinapis alba Plant Nutrient Metabolism. Int. J. Environ. Res. Public Health 2021, 18, 12947. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Lee, Y.B.; Lee, C.H.; Hong, C.O.; Kim, P.J.; Yu, C. Characteristics of boron accumulation by fly ash application in paddy soil. Bioresour. Technol. 2008, 99, 5928–5932. [Google Scholar] [CrossRef]
- Hussain, Z.; Alam, M.; Khan, M.A.; Asif, M.; Shah, M.A.; Khan, S.; Nawab, J. Bioaccumulation of potentially toxic elements in spinach grown on contaminated soils amended with organic fertilizers and their subsequent human health risk. Arab. J. Geosci. 2020, 13, 945. [Google Scholar] [CrossRef]
- Uchimiya, M.; Bannon, D.; Nakanishi, H.; McBride, M.B.; Williams, M.A.; Yoshihara, T. Chemical speciation, plant uptake, and toxicity of heavy metals in agricultural soils. J. Agric. Food Chem. 2020, 68, 12856–12869. [Google Scholar] [CrossRef] [PubMed]
- Elbehiry, F.; Elbasiouny, H.; Ali, R. Enhanced Immobilization and Phytoremediation of Heavy Metals in Landfill Contaminated Soils. Water Air Soil Pollut. 2020, 231, 204. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Soil–plant transfer of trace elements—An environmental issue. Geoderma 2004, 122, 143–149. [Google Scholar] [CrossRef]
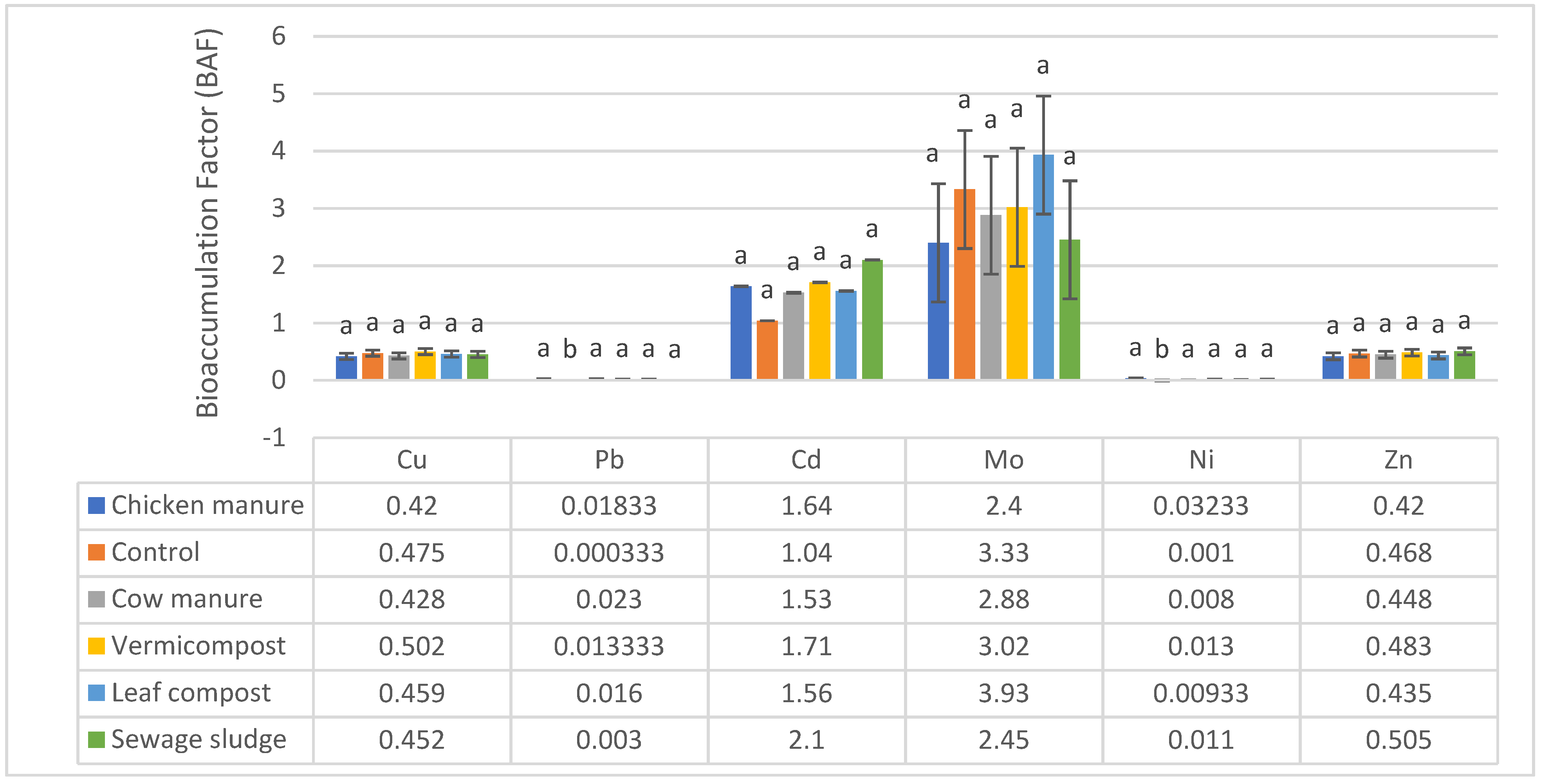
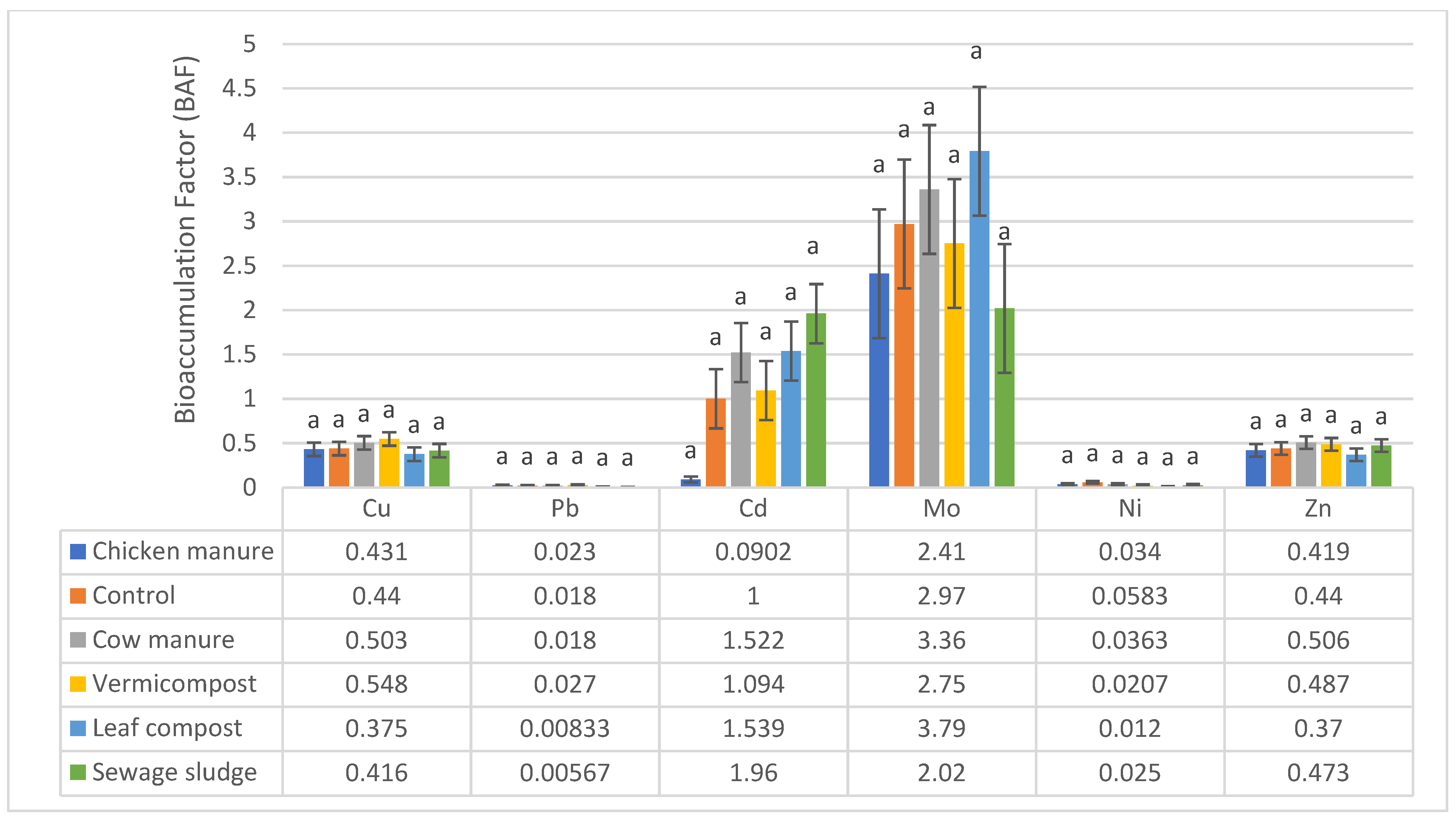
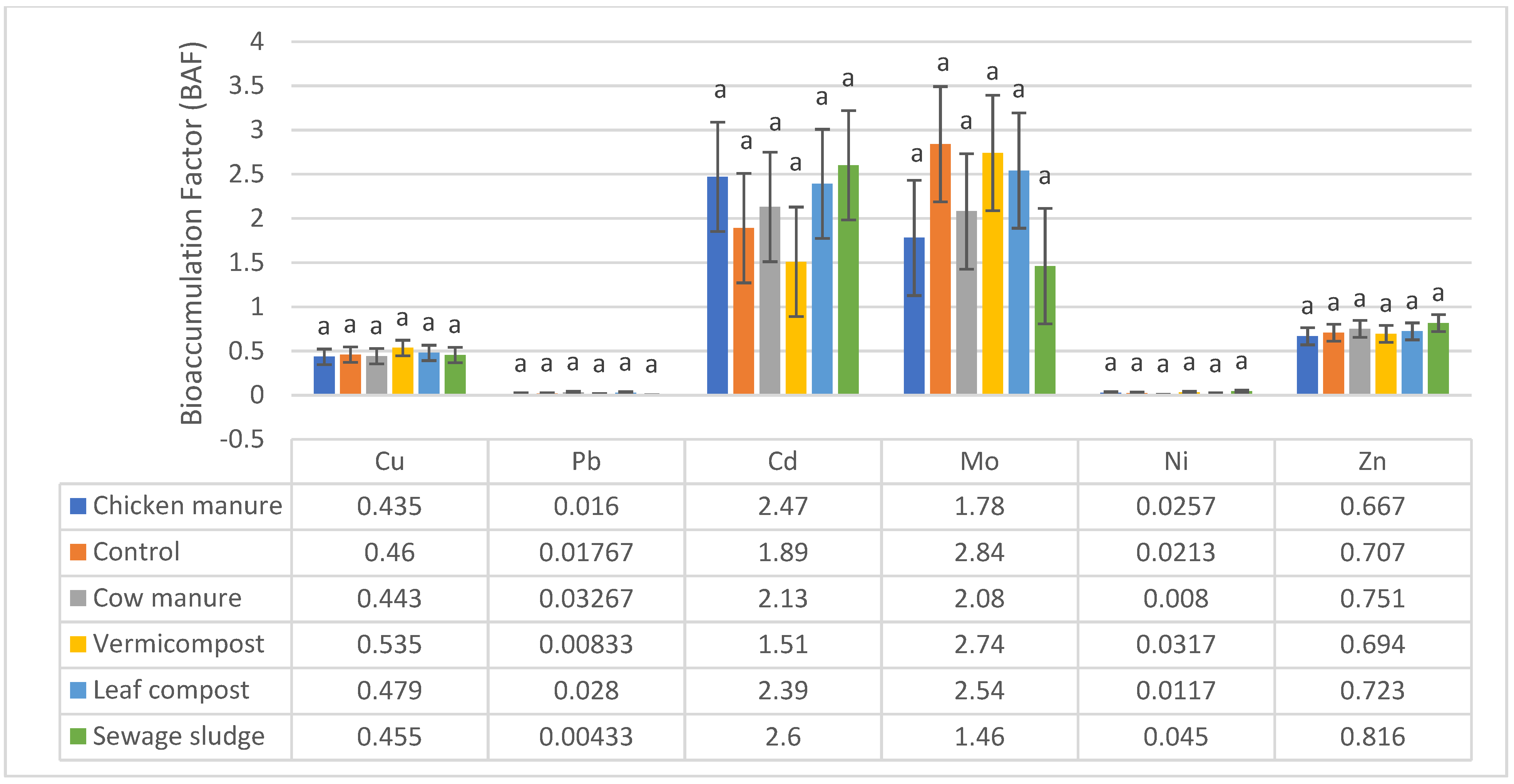
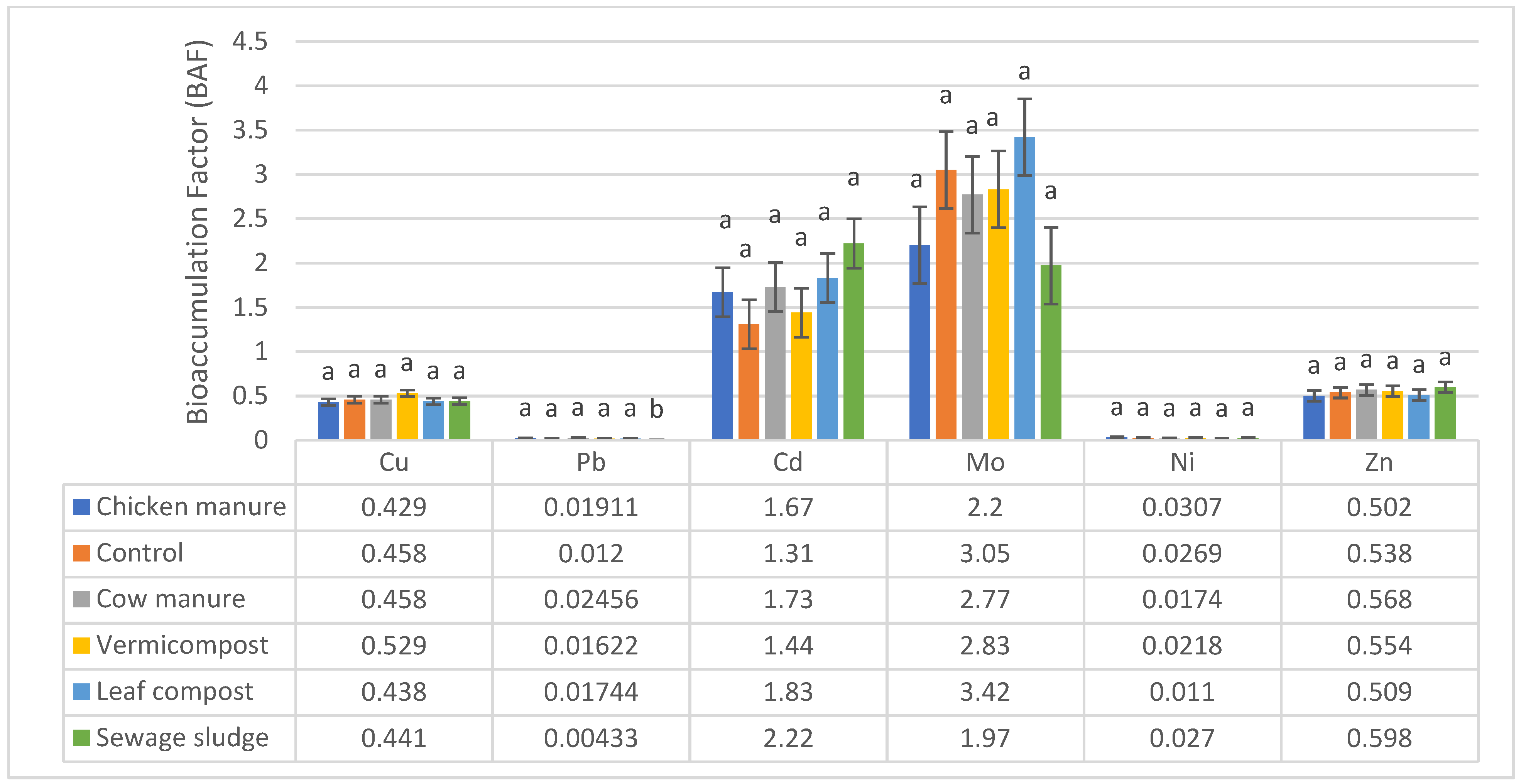
| Soil Parameters | Cow Manure | Sewage Sludge | Leaf Compost | Vermicompost | Chicken Manure | Native Soil (Control) |
|---|---|---|---|---|---|---|
| N (%) | 1.86 b | 0.58 c | 0.32 c | 1.50 b | 4.23 a | 0.15 c |
| P (%) | 0.74 ab | 0.32 b | 0.25 b | 1.27 a | 0.8 | 0.17 c |
| K (%) | 1.25 a | 0.24 b | 0.28 b | 0.56 ab | 0.5 ab | 0.26 b |
| C (%) | 26.2 a | 3.7 c | 3.8 c | 12.2 b | 17.8 b | 1.6 c |
| OM (%) | 5.7 a | 3.2 b | 7.5 a | 7.6 a | 6.3 a | 2.6 b |
| C/N ratio | 14.08 a | 6.4 c | 11.9 b | 8.13 bc | 4.21 c | 10.6 b |
