Life Cycle Assessment of Immobilised and Slurry Photocatalytic Systems for Removal of Natural Organic Matter in Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Photocatalytic Experiments
2.3. LCA Methodology
2.3.1. Goal and Scope
2.3.2. Functional Unit
2.3.3. System Boundary
2.3.4. Life Cycle Inventory
2.3.5. Life Cycle Impact Assessment
3. Results and Discussion
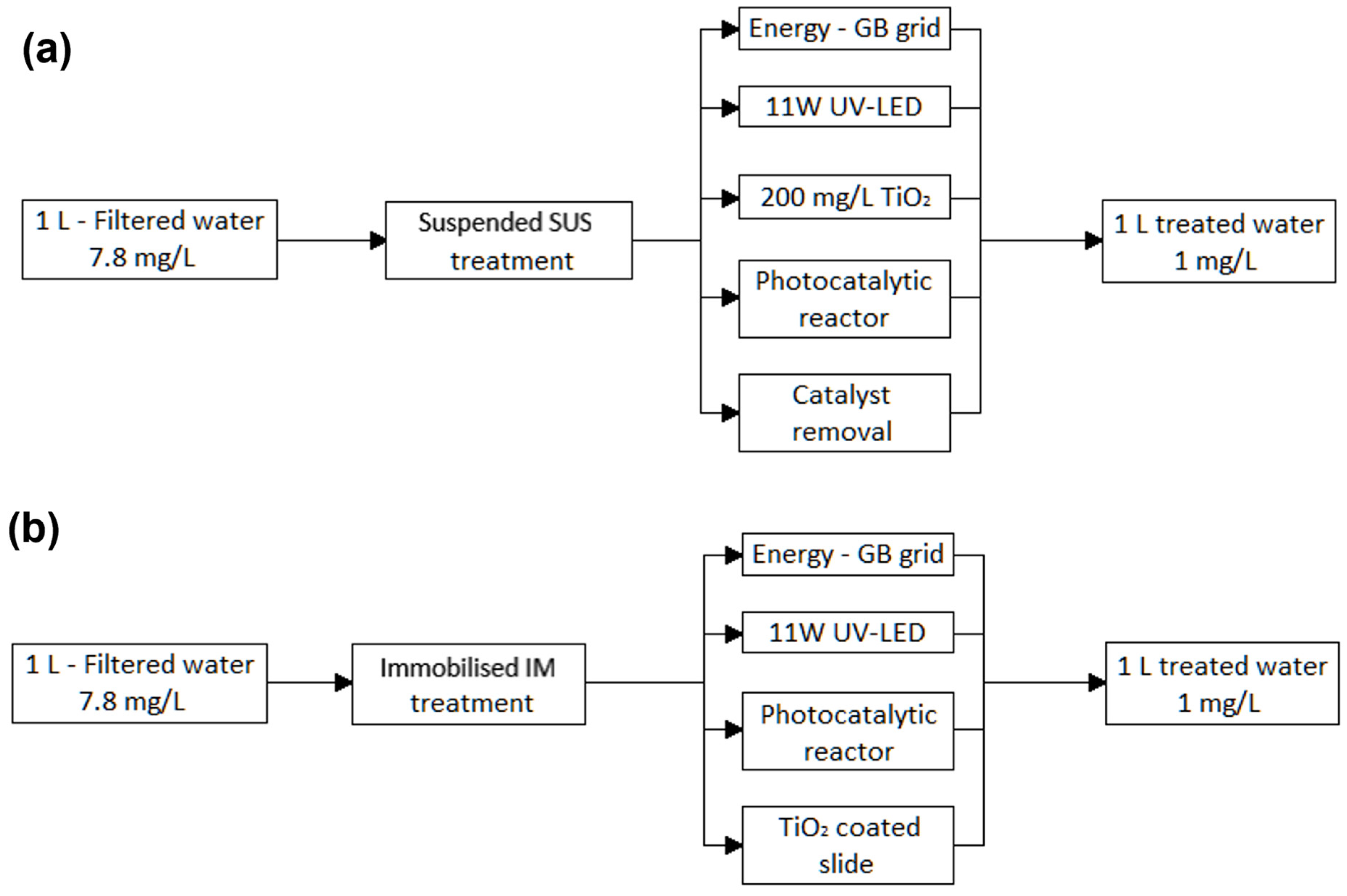
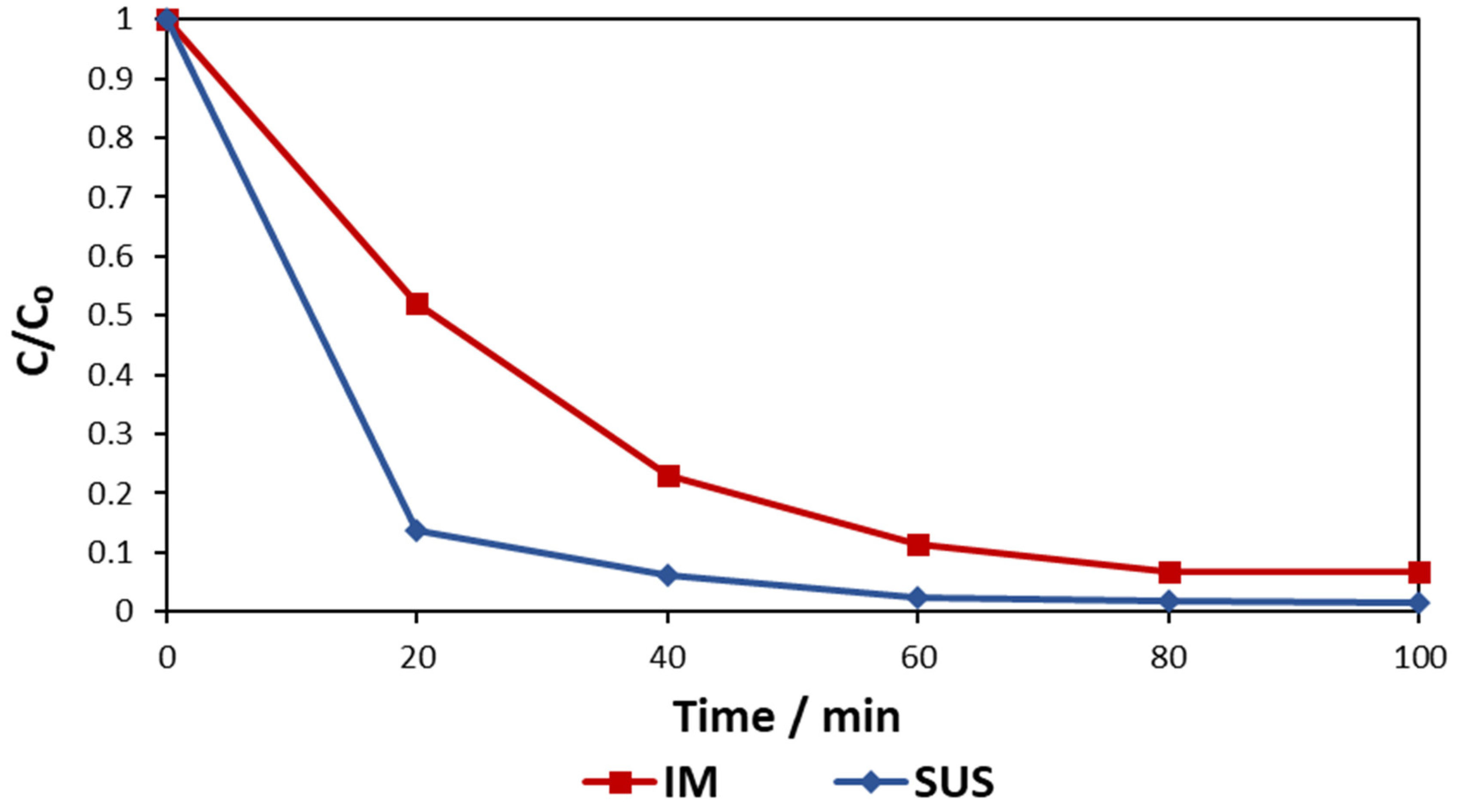
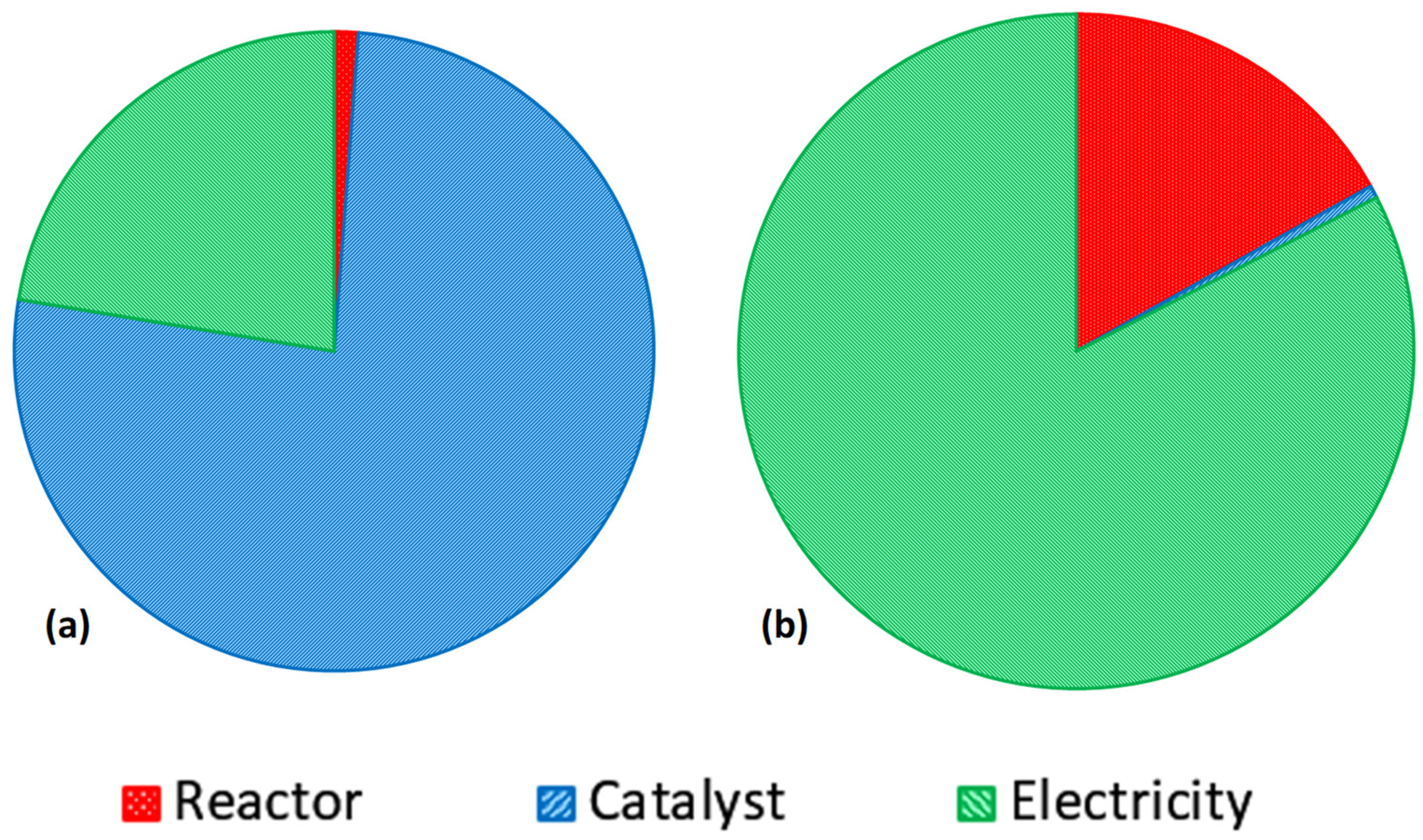
3.1. Suspended vs. Immobilised Photocatalysis
3.2. LCA Results
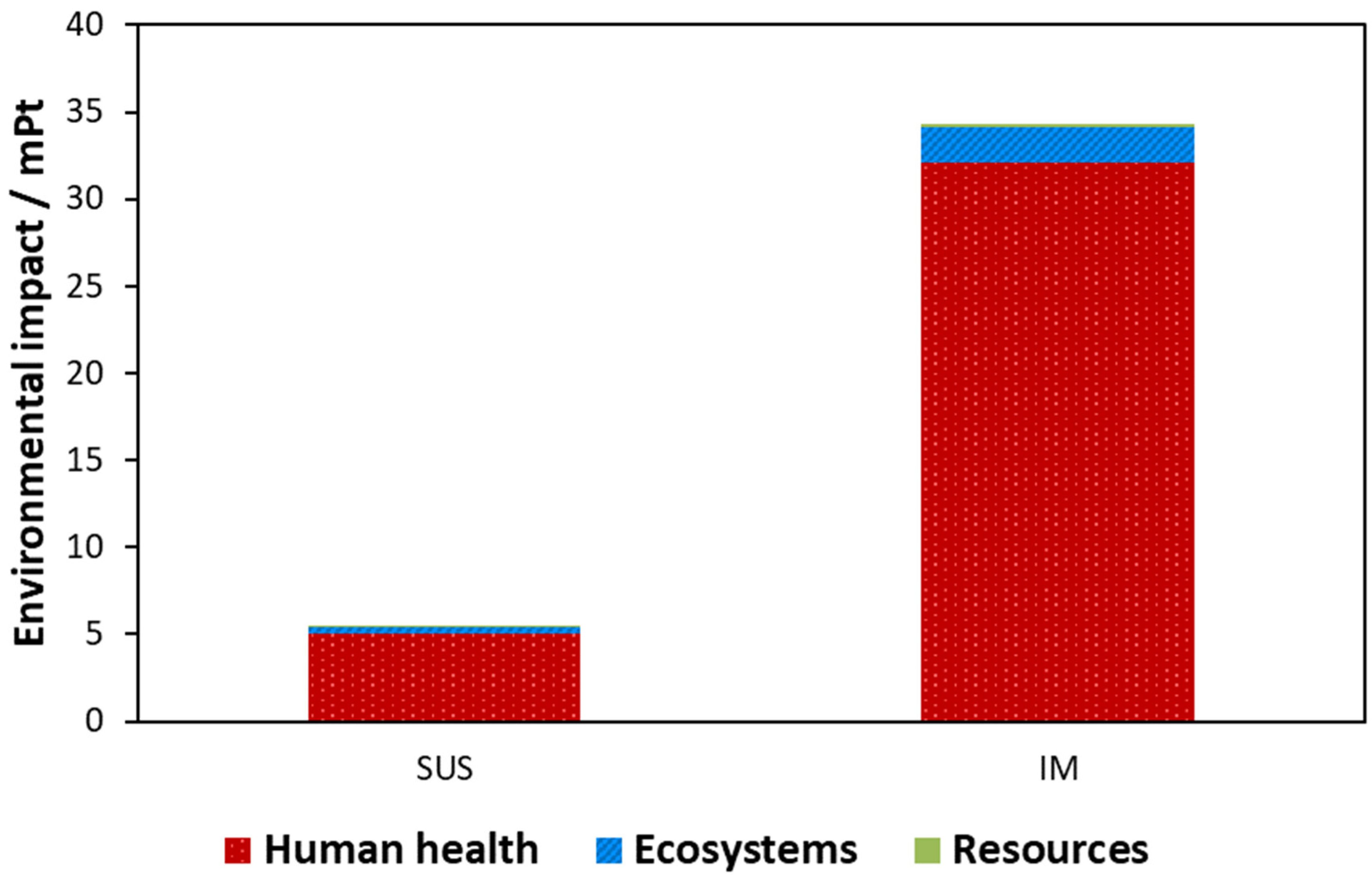
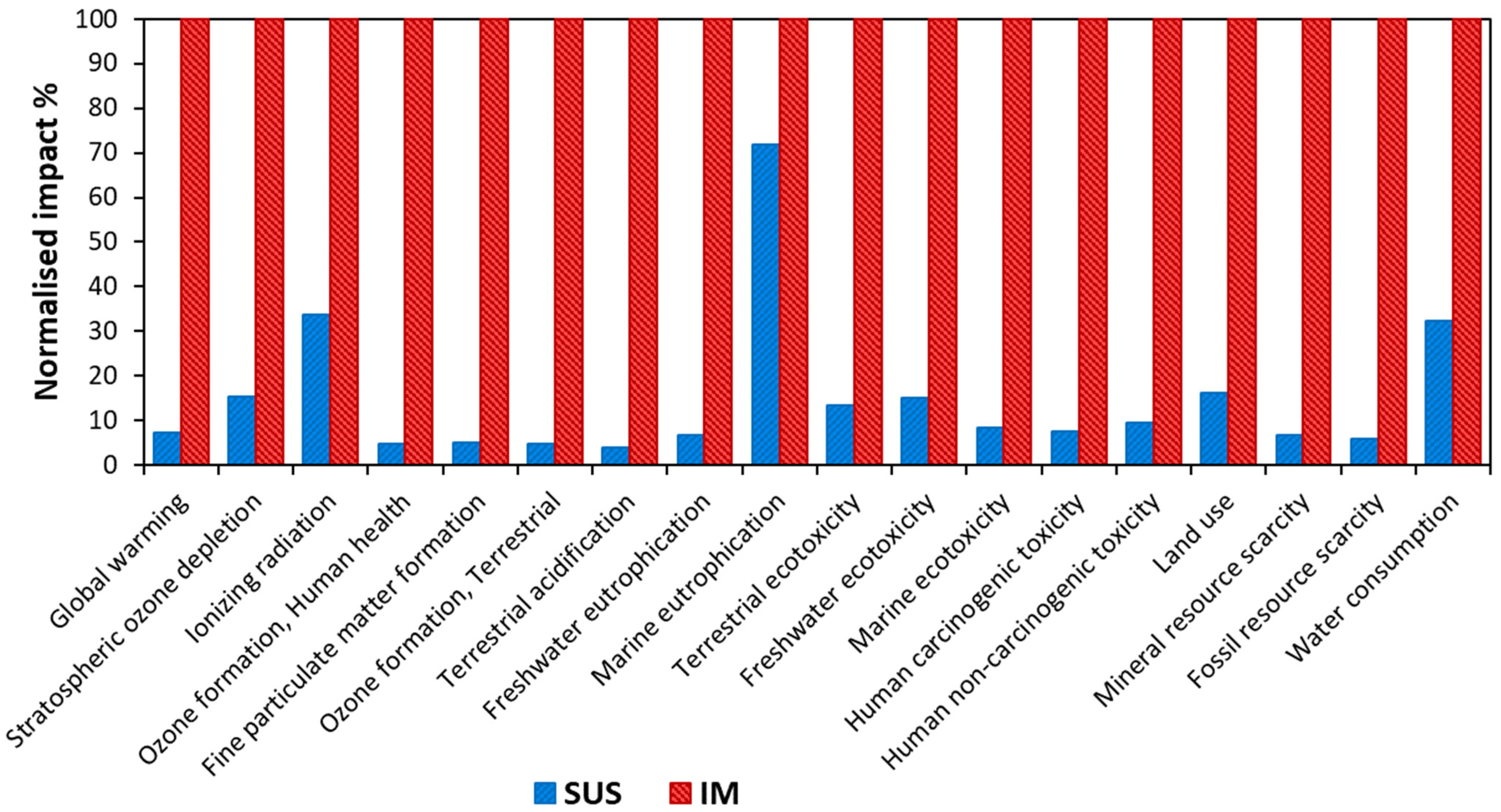
3.2.1. LCIA Results Using IPCC Framework
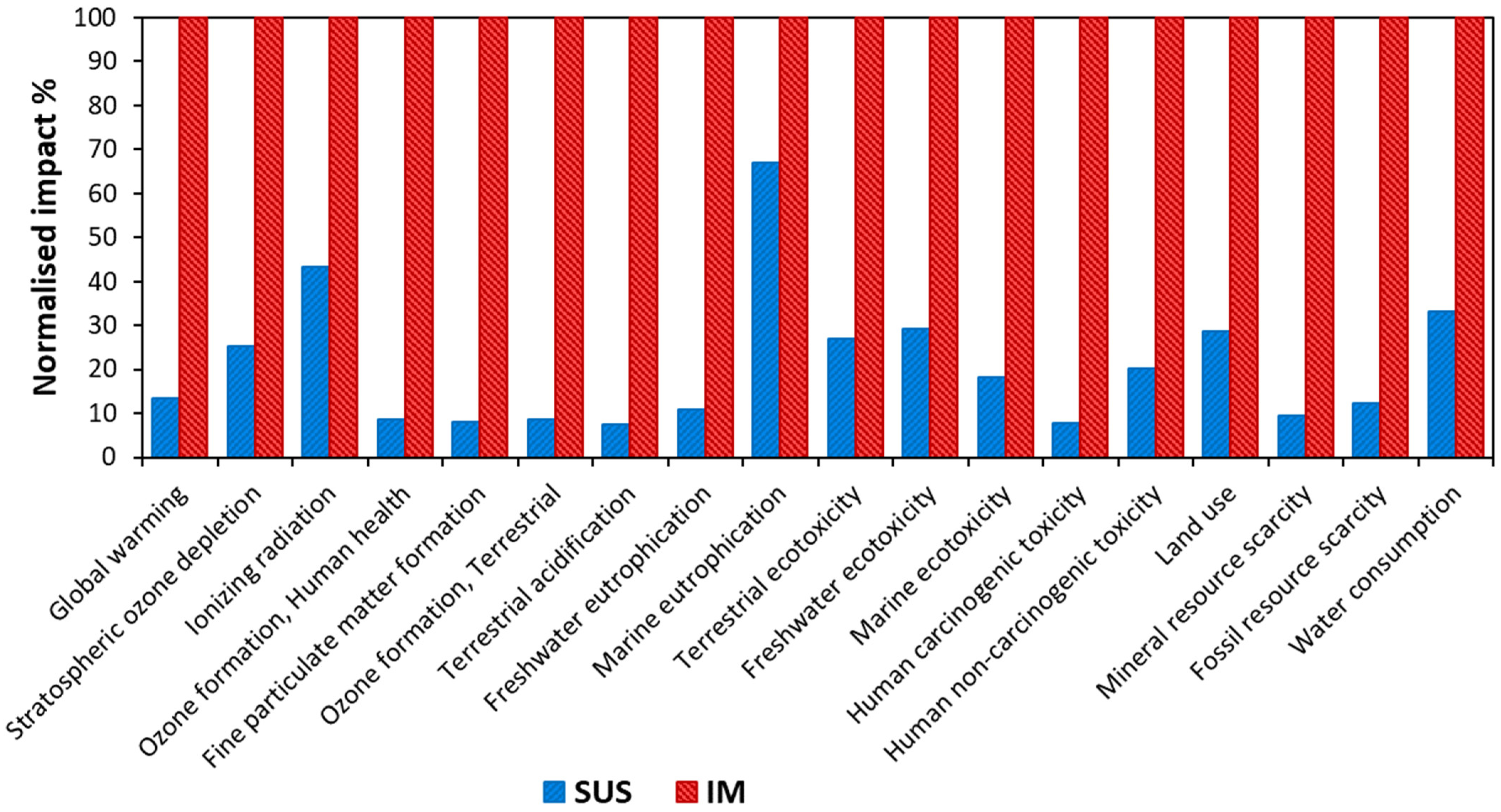
3.2.2. LCIA Results Using ReCiPe Framework
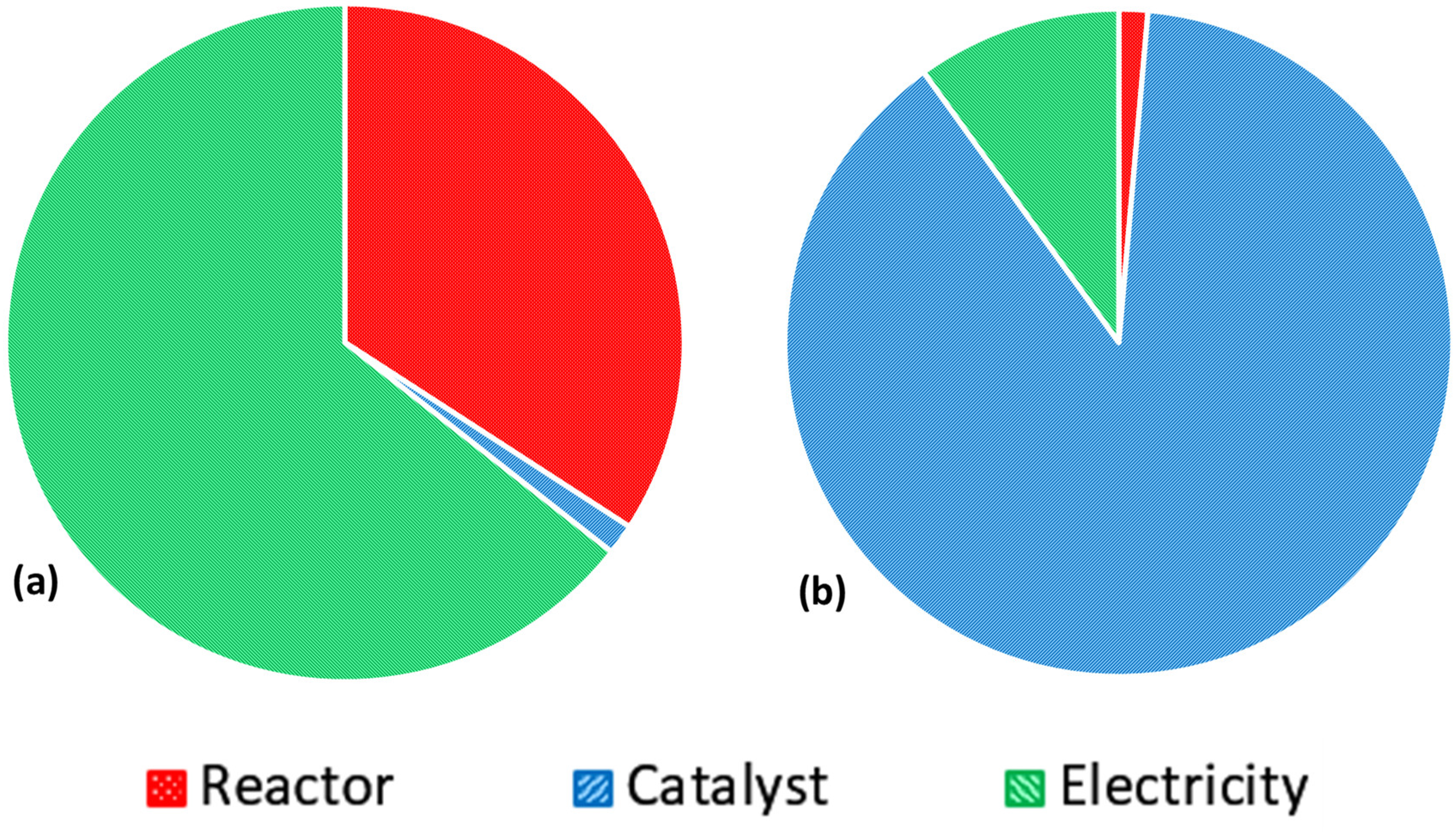
3.2.3. Alternative Scenario 1: Renewable Electricity Mix
Scenario 1—LCIA Results Using IPCC Framework
Scenario 1—LCIA Results Using ReCiPe Framework
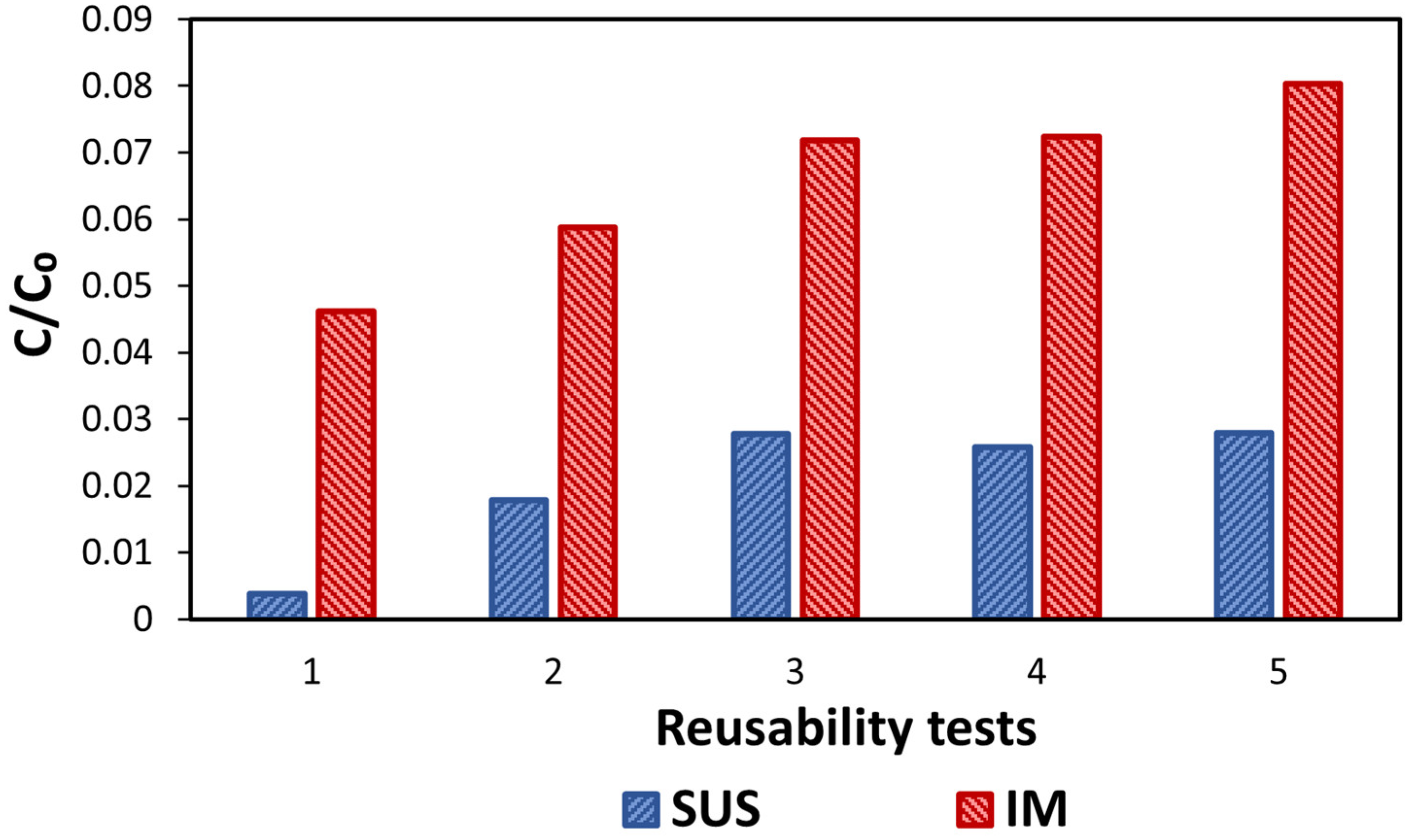
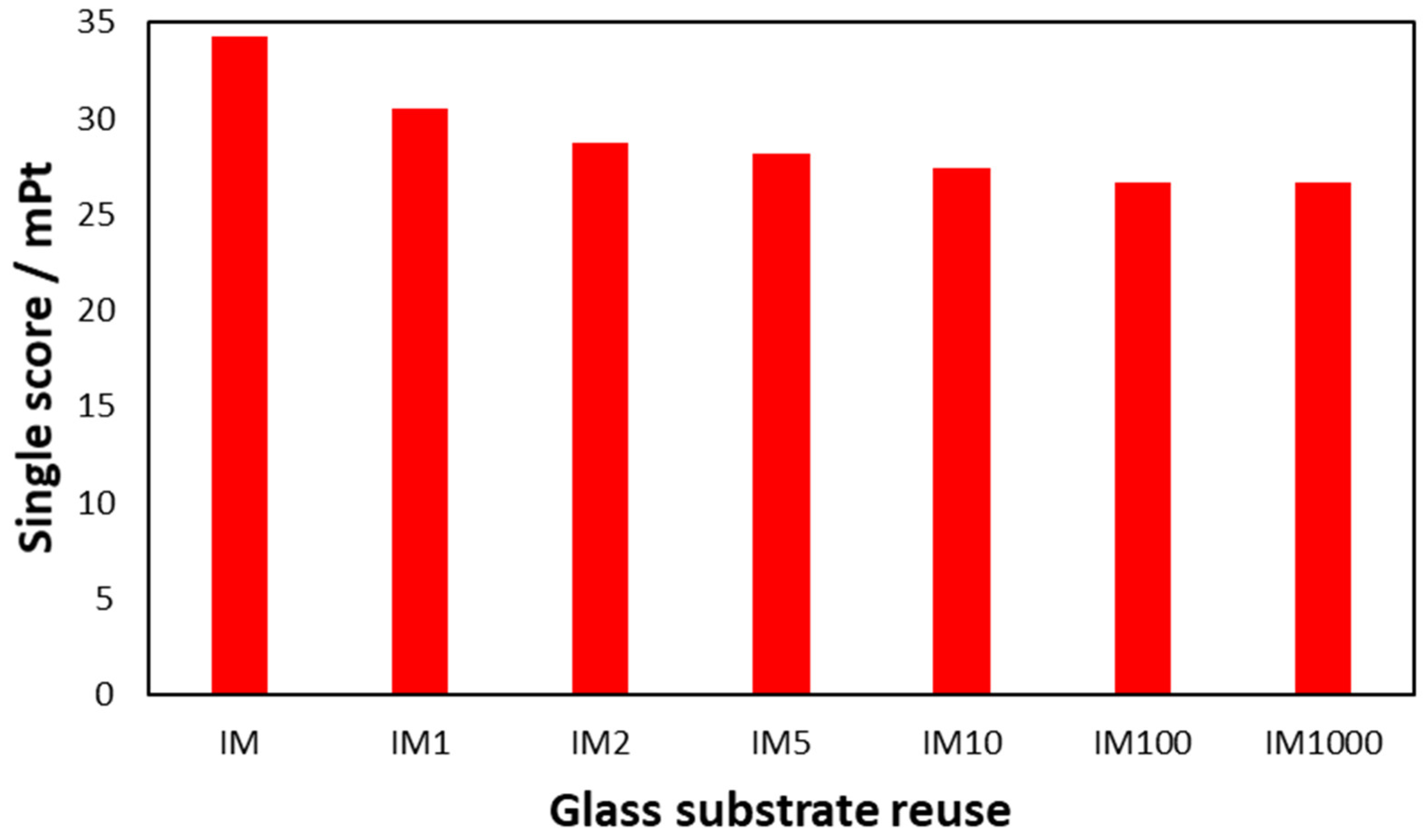
3.2.4. Alternative Scenario 2: Reuse of the Catalyst’s Glass Substrate
Scenario 2—LCIA Results Using IPCC Framework
Scenario 2—LCIA Results Using ReCiPe Framework
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.; Zheng, H.; Tratnyek, P.G. Advanced redox processes for sustainable water treatment. Nat. Water 2023, 1, 666–681. [Google Scholar] [CrossRef]
- Dong, G.; Chen, B.; Liu, B.; Hounjet, L.J.; Cao, Y.; Stoyanov, S.R.; Yang, M.; Zhang, B. Advanced oxidation processes in microreactors for water and wastewater treatment: Development, challenges, and opportunities. Water Res. 2022, 211, 118047. [Google Scholar] [CrossRef] [PubMed]
