The Origin of Phthalates in Algae: Biosynthesis and Environmental Bioaccumulation
Abstract
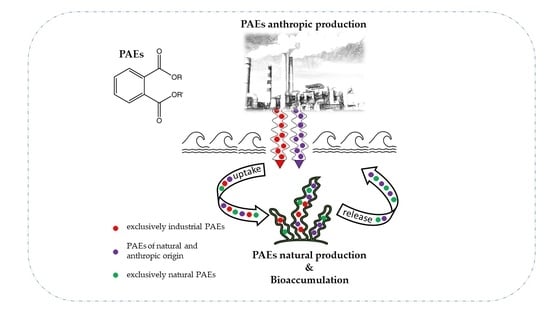
:1. Introduction
2. PAEs and Algal Matrices
2.1. Principal PAEs Studies in Algae
| Species | PAEs (µg/g Dry Weight) | Context | Hypothesis of PAEs’ Origin and Reference | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DnBP | DEHP | MEHP | |||||||||||
| Bangia atropurpurea 8 | 62.14 | 34.74 | L.C. | Biosynthesis [33] | |||||||||
| Porphyra angusta 8 | 22.16 | 6.35 | L.C. | ||||||||||
| Porphyra angusta 8 | 11.67 * | 6.86 * | N.E. | ||||||||||
| Porphyra dentata 8 | 33.47 | 18.53 | L.C. | ||||||||||
| Ulva fasciata 8 | 17.56 * | 11.59 * | N.E. | ||||||||||
| Enteromorpha intestinalis 8 | 18.32 * | 15.77 * | N.E. | ||||||||||
| Cladophora fracta 7 | 77.77 ** | 354.20 | N.E. | Biosynthesis [34] | |||||||||
| Spirogyra sp. 7 | 147.83 | 339.59 ** | N.E. | ||||||||||
| Botryococcus braunii 7 | - | 191.97 | N.E. | ||||||||||
| Chlorella sp. 7 | 41.36 | 376.10 | N.E. | ||||||||||
| Hydrodictyon reticulatum 7 | 165.21 | - | N.E. | ||||||||||
| Ulva lactuca 1 | DEP, DMP, DBP, BBP, DEHP, and DnOP at concentration less than 1 µg/g | L.C. | Bioaccumulation [8] | ||||||||||
| PAEs (compositional %) | |||||||||||||
| DEP | DnBP | DIBP | DEHP | MEHP | DnOP | DIOP | |||||||
| Nizamuddinia zanardinii 9 | 0.70 | 5.10 | N.E. | Biosynthesis [27] | |||||||||
| Padina pavonica 9 | 0.92 | 1.26 | 19.75 | 0.38 | N.E. | Not assessed [24] | |||||||
| Hydroclanthrus clathratus 9 | 0.34 | 40.22 | 1.16 | N.E. | |||||||||
| Cladophora glomerata 6 | 27.70 | N.E. | Not assessed [21] | ||||||||||
| Gelidium crinale 6 | - | - | - | - | - | - | 0.86 | N.E. | Not assessed [30] | ||||
| Sargassum hornschuchii 6 | - | - | - | - | - | - | traces | N.E. | |||||
| Ulva linza 6 | - | - | - | - | - | - | 7.58 | N.E. | |||||
| Sarconema filiforme 3 | 3.67 | 15.67 | N.E. | Not assessed [12] | |||||||||
| Sarconema filiforme 2 | 41.62 | N.E. | |||||||||||
| Laurencia obtusa 3 | 16.12 | N.E. | |||||||||||
| Laurencia obtusa 2 | 54.25 | N.E. | |||||||||||
| Presence (P) or absence (A) of PAEs in algal surface (S), inner part (I), rhizoids (R) or whole (W) | |||||||||||||
| DEP | DnBP | DIBP | BIBP | DEHP | DMBP | MEHP | DnOP | DnDP | DINP | DIOP | |||
| Ulva lactuca 5 | A (W) | P (W) | P (W) | P (W) | N.E. | Bioaccumulation | |||||||
| Enteromorpha linza 5 | A (S) P (I) | P (W) | P (W) | P (W) | N.E. | [35] | |||||||
| Cystoseira barbata 5 | P (S) A (I) | P (W) | P (W) | P (W) | N.E. | ||||||||
| Pterocladia capillacea 5 | P (S) A (I) | P (W) | P (W) | P (W) | N.E. | ||||||||
| Ceramium rubrum 5 | A (W) | P (W) | P (W) | P (W) | N.E. | ||||||||
| Ulva rigida 5 | A (W) | A(W) | P (W) | P (W) | N.E. | Bioaccumulation [36] | |||||||
| Enteromorpha muscoides 5 | P (W) | P (W) | A (W) | A (W) | N.E. | ||||||||
| Enteromorpha linza 5 | A (W) | A(W) | P (W) | P (W) | N.E. | ||||||||
| Gelidium pulchellum 5 | A (W) | A(W) | P (W) | A (W) | N.E. | Bioaccumulation [37] *** | |||||||
| Polysiphonia elongata 5 | A (W) | A(W) | A (W) | P (W) | N.E. | ||||||||
| Coralligena elongata 5 | A (W) | A(W) | A (W) | A (W) | N.E. | ||||||||
| Codium fragile 5 | A (W) | A (W) | A (W) | A (W) | N.E. | ||||||||
| Peyssonnelia squamaria 5 | A (W) | A (W) | P (W) | A (W) | N.E. | ||||||||