| pH | 7.95 a | 8.4 a | 7.4 a | 5.71 a | 6.15 a | 6.8 a |
| Cd (mg kg−1) | 0.22 a | 0.23 a | 0.19 a | 0.23 a | 0.24 a | 0.23 a |
| Cu (mg kg−1) | 9.23 a | 9.63 a | 10.2 a | 9.8 a | 9.9 a | 10.17 a |
| Mo (mg kg−1) | 0.66 a | 0.78 a | 0.74 a | 0.74 a | 0.84 a | 0.64 a |
| Ni (mg kg−1) | 15.8 a | 16.4 a | 17.1 a | 16.2 a | 18.4 a | 17.5 a |
| Pb (mg kg−1) | 27.9 a | 28.12 a | 28.1 a | 28.7 a | 30.7 a | 31.2 a |
| Zn (mg kg−1) | 52.5 b | 57.8 a | 59.3 a | 60.9 a | 63.4 a | 59.5 a |
| Soil Amendments | Rate (g m−2) |
|---|---|
| Vermicompost (Vermi.) | 1120.52 |
| Sewage sludge (SS) | 224.54 |
| Chicken manure (CM) | 1022.57 |
| Cow manure (Cow) | 1937.5 |
| Leaf compost (Leaf) | 322.92 |
| Soil Amendments | Total Metal Content in Mustard (Mean of Three Varieties) | Total Metal Content in Soil (Mean of Three Replicates) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ni | Cd | Pb | Mo | Cu | Zn | Cd | Mo | Cu | Ni | Pb | Zn | |