- McKee, S.M.; Chatzisymeon, E. Assessing the environmental sustainability of light emitting diodes and conventional blacklight lamps for the treatment of bisphenol-A in water. Environ. Impact Assess. Rev. 2022, 97, 106886. [Google Scholar] [CrossRef]
- Sundar, K.P.; Kanmani, S. Progression of Photocatalytic reactors and it’s comparison: A Review. Chem. Eng. Res. Des. 2020, 154, 135–150. [Google Scholar] [CrossRef]
- Gowland, D.C.A.; Robertson, N.; Chatzisymeon, E. Photocatalytic Oxidation of Natural Organic Matter in Water. Water 2021, 13, 288. [Google Scholar] [CrossRef]
- Musial, J.; Mlynarczyk, D.T.; Stanisz, B.J. Photocatalytic degradation of sulfamethoxazole using TiO2-based materials—Perspectives for the development of a sustainable water treatment technology. Sci. Total Environ. 2023, 856, 159122. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-E.; Kim, M.-K.; Danish, M.; Jo, W.-K. State-of-the-art review on photocatalysis for efficient wastewater treatment: Attractive approach in photocatalyst design and parameters affecting the photocatalytic degradation. Catal. Commun. 2023, 183, 106764. [Google Scholar] [CrossRef]
- Dijkstra, M.F.J.; Michorius, A.; Buwalda, H.; Panneman, H.J.; Winkelman, J.G.M.; Beenackers, A.A.C.M. Comparison of the efficiency of immobilized and suspended systems in photocatalytic degradation. Catal. Today 2001, 66, 487–494. [Google Scholar] [CrossRef]
- Wei, X.; Liu, H.; Gao, S.; Jia, K.; Wang, Z.; Chen, J.J. Photocatalyst: To Be Dispersed or To Be Immobilized? The Crucial Role of Electron Transport in Photocatalytic Fixed Bed Reaction. Phys. Chem. Lett. 2022, 13, 9642–9648. [Google Scholar] [CrossRef]