| Rhodymenia corrallina 5 | A (W) | A (W) | A (W) | P (W) | N.E. | ||||||||
| Phyllophora nervosa 5 | A (W) | P (W) | P (W) | A (W) | N.E. | ||||||||
| Colpomenia peregrina 5 | A (W) | A (W) | P (W) | P (W) | N.E. | ||||||||
| Cystoseira barbata 5 | A (W) | P (W) | P (W) | A (W) | N.E. | ||||||||
| Zanardina prototypus 5 | A (W) | P (W) | P (W) | A (W) | N.E. | ||||||||
| Gracilaria verrucosa 5 | A (W) | P (W) | A (W) | A (W) | N.E. | ||||||||
| Gracilaria verrucosa 5 | P (W) | N.E. | |||||||||||
| Polysiphonia elongata 5 | P (W) | N.E. | |||||||||||
| Phyllophora nervosa 5 | P (W) | P (W) | N.E. | ||||||||||
| Rhodymenia corrallina 5 | P (W) | N.E. | |||||||||||
| Ceramium rubrum 5 | P (W) | P (W) | P (W) | N.E. | |||||||||
| Gelidium pulchellum 5 | P (W) | P (W) | P (W) | N.E. | |||||||||
| Cystoseira barbata 5 | P (W) | P (W) | P (W) | P (W) | N.E. | ||||||||
| Zanardina prototypus 5 | P (W) | P (W) | N.E. | ||||||||||
| Colpomenia peregrina 5 | P (W) | P (W) | N.E. | ||||||||||
| Ulva rigida 5 | P (W) | N.E. | |||||||||||
| Ulva lactuca 5 | P (W) | P (W) | N.E. | ||||||||||
| Enteromorpha muscoides 5 | P (W) | N.E. | |||||||||||
| Enteromorpha linza 5 | P (W) | P (W) | P (W) | P (W) | N.E. | ||||||||
| Gracilaria lemaneiformis | P (W) | N.E. | Biosynthesis [38] | ||||||||||
| Cladophora fracta | P (W) | P (W) | N.E. | Biosynthesis [39] | |||||||||
| Chaetomorpha basiretorsa | P (W) | P (W) | N.E. | Biosynthesis [40] | |||||||||
| Ulva tepida 5 | P (W) | N.E. | Uncertain [29] | ||||||||||
| Desmarestia anceps 2 | A (W) | A(W) | P (W) | A (W) | A(W) | N.E. | Not assessed [41] | ||||||
| Desmarestia anceps 3 | A (W) | A(W) | P (W) | A (W) | A(W) | N.E. | |||||||
| Pyropia endiviifolia 1 | A (W) | A(W) | P (W) | A (W) | P (W) | N.E. | |||||||
| Pyropia endiviifolia 2 | A (W) | P (W) | P (W) | A (W) | P (W) | P (W) | N.E. | ||||||
| Pyropia endiviifolia 3 | A (W) | P (W) | P (W) | A (W) | P (W) | P (W) | N.E. | ||||||
| Sargassum wightii 4 | P (W) | N.E. | Biosynthesis [23] | ||||||||||
| Caulerpa racemosa | P (W) | P (W) | P (W) | P (W) | N.E. | Uncertain [26] | |||||||
| Codium tomentosum | - | P (W) | P (W) | P (W) | N.E. | ||||||||
| Sargassum confusum 1,6 | P (W) | P (W) | P (W) | N.E. | Biosynthesis [18] | ||||||||
| Stoechospermum marginatum 10 | P (W) | N.E. | Bioaccumulation [17] | ||||||||||
| Sargassum wightii 2,6 | P (W) | N.E. | Biosynthesis [42] | ||||||||||
| Sargassum muticum | P (W) | P (W) | N.E. | Uncertain [43] | |||||||||
| Laminaria japonica 5,6 | P (W) | P (W) | L.C. | Biosynthesis [44] | |||||||||
| Ulva sp. 5,6 | P (W) | P (W) | L.C. | ||||||||||
| Undaria pinnatifida 5,6 | P (W) | P (W) | L.C. | ||||||||||
| Croisetta sp. | P (W) | N.E. | Uncertain [45] | ||||||||||
| Laminaria japonica 3 | P (R) | N.E. | Not assessed [20] | ||||||||||
| Iridea cordata 2,3 | P (W) | P (W) | P (W) | N.E. | Uncertain [31] | ||||||||
2.2. Hypothesis of PAEs Origin
2.2.1. Biosynthesis
2.2.2. Bioaccumulation
2.2.3. Critical Analysis of Undefined PAEs’ Origin
2.3. Algae as the Future of PAEs’ Bioremediation
2.4. Photodegradation of Phthalates
3. Future Directions
- (1)
- Phthalate is present in all individuals;
- (2)
- Phthalate is not present in any individual;
- (3)
