Nitrogen and Phosphorus Removal from Wastewater Using Calcareous Waste Shells—A Systematic Literature Review
Abstract
1. Introduction
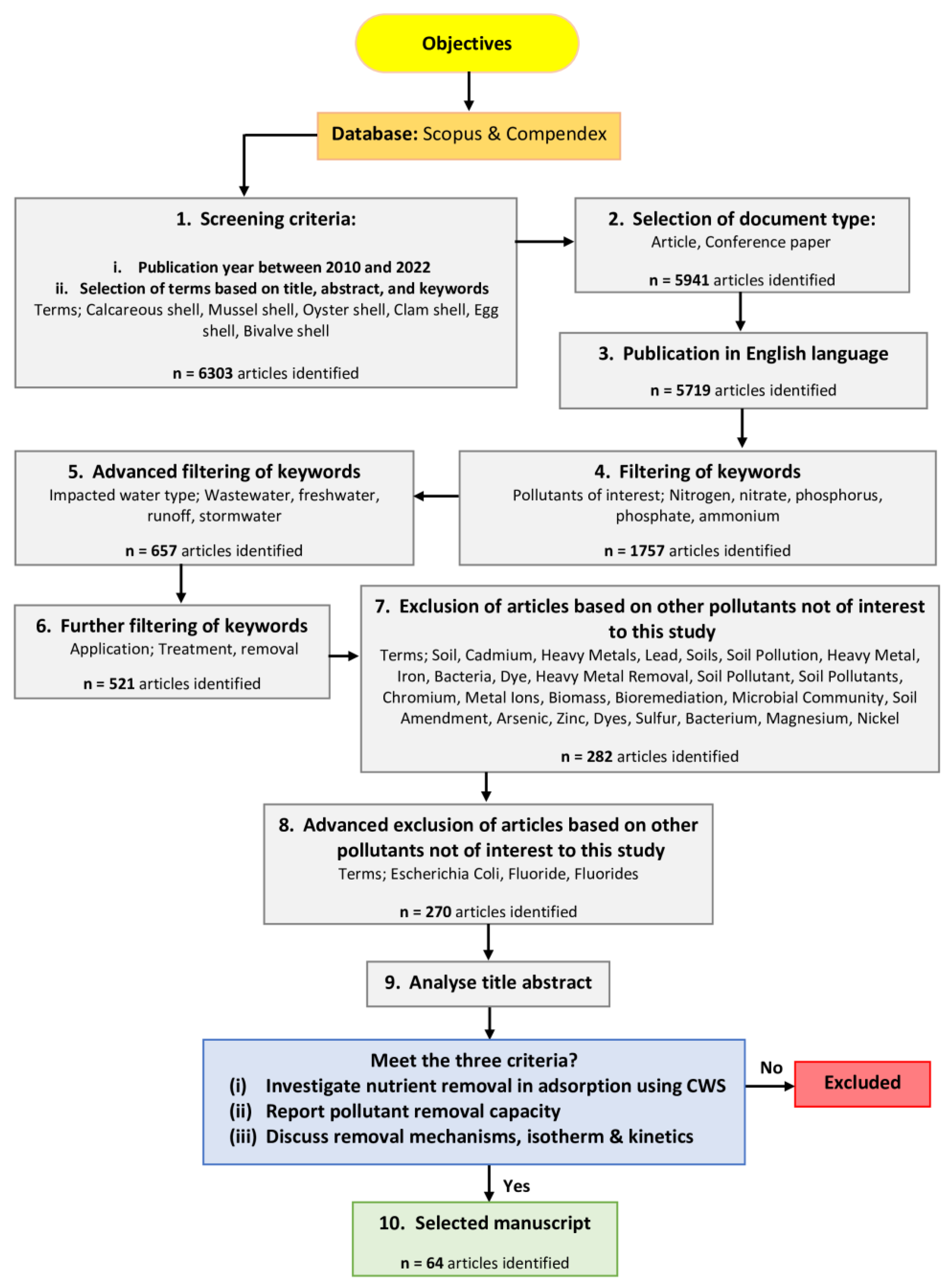
2. Materials and Methods
3. Results
3.1. Regional and Temporal Trends
| CWS Type | Common (English) Name | Scientific (Latin) Name | Wastewater Type and Target Pollutant | Reference | ||
|---|---|---|---|---|---|---|
| Domestic