- Chenab, K.K.; Sohrabi, B.; Jafari, A.; Ramakrishna, S. Water treatment: Functional nanomaterials and applications from adsorption to photodegradation. Mater. Today Chem. 2020, 16, 100262. [Google Scholar] [CrossRef]
- Manassero, A.; Satuf, M.L.; Alfano, O.M. Photocatalytic reactors with suspended and immobilized TiO2: Comparative efficiency evaluation. Chem. Eng. J. 2017, 326, 29–36. [Google Scholar] [CrossRef]
- Grčić, I.; Koprivanac, N.; Puma, G.L. Modeling the photocatalytic oxidation of carboxylic acids on aqueous TiO2 suspensions and on immobilized TiO2-chitosan thin films in different reactor geometries irradiated by UVA or UVC light sources. Chem. Eng. J. 2021, 422, 130104. [Google Scholar] [CrossRef]
- Vezzoli, M.; Farrell, T.; Baker, A.; Psaltis, S.; Martens, W.N.; Bell, J.M. Optimal catalyst thickness in titanium dioxide fixed film reactors: Mathematical modelling and experimental validation. Chem. Eng. J. 2013, 234, 57–65. [Google Scholar] [CrossRef]
- Binjhade, R.; Mondal, R.; Mondal, S. Continuous photocatalytic reactor: Critical review on the design and performance. J. Environ. Chem. Eng. 2022, 10, 107746. [Google Scholar] [CrossRef]
- Chen, D.; Li, F.; Ray, A.K. External and internal mass transfer effect on photocatalytic degradation. Catal. Today 2001, 66, 475–485. [Google Scholar] [CrossRef]
- Ray, A.K.; Beenackers, A.A.C.M. Novel swirl-flow reactor for kinetic studies of semiconductor photocatalysis. AIChE J. 1997, 43, 2571–2578. [Google Scholar] [CrossRef]