- Phthalate is present with variable frequency between individuals.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gao, J.; Chi, J. Biodegradation of Phthalate Acid Esters by Different Marine Microalgal Species. Mar. Pollut. Bull. 2015, 99, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Das, M.T.; Kumar, S.S.; Ghosh, P.; Shah, G.; Malyan, S.K.; Bajar, S.; Thakur, I.S.; Singh, L. Remediation Strategies for Mitigation of Phthalate Pollution: Challenges and Future Perspectives. J. Hazard. Mater. 2021, 409, 124496. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhu, X.; Zhou, S.; Cheng, Z.; Shi, K.; Zhang, C.; Shao, H. Phthalic Acid Esters: Natural Sources and Biological Activities. Toxins 2021, 13, 495. [Google Scholar] [CrossRef]
- Savoca, D.; Barreca, S.; Lo Coco, R.; Punginelli, D.; Orecchio, S.; Maccotta, A. Environmental Aspect Concerning Phthalates Contamination: Analytical Approaches and Assessment of Biomonitoring in the Aquatic Environment. Environments 2023, 10, 99. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 Update. Nucleic Acids Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.; Fasano, M.; Palandri, L.; Righi, E. A review of European and international phthalates regulation: Focus on daily use products. Eur. J. Public Health 2022, 32, ckac131-226. [Google Scholar] [CrossRef]
- Zhao, E.; Xiong, X.; Hu, H.; Li, X.; Wu, C. Phthalates in Plastic Stationery in China and Their Exposure Risks to School-Aged Children. Chemosphere 2023, 339, 139763. [Google Scholar] [CrossRef] [PubMed]
- Savoca, D.; Lo Coco, R.; Melfi, R.; Pace, A. Uptake and Photoinduced Degradation of Phthalic Acid Esters (PAEs) in Ulva Lactuca Highlight Its Potential Application in Environmental Bioremediation. Environ. Sci. Pollut. Res. 2022, 29, 90887–90897. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.L. Algae: The World’s Most Important “Plants”—An Introduction. Mitig. Adapt. Strateg. Glob. Chang. 2013, 18, 5–12. [Google Scholar] [CrossRef]
- Pereira, L. Macroalgae. Encyclopedia 2021, 1, 177–188. [Google Scholar] [CrossRef]
- Correa, I.; Drews, P.; Botelho, S.; De Souza, M.S.; Tavano, V.M. Deep Learning for Microalgae Classification. In Proceedings of the 2017 16th IEEE International Conference on Machine Learning and Applications (ICMLA), Cancun, Mexico, 18–21 December 2017; IEEE: New York, NY, USA; pp. 20–25. [Google Scholar]
- Teleb, W.K.; Tantawy, M.A.; Osman, N.A.H.K.; Abdel-Rahman, M.A.; Hussein, A.A. Structural and Cytotoxic Characterization of the Marine Red Algae Sarconema Filiforme and Laurencia Obtusa. Egypt. J. Aquat. Biol. Fish 2022, 26, 549–573. [Google Scholar] [CrossRef]
- Cheney, D.; Rajic, L.; Sly, E.; Meric, D.; Sheahan, T. Uptake of PCBs Contained in Marine Sediments by the Green Macroalga Ulva Rigida. Mar. Pollut. Bull. 2014, 88, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Roccuzzo, S.; Beckerman, A.P.; Trögl, J. New Perspectives on the Bioremediation of Endocrine Disrupting Compounds from Wastewater Using Algae-, Bacteria- and Fungi-Based Technologies. Int. J. Environ. Sci. Technol. 2021, 18, 89–106. [Google Scholar] [CrossRef]
- Astrahan, P.; Korzen, L.; Khanin, M.; Sharoni, Y.; Israel, Á. Seaweeds Fast EDC Bioremediation: Supporting Evidence of EE2 and BPA Degradation by the Red Seaweed Gracilaria Sp., and a Proposed Model for the Remedy of Marine-Borne Phenol Pollutants. Environ. Pollut. 2021, 278, 116853. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.N. Bioactive Natural Derivatives of Phthalate Ester. Crit. Rev. Biotechnol. 2020, 40, 913–929. [Google Scholar] [CrossRef] [PubMed]