| Leaf compost | 0.204 ± 0.13 b | 0.48 ± 0.2 a | 0.493 ± 0.16 b | 2.06 ±0.6 a | 4.49 ± 0.77 a | 30.2 ± 10.76 a | 0.263 ± 0.03 a | 0.747 ± 0.31 a | 10.46 ± 0.58 a | 18 ± 2.38 a | 29 ± 3.55 a | 60.3 ± 3.34 ab |
| Cow manure | 0.301 ± 0.14 b | 0.434 ± 0.12 a | 0.689 ± 0.54 a | 1.76 ± 0.51 a | 4.31 ± 0.73 a | 30.3 ± 12.02 a | 0.253 ± 0.03 a | 0.66 ± 0.1 a | 9.58 ± 0.58 a | 16.8 ± 0.92 a | 28.9 ± 2.65 a | 53.5 ± 3.34 b |
| Chicken manure | 0.565 ± 0.51 a | 0.417 ± 0.28 a | 0.632 ± 0.33 a | 1.76 ± 0.6 a | 4.72 ±1.17 a | 32.9 ± 9.74 a | 0.25 ± 0.04 a | 0.84 ± 0.19 a | 10.98 ± 0.58 a | 18.7 ± 1.57 a | 32.7 ± 1.24 a | 66.4 ± 3.34 a |
| Vermicompost | 0.373 ± 0.32 b | 0.332 ± 0.17 a | 0.484 ± 0.16 b | 2.06 ± 0.49 a | 5.2 ± 2.00 a | 34.2 ± 9.3 a | 0.24 ± 0.03 a | 0.74 ± 0.06 a | 9.8 ± 0.58 a | 17.2 ± 0.87 a | 29.7 ± 1.12 a | 61.9 ± 3.34 ab |