- Ballari, M.D.L.M.; Brandi, R.; Alfano, O.; Cassano, A. Mass transfer limitations in photocatalytic reactors employing titanium dioxide suspensions: II. External and internal particle constrains for the reaction. Chem. Eng. J. 2008, 136, 242–255. [Google Scholar] [CrossRef]
- Chen, D.; Li, F.; Ray, A.K. Effect of mass transfer and catalyst layer thickness on photocatalytic reaction. AIChE J. 2000, 46, 1034–1045. [Google Scholar] [CrossRef]
- Soleymani, A.R.; Tavassoli, A.M.; Rezaei-Vahidian, H. Assessment of back-side activation of titania thin film using a fixed-bed photocatalytic-reactor: Kinetic study, operating cost and ANN modeling. Chem. Eng. Res. Des. 2023, 190, 759–776. [Google Scholar] [CrossRef]
- van Grieken, R.; Marugán, J.; Sordo, C.; Pablos, C. Comparison of the photocatalytic disinfection of E. coli suspensions in slurry, wall and fixed-bed reactors. Catal. Today 2009, 144, 48–54. [Google Scholar] [CrossRef]
- Rachel, A.; Subrahmanyam, M.; Boule, P. Comparison of photocatalytic efficiencies of TiO2 in suspended and immobilised form for the photocatalytic degradation of nitrobenzenesulfonic acids. Appl. Catal. B Environ. 2002, 37, 301–308. [Google Scholar] [CrossRef]
- Rani, C.N.; Karthikeyan, S.; Doss, S.P.A. Photocatalytic ultrafiltration membrane reactors in water and wastewater treatment—A review. Chem. Eng. Process.-Process Intensif. 2021, 165, 108445. [Google Scholar] [CrossRef]
- Pesqueira, J.F.J.R.; Pereira, M.F.R.; Silva, A.M.T. A life cycle assessment of solar-based treatments (H2O2, TiO2 photocatalysis, circumneutral photo-Fenton) for the removal of organic micropollutants. Sci. Total Environ. 2021, 761, 143258. [Google Scholar] [CrossRef] [PubMed]