- Wahidulla, S.; De Souza, L. Phthalate Esters from the Brown Alga Stoechospermum Marginatum (C. Agardh) Kuetzing. Bot. Mar. 1995, 38, 333–334. [Google Scholar] [CrossRef]
- Ganti, V.S.; Kim, K.H.; Bhattarai, H.D.; Shin, H.W. Isolation and Characterisation of Some Antifouling Agents from the Brown Alga Sargassum Confusum. J. Asian Nat. Prod. Res. 2006, 8, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraj, R.; Altaff, K.; Kumaran, N.S. Volatile Bioactive Compounds from Marine Macro-Algae and Their Pharmacological Properties. Indian J. Geo-Marine Sci. 2022, 51, 832–842. [Google Scholar] [CrossRef]
- Bu, T.; Liu, M.; Zheng, L.; Guo, Y.; Lin, X. A-glucosidase Inhibition and the in Vivo Hypoglycemic Effect of Butyl-isobutyl-phthalate Derived from the Laminaria Japonica Rhizoid. Phytother. Res. 2010, 24, 1588–1591. [Google Scholar] [CrossRef] [PubMed]
- Usha, N.S.; Sabari Anand, J.V. Mangaiyarkarasi Preliminary Phytochemical Screening and GC-MS Analysis of Cladophora Glomerata: Green Marine Algae. Int. J. Basic Clin. Pharmacol. 2019, 8, 732. [Google Scholar] [CrossRef]
- El Shouny, W.A.; Gaafar, R.M.; Ismail, G.A.; Elzanaty, M.M. Antibacterial Activity of Some Seaweed Extracts against Multidrug Resistant Urinary Tract Bacteria and Analysis of Their Virulence Genes. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 2569–2586. [Google Scholar] [CrossRef]
- Rosaline, D.; Sakthivelkumar, S.; Rajendran, K.; Janarthanan, S. Detection of Antibacterial Activity and Its Characterization from the Marine Macro-Algae Sargassum Wightii (Greville Ex J. Agardh 1848). Indian J. Mar. Sci. 2016, 45, 1365–1371. [Google Scholar]
- Awad, N.E.; Motawe, H.M.; Selim, M.A.; Matloub, A.A. Volatile Constituents of the Brown Algae Padina Pavonia (L.) Gaill. and Hydroclathrus Clathratus (C. Agardh) Howe and Their Antimicrobial Activity. Med. Aromat. Plant Sci. Biotechnol. 2009, 3, 12–15. [Google Scholar]
- Awad, N.E.; Selim, M.A.; Saleh, M.M.; Matloub, A.A. Seasonal variation of the lipoidal matters and hypolipidaemic activity of the red alga Corallina officinalis L. Phytother. Res. 2003, 17, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Awad, N.E. Volatile constituents, carbohydrate compositions and topical anti-inflammatory of the two green algae Caulerpa racemosa (O. Dargent) and Codium tomentosum (Stackhouse). Bull. Fac. Pharm. 2002, 40, 233–243. [Google Scholar]
- Firouzi, J.; Gohari, A.R.; Rustaiyan, A.; Larijani, K.; Saeidnia, S. Composition of the Essential Oil of Nizamuddinia Zanardinii, a Brown Alga Collected from Oman Gulf. J. Essent. Oil Bear. Plants 2013, 16, 689–692. [Google Scholar] [CrossRef]
- Noguchi, T.; Ikawa, M.; Uebel, J.J.; Andersen, K.K. Lipid constituents of the red algae Ceramium rubrum. A search for antimicrobial and chemical defense substances. In Marine Algae in Pharmaceutical Science; Hoppe, H.A., Levring, T., Tanaka, Y., Eds.; Walter de Gruyter & Co: New York, NY, USA, 1979; pp. 711–718. [Google Scholar]
- Agusman; Qi, Y.; Wu, Z.; He, J.; Rittschof, D.; Su, P.; Ke, C.; Feng, D. Conspecific Cues That Induce Spore Settlement in the Biofouling and Green Tide-Forming Alga Ulva Tepida Provide a Potential Aggregation Mechanism. Int. Biodeterior. Biodegrad. 2019, 145, 104807. [Google Scholar] [CrossRef]