| Sewage sludge | 0.47 ± 0.41 ab | 0.518 ± 0.21 a | 0.119 ± 0.11 c | 1.52 ± 0.41 a | 4.39 ± 0.45 a | 34.5 ± 10.99 a | 0.23 ± 0.04 a | 0.787 ± 0.127 a | 9.97 ± 0.58 a | 17.3 ± 0.4 a | 29.1 ± 0.53 a | 57.8 ± 3.34 ab |
| Control | 0.47 ± 0.13 ab | 0.338 ± 0.23 a | 0.363 ± 0.36 b | 1.86 ± 0.28 a | 4.58 ± 0.7 a | 30.9 ± 9.15 a | 0.26 ± 0.03 a | 0.673 ± 0.20 a | 10.06 ± 0.58 a | 17.5 ± 0.91 a | 30 ± 1.32 a | 57.7 ± 3.34 ab |
| Heavy Metals | Allowable Limit in Soil (mg kg−1) | Allowable Limit in Vegetables (mg kg−1) | Total Metal Content in Soil from the Study (mg kg−1) | Total Metal Content in Mustard from the Study (mg kg−1) |
|---|---|---|---|---|
| Cd | 3 | 0.1 | 0.250 | 0.420 |
| Zn | 300 | 100 | 56 | 32.184 |
| Cu | 100 | 73 | 9.5 | 4.610 |
| Mo | NA | NA | 0.650 | 0.015 |
| Pb | 100 | 0.3 | 30 | 0.463 |
| Ni | 50 | 67 | 16.5 | 0.397 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nepal, A.; Antonious, G.F.; Bebe, F.N.; Webster, T.C.; Gyawali, B.R.; Neupane, B. Heavy Metal Accumulation in Three Varieties of Mustard Grown under Five Soil Management Practices. Environments 2024, 11, 77. https://doi.org/10.3390/environments11040077
Nepal A, Antonious GF, Bebe FN, Webster TC, Gyawali BR, Neupane B. Heavy Metal Accumulation in Three Varieties of Mustard Grown under Five Soil Management Practices. Environments. 2024; 11(4):77. https://doi.org/10.3390/environments11040077
Chicago/Turabian StyleNepal, Anjan, George F. Antonious, Frederick N. Bebe, Thomas C. Webster, Buddhi R. Gyawali, and Basanta Neupane. 2024. "Heavy Metal Accumulation in Three Varieties of Mustard Grown under Five Soil Management Practices" Environments 11, no. 4: 77. https://doi.org/10.3390/environments11040077
APA StyleNepal, A., Antonious, G. F., Bebe, F. N., Webster, T. C., Gyawali, B. R., & Neupane, B. (2024). Heavy Metal Accumulation in Three Varieties of Mustard Grown under Five Soil Management Practices. Environments, 11(4), 77. https://doi.org/10.3390/environments11040077