- Elhami, M.; Bahramifar, N.; Bijanzadeh, H.R.; Abyar, H. Process efficiency and life cycle assessment of novel waste-derived Chl/TiO2 photocatalyst for rhodamine B removal. J. Water Process Eng. 2023, 56, 104425. [Google Scholar] [CrossRef]
- Lutterbeck, C.A.; Colares, G.S.; Dell’Osbel, N.; da Silva, F.P.; Kist, L.T.; Machado, Ê.L. Hospital laundry wastewaters: A review on treatment alternatives, life cycle assessment and prognosis scenarios. J. Clean. Prod. 2020, 273, 122851. [Google Scholar] [CrossRef]
- Yoonus, H.; Al-Ghamdi, S.G. Environmental performance of building integrated grey water reuse systems based on Life-Cycle Assessment: A systematic and bibliographic analysis. Sci. Total Environ. 2020, 712, 136535. [Google Scholar] [CrossRef] [PubMed]
- Foteinis, S.; Borthwick, A.G.L.; Frontistis, Z.; Mantzavinos, D.; Chatzisymeon, E.J. Environmental sustainability of light-driven processes for wastewater treatment applications. J. Clean. Prod. 2018, 182, 8–15. [Google Scholar] [CrossRef]
- Costamagna, M.; Ciacci, L.; Paganini, M.C.; Calza, P.; Passarini, F.J. Combining the highest degradation efficiency with the lowest environmental impact in zinc oxide based photocatalytic systems. J. Clean. Prod. 2020, 252, 119762. [Google Scholar] [CrossRef]
- Juvakoski, A.; Singhal, G.; Manzano, M.A.; Moriñigo, M.Á.; Vahala, R.; Levchuk, I. Solar disinfection—An appropriate water treatment method to inactivate faecal bacteria in cold climates. Sci. Total Environ. 2022, 827, 154086. [Google Scholar] [CrossRef]