- Mohy El-Din, S.M.; Alagawany, N.I. Phytochemical Constituents and Anticoagulation Property of Marine Algae Gelidium Crinale, Sargassum Hornschuchii and Ulva Linza. Thalassas 2019, 35, 381–397. [Google Scholar] [CrossRef]
- Rangel, K.C.; Debonsi, H.M.; Clementino, L.C.; Graminha, M.A.S.; Vilela, L.Z.; Colepicolo, P.; Gaspar, L.R. Antileishmanial Activity of the Antarctic Red Algae Iridaea Cordata (Gigartinaceae; Rhodophyta). J. Appl. Phycol. 2019, 31, 825–834. [Google Scholar] [CrossRef]
- Gu¨ven, K.C.; Reisch, J.; Kizil, Z.; Gu¨vener, B.; Cevher, E. Dimethyl Terephthalate Pollution in Red Algae. Phytochemistry 1990, 29, 3115. [Google Scholar] [CrossRef]
- Chen, C.Y. Biosynthesis of Di-(2-Ethylhexyl) Phthalate (DEHP) and Di-n-Butyl Phthalate (DBP) from Red Alga—Bangia Atropurpurea. Water Res. 2004, 38, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Babu, B.; Wu, J.-T. Production of Phthalate Esters by Nuisance Freshwater Algae and Cyanobacteria. Sci. Total Environ. 2010, 408, 4969–4975. [Google Scholar] [CrossRef] [PubMed]
- Gezgin, T.; Güven, K.C.; Akçin, G. Phthalate esters in marine algae. Turk. J. Mar. Sci. 2001, 7, 119–130. [Google Scholar]
- Erakin, S.; Güven, K.C. The volatile petroleum hydrocarbons in marine algae around Turkish Coasts. Acta Pharm. Sci. 2008, 50, 167–182. [Google Scholar]
- Erakin, S.; Binark, N.; Güven, K.C.; Coban, B.; Erduğan, H. Phthalates pollution in algae of Turkish coast. J Black Sea/Medit. 2014, 20, 122–126. [Google Scholar]
- Lu, H.; Xie, H.; Yang, Y.; Wei, X. Chemical Constituents from the Macroalga Gracilaria lemaneiformis. J. Trop. Subtrop. Bot. 2011, 19, 166–170. [Google Scholar]
- Dong, S.J.; Bi, X.D.; Wang, N.; Song, L.; Dai, W.; Zhang, S.L. Algicidal activities of Cladophora fracta on red tide-forming microalgae Heterosigma akashiwo and Gymnodinium breve. Allelopath. J. 2016, 37, 231–240. [Google Scholar]
- Shi, D.; Han, L.; Sun, J.; Wang, Y.; Yang, Y.; Shi, J.; Fan, X. Chemical constituents from marine alga Chaetomorpha basiretorsa. China J. Chin. Mater. Med. 2005, 30, 347–350. [Google Scholar]
- De Souza, P.O.; Silva, F.A.; Frozza, C.O.D.S.; Frassini, R.; Roesch-Ely, M.; Dos Santos, M.A.Z.; Freitag, R.A.; Colepicolo, P.; Pereira, C.M.P.; Braganhol, E. Bioprospecting of New Antarctic Seaweed Selective Antitumor Molecules: Chemical Characterization and in Vitro Analysis. Phytomed. Plus 2022, 2, 100246. [Google Scholar] [CrossRef]
- Sastry, V.M.V.S.; Rao, G.R.K. Dioctyl Phthalate, and Antibacterial Compound from the Marine Brown Alga? Sargassum Wightii. J. Appl. Phycol. 1995, 7, 185–186. [Google Scholar] [CrossRef]
- Bazes, A.; Silkina, A.; Douzenel, P.; Faÿ, F.; Kervarec, N.; Morin, D.; Berge, J.-P.; Bourgougnon, N. Investigation of the Antifouling Constituents from the Brown Alga Sargassum Muticum (Yendo) Fensholt. J. Appl. Phycol. 2009, 21, 395–403. [Google Scholar] [CrossRef]
- Namikoshi, M.; Fujiwara, T.; Nishikawa, T.; Ukai, K. Natural Abundance 14C Content of Dibutyl Phthalate (DBP) from Three Marine Algae. Mar. Drugs 2006, 4, 290–297. [Google Scholar] [CrossRef]
- Kasanah, N.; Seto, D.S.; Ulfah, M.; Peng, Z. Triyanto First Report on Chemistry of a Red Seaweed Croisettea Sp. from the Coastal Area of Yogyakarta, Indonesia. Mar. Biol. Res. 2022, 18, 215–221. [Google Scholar] [CrossRef]
- Thiemann, T. Isolation of Phthalates and Terephthalates from Plant Material—Natural Products or Contaminants? Open Chem. J. 2021, 8, 1–36. [Google Scholar] [CrossRef]
- Masojídek, J.; Ranglová, K.; Lakatos, G.E.; Silva Benavides, A.M.; Torzillo, G. Variables Governing Photosynthesis and Growth in Microalgae Mass Cultures. Processes 2021, 9, 820. [Google Scholar] [CrossRef]
- Chan, H.W.; Lau, T.C.; Ang, P.O.; Wu, M.; Wong, P.K. Biosorption of Di(2-Ethylhexyl)Phthalate by Seaweed Biomass. J. Appl. Phycol. 2004, 16, 263–274. [Google Scholar] [CrossRef]
- Semenov, A.A.; Enikeev, A.G.; Babenko, T.A.; Shafikova, T.N.; Gorshkov, A.G. Phthalates—A Strange Delusion of Ecologists. Theor. Appl. Ecol. 2021, 1, 16–21. [Google Scholar] [CrossRef]
- Cho, J.-Y.; Choi, J.-S.; Kang, S.-E.; Kim, J.-K.; Shin, H.-W.; Hong, Y.-K. Isolation of Antifouling Active Pyroglutamic Acid, Triethyl Citrate and Di-n-Octylphthalate from the Brown Seaweed Ishige Okamurae. J. Appl. Phycol. 2005, 17, 431–435. [Google Scholar] [CrossRef]
- Osman, M.E.H.; Aboshady, A.M.; Elshobary, M.E. Production and Characterization of Antimicrobial Active Substance from Some Macroalgae Collected from Abu-Qir Bay Alexandria, Egypt. Afr. J. Biotechnol. 2013, 12, 6847–6858. [Google Scholar]
- Sarkar, A.; Bagavananthem Andavan, G.S. Computational Optimization of in Vitro Parameters for Di-(2-ethylhexyl) Phthalate Production from Anabaena Circinalis. J. Cell. Biochem. 2019, 120, 790–798. [Google Scholar] [CrossRef]
- Staples, C.A.; Peterson, D.R.; Parkerton, T.F.; Adams, W.J. The Environmental Fate of Phthalate Esters: A Literature Review. Chemosphere 1997, 35, 667–749. [Google Scholar] [CrossRef]
- Seng, N.; Lai, S.; Fong, J.; Saleh, M.F.; Cheng, C.; Cheok, Z.Y.; Todd, P.A. Early evidence of microplastics on seagrass and macroalgae. Mar. Freshw. 2020, 71, 922–928. [Google Scholar] [CrossRef]
- Castro-Jiménez, J.; Ratola, N. An Innovative Approach for the Simultaneous Quantitative Screening of Organic Plastic Additives in Complex Matrices in Marine Coastal Areas. Environ. Sci. Pollut. Res. 2020, 27, 11450–11457. [Google Scholar] [CrossRef] [PubMed]
- Jebara, A.; Albergamo, A.; Rando, R.; Potortì, A.G.; Lo Turco, V.; Mansour, H.B.; Di Bella, G. Phthalates and Non-Phthalate Plasticizers in Tunisian Marine Samples: Occurrence, Spatial Distribution and Seasonal Variation. Mar. Pollut. Bull. 2021, 163, 111967. [Google Scholar] [CrossRef]
- Liu, B.; Lv, L.; Ding, L.; Gao, L.; Li, J.; Ma, X.; Yu, Y. Comparison of Phthalate Esters (PAEs) in Freshwater and Marine Food Webs: Occurrence, Bioaccumulation, and Trophodynamics. J. Hazard. Mater. 2024, 466, 133534. [Google Scholar] [CrossRef] [PubMed]
- Berneira, L.; Da Silva, C.; Poletti, T.; Ritter, M.; Dos Santos, M.; Colepicolo, P.; De Pereira, C.M.P. Evaluation of the Volatile Composition and Fatty Acid Profile of Seven Antarctic Macroalgae. J. Appl. Phycol. 2020, 32, 3319–3329. [Google Scholar] [CrossRef]
- Sun, S.-M.; Chung, G.-H.; Shin, T.-S. Volatile Compounds of the Green Alga, Capsosiphon Fulvescens. J. Appl. Phycol. 2012, 24, 1003–1013. [Google Scholar] [CrossRef]