- Moreno-SanSegundo, J.; Giannakis, S.; Samoili, S.; Farinelli, G.; McGuigan, K.G.; Pulgarín, C.; Marugán, J. SODIS potential: A novel parameter to assess the suitability of solar water disinfection worldwide. Chem. Eng. J. 2021, 419, 129889. [Google Scholar] [CrossRef]
- Odling, G.; Chatzisymeon, E.; Robertson, N. Sequential ionic layer adsorption and reaction (SILAR) deposition of Bi4Ti3O12 on TiO2: An enhanced and stable photocatalytic system for water purification. Catal. Sci. Technol. 2018, 8, 829–839. [Google Scholar] [CrossRef]
- ISO 14040:2006; Environmental Management—Life Cycle Assessment—Principles and Framework. International Organisation for Standardisation (ISO): Geneva, Switzerland, 2006.
- ISO 14044:2006; Environmental Management—Life Cycle Assessment—Requirements and Guidelines. International Organisation for Standardisation (ISO): Geneva, Switzerland, 2006.
- Ferretto, A.; Brooker, R.; Matthews, R.; Smith, P. Climate change and drinking water from Scottish peatlands: Where increasing DOC is an issue? J. Environ. Manag. 2021, 300, 113688. [Google Scholar] [CrossRef] [PubMed]
- Hashempour, Y.; Nasseri, M.; Mohseni-Bandpei, A.; Motesaddi, S.; Eslamizadeh, M. Assessing vulnerability to climate change for total organic carbon in a system of drinking water supply. Sustain. Cities Soc. 2020, 53, 101904. [Google Scholar] [CrossRef]
- Climate & Clean Air Coaltion. METHANE The World’S Second-Largest Contributor to Global Warming after Carbon Dioxide and a Key Ingredient in Ground-Level Ozone Pollution. Available online: https://www.ccacoalition.org/short-lived-climate-pollutants/methane (accessed on 20 April 2024).