- Li, F.; Wu, M.; Yao, Y.; Zheng, X.; Zhao, J.; Wang, Z.; Xing, B. Inhibitory Effects and Oxidative Target Site of Dibutyl Phthalate on Karenia Brevis. Chemosphere 2015, 132, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yi, X.; Zhou, H.; Chi, T.; Li, W.; Yang, K. Combined Effect of Polystyrene Microplastics and Dibutyl Phthalate on the Microalgae Chlorella pyrenoidosa. Environ. Pollut. 2020, 257, 113604. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Q.J.; Zhao, W.Y.; Cheng, S.P. Toxicity of Dibutyl Phthalate to Algae. Bull. Environ. Contam. Toxicol. 2003, 71, 602–608. [Google Scholar] [CrossRef]
- Melin, C.; Egnéus, H. Effects of Di- n -butyl Phthalate on Growth and Photosynthesis in Algae and on Isolated Organelles from Higher Plants. Physiol. Plant. 1983, 59, 461–466. [Google Scholar] [CrossRef]
- Gu, S.; Zheng, H.; Xu, Q.; Sun, C.; Shi, M.; Wang, Z.; Li, F. Comparative Toxicity of the Plasticizer Dibutyl Phthalate to Two Freshwater Algae. Aquat. Toxicol. 2017, 191, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, G.; Zheng, H.; Liu, N.; Shi, M.; Luo, X.; Chen, L.; Li, F.; Hu, S. Fate of Four Phthalate Esters with Presence of Karenia Brevis: Uptake and Biodegradation. Aquat. Toxicol. 2019, 206, 81–90. [Google Scholar] [CrossRef]
- Chi, J.; Liu, H.; Li, B.; Huang, G.-L. Accumulation and Biodegradation of Dibutyl Phthalate in Chlorella vulgaris. Bull. Environ. Contam. Toxicol. 2006, 77, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Pan, G. Increase in Biodegradation of Dimethyl Phthalate by Closterium Lunula Using Inorganic Carbon. Chemosphere 2004, 55, 1281–1285. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.; Li, B.; Wang, Q.Y.; Liu, H. Influence of Nutrient Level on Biodegradation and Bioconcentration of Phthalate Acid Esters in Chlorella vulgaris. J. Environ. Sci. Health Part A 2007, 42, 179–183. [Google Scholar] [CrossRef]
- Chi, J.; Li, Y.; Gao, J. Interaction between Three Marine Microalgae and Two Phthalate Acid Esters. Ecotoxicol. Environ. Saf. 2019, 170, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, C.J. Ultraviolet radiation and phytoplankton photosynthesis 1. Limnol. Oceanogr. 1979, 24, 1117–1120. [Google Scholar] [CrossRef]
- Gledhill, W.E.; Kaley, R.G.; Adams, W.J.; Hicks, O.; Michael, P.R.; Saeger, V.W.; LeBlanc, G.A. An environmental safety assessment of butyl benzyl phthalate. Environ. Sci. Technol. 1980, 14, 301–305. [Google Scholar] [CrossRef]
- Lertsirisopon, R.; Soda, S.; Sei, K.; Ike, M. Abiotic degradation of four phthalic acid esters in aqueous phase under natural sunlight irradiation. J. Environ. Sci. 2009, 21, 285–290. [Google Scholar] [CrossRef]
- Feng, J.R.; Deng, Q.X.; Ni, H.G. Photodegradation of phthalic acid esters under simulated sunlight: Mechanism, kinetics, and toxicity change. Chemosphere 2022, 299, 134475. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Li, X.; Feng, L. Behavior of stable carbon isotope of phthalate acid esters during photolysis under ultraviolet irradiation. Chemosphere 2013, 92, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, K.; Branchu, P.; Boudahmane, L.; Caupos, E.; Demare, D.; Deshayes, S.; Dubois, P.; Kajeiou, M.; Meffray, L.; Partibane, C.; et al. Mass Balance of Micropollutants over the First Year of Operation in a Stormwater Biofilter: A Field Approach Integrating Water and Soil. 2019. Available online: https://enpc.hal.science/hal-02190391/document (accessed on 20 February 2024).