- Constantino, D.S.M.; Dias, M.M.; Silva, A.M.T.; Faria, J.L.; Silva, C.G. Intensification strategies for improving the performance of photocatalytic processes: A review. J. Clean. Prod. 2022, 340, 130800. [Google Scholar] [CrossRef]
- Sun, Y.; Bai, S.; Wang, X.; Ren, N.; You, S. Prospective Life Cycle Assessment for the Electrochemical Oxidation Wastewater Treatment Process: From Laboratory to Industrial Scale. Environ. Sci. Technol. 2023, 57, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Pesqueira, J.F.J.R.; Marugán, J.; Pereira, M.F.R.; Silva, A.M.T. Selecting the most environmentally friendly oxidant for UVC degradation of micropollutants in urban wastewater by assessing life cycle impacts: Hydrogen peroxide, peroxymonosulfate or persulfate? Sci. Total Environ. 2022, 808, 152050. [Google Scholar] [CrossRef]
- de Simone Souza, H.H.; Falqui, L.; Xuereb, R.; Mamo, J.; Abela, S.; Grech, M.; Refalo, P. Environmental assessment of a single-family photocatalytic greywater treatment system based on the design and operating conditions. Sustain. Prod. Consum. 2023, 35, 483–494. [Google Scholar] [CrossRef]
- Department for Energy Security & Net Zero. Electricity Generation and Supply in Scotland, Wales, Northern Ireland and England, 2018 to 2022. Available online: https://assets.publishing.service.gov.uk/media/6581b11eed3c3400133bfbb1/Electricity_generation_and_supply_in_Scotland__Wales__Northern_Ireland__and_England__2018_to_2022.pdf (accessed on 1 February 2024).
- Scottish Energy Statistics Hub. Share of Renewable Electricity in Gross Final Consumption. Available online: https://scotland.shinyapps.io/sg-scottish-energy-statistics/?Section=RenLowCarbon&Subsection=RenElec&Chart=RenElecTarget (accessed on 1 February 2024).
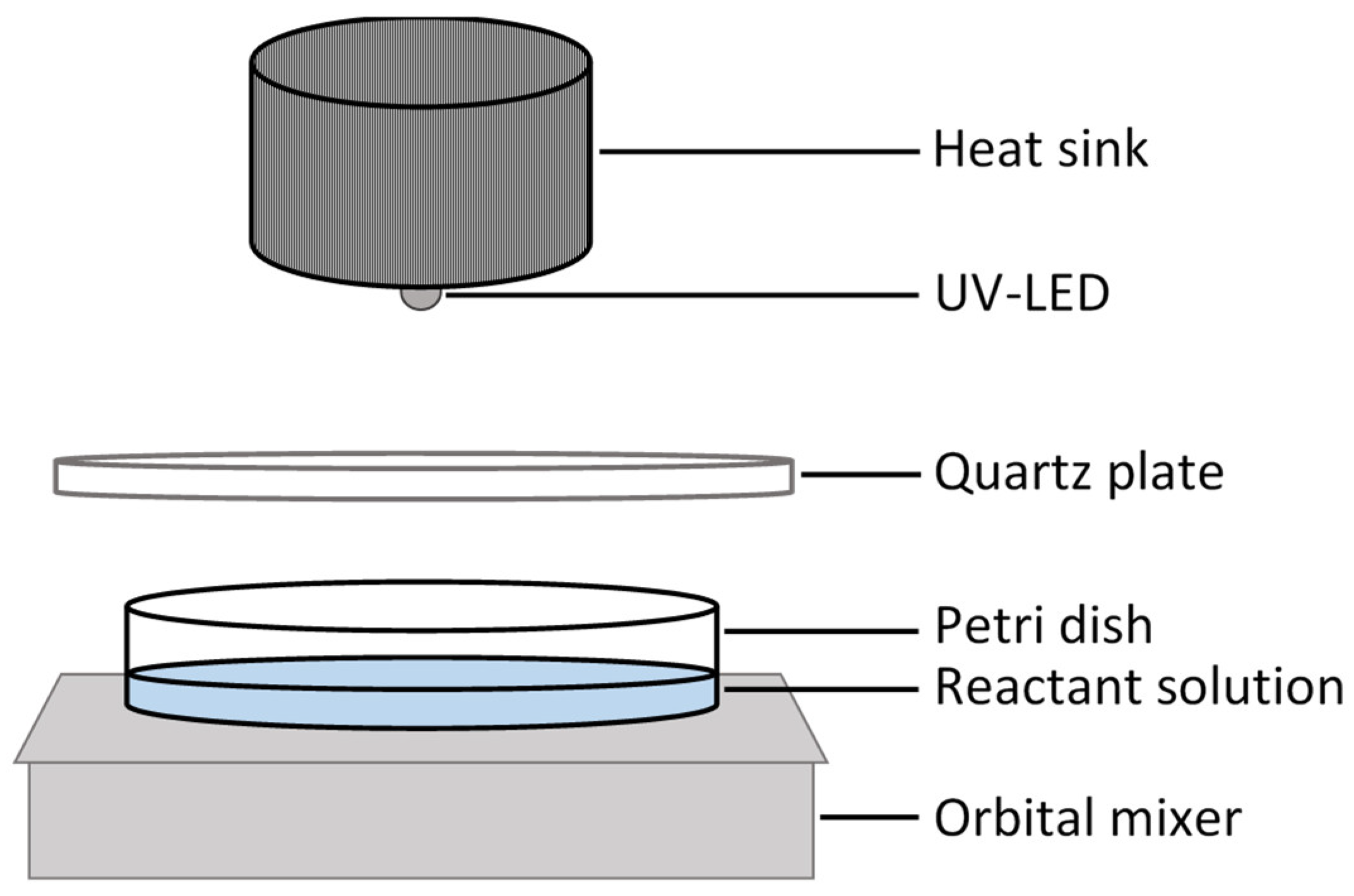


| Catalyst | Description | Catalyst Properties |
|---|---|---|
| Immobilised (IM) | P25 TiO2 immobilised on a glass medium | Bandgap = 3.18 eV; particle sizes of 20–30 nm |
| Suspended (SUS) | P25 TiO2 suspended in the water matrix, without a support medium | Bandgap = 3.18 eV; particle sizes of ~25 nm; SBET = 54–55 m2 g−1 |
| Process | Materials/Assemblies | Items | SimaPro Component | Quantity (per Functional Unit) |
|---|---|---|---|---|
| Suspended and immobilised | Light source | Light-emitting diode | Light-emitting diode [GLO]|production|Cut-off, S | 0.200 kg |
| Heat sink | Aluminium alloy, AlMg3 [RER]|production|Cut-off, S | 3.69 kg | ||
| Starboard | Copper-rich materials [GLO]|market for copper-rich materials|Cut-off, S Aluminium alloy, AlMg3 [RER]|production|Cut-off, S | 0.400 kg | ||
| Reactor vessel | Glass beaker | Glass tube, borosilicate [GLO]|market for|Cut-off, S | 0.550 kg | |
| Quartz plate | Silicon, metallurgical grade [RoW]| production|Cut-off, S | 0.285 kg | ||
| Lab equipment | Orbital mixer | Permanent magnet, for electric motor [GLO]| production|Cut-off, S | 0.407 kg | |
| Suspended | Catalyst | TiO2 catalyst | Titanium dioxide [RER]| market for |Cut-off, S | 200 mg |
| Catalyst separation | Filter | Ultrafiltration module [GLO]|ultrafiltration module production, hollow fibre|Cut-off, S | 1 P | |
| Pump | Water pump operation, electric [RoW]|water pump operation, electric|Cut-off, S | 3.6 kJ | ||
| Electricity | Electrical connection | Electricity, low voltage [GB]|market for electricity, low voltage|Cut-off, S | 214 kJ | |
| Immobilised | Catalyst | Glass slide | Flat glass, uncoated [RoW]|market for flat glass, uncoated|Cut-off, S | 0.25 kg |
| Titanium dioxide | Titanium dioxide [RER]| market for |Cut-off, S | 3.03 g | ||
| Titanium butoxide | Titanium butoxide | 4.54 cm3 | ||
| n-Butanol | 1-butanol [GLO]|market for|Cut-off, S | 0.077 kg | ||
| Hydrochloric acid | Hydrochloric acid, Mannheim process (30% HCl), at plant/RER mass | 1.05 g | ||
| Electricity | Electrical connection | Electricity, low voltage [GB]|market for electricity, low voltage|Cut-off, S | 432 kJ |
| Energy Source | Original Scenario: SimaPro Low-Voltage GB Electricity Mix (%) | Scenario 1: Scottish Renewable Electricity Mix in 2020 (%) |
|---|---|---|
| Onshore wind | 14.15 | 61.20 |
| Offshore wind | 11.69 | 10.90 |
| Hydro | 1.47 | 19.60 |
| Bioenergy and Waste | 13.81 | 7.18 |
| Solar PV | 2.43 | 1.02 |
| Wave and tidal | 0.00 | 0.03 |
| Nuclear | 17.05 | - |
| Coal | 7.22 | - |
| Natural gas | 31.29 | - |
| Oil | 0.89 | - |
| Name | Reuses of Glass Substrate before Recycling |
|---|---|
| IM1 | 1 |
| IM2 | 2 |
| IM5 | 5 |
| IM10 IM100 | 10 100 |
| IM1000 | 1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gowland, D.C.A.; Robertson, N.; Chatzisymeon, E. Life Cycle Assessment of Immobilised and Slurry Photocatalytic Systems for Removal of Natural Organic Matter in Water. Environments 2024, 11, 114. https://doi.org/10.3390/environments11060114
Gowland DCA, Robertson N, Chatzisymeon E. Life Cycle Assessment of Immobilised and Slurry Photocatalytic Systems for Removal of Natural Organic Matter in Water. Environments. 2024; 11(6):114. https://doi.org/10.3390/environments11060114
Chicago/Turabian StyleGowland, Dan C. A., Neil Robertson, and Efthalia Chatzisymeon. 2024. "Life Cycle Assessment of Immobilised and Slurry Photocatalytic Systems for Removal of Natural Organic Matter in Water" Environments 11, no. 6: 114. https://doi.org/10.3390/environments11060114
APA StyleGowland, D. C. A., Robertson, N., & Chatzisymeon, E. (2024). Life Cycle Assessment of Immobilised and Slurry Photocatalytic Systems for Removal of Natural Organic Matter in Water. Environments, 11(6), 114. https://doi.org/10.3390/environments11060114