- Tian, C.; Ni, J.; Chang, F.; Liu, S.; Xu, N.; Sun, W.; Xie, Y.; Guo, Y.; Ma, Y.; Yang, Z.; et al. Bio-Source of di-n-butyl Phthalate Production by Filamentous Fungi. Sci. Rep. 2016, 6, 19791. [Google Scholar] [CrossRef] [PubMed]

| PAEs | Acronym | Molecular Formula | R | R′ | Log Kow |
|---|---|---|---|---|---|
| dimethyl phthalate | DMP | C10H10O4 | CH3 | CH3 | 1.60 |
| diethyl phthalate | DEP | C12H14O4 | CH2CH3 | CH2CH3 | 2.47 |
| mono(2-ethylhexyl) phthalate | MEHP | C16H22O4 | CH(CH2)5(CH3)2 | H | 4 * |
| dibutyl phthalate | DnBP | C16H22O4 | CH2CH2CH2CH3 | CH2CH2CH2CH3 | 4.50 |
| diisobutyl phthalate | DIBP | C16H22O4 | CH2CH(CH3)2 | CH2CH(CH3)2 | 4.11 |
| butyl isobutyl phthalate | BIBP | C16H22O4 | CH2CH(CH3)2 | CH2CH2CH2CH3 | 4.8 * |
| di(2-methylbutyl) phthalate | DMBP | C18H26O4 | CH(CH2)2(CH3)2 | CH(CH2)2(CH3)2 | - |
| benzyl butyl phthalate | BBzP | C19H20O4 | CH2C6H5 | CH2C6H5 | 4.73 |
| di(2-ethylhexyl) phthalate | DEHP | C24H38O4 | CH(CH2)5(CH3)2 | CH(CH2)5(CH3)2 | 7.60 |
| di-n-octyl phthalate | DnOP | C24H38O4 | (CH2)7CH3 | (CH2)7CH3 | 8.10 |
| diisooctyl phthalate | DIOP | C24H38O4 | CH(CH2)5(CH3)2 | CH(CH2)5(CH3)2 | 8.5 * |
| di-isononyl phthalate | DINP | C26H42O4 | CH(CH2)6(CH3)2 | CH(CH2)6(CH3)2 | 9.6 * |
| di-n-decyl phthalate | DnDP | C28H46O4 | (CH2)8CH3 | (CH2)8CH3 | 11.2 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pace, A.; Vaglica, A.; Maccotta, A.; Savoca, D. The Origin of Phthalates in Algae: Biosynthesis and Environmental Bioaccumulation. Environments 2024, 11, 78. https://doi.org/10.3390/environments11040078
Pace A, Vaglica A, Maccotta A, Savoca D. The Origin of Phthalates in Algae: Biosynthesis and Environmental Bioaccumulation. Environments. 2024; 11(4):78. https://doi.org/10.3390/environments11040078
Chicago/Turabian StylePace, Andrea, Alessandro Vaglica, Antonella Maccotta, and Dario Savoca. 2024. "The Origin of Phthalates in Algae: Biosynthesis and Environmental Bioaccumulation" Environments 11, no. 4: 78. https://doi.org/10.3390/environments11040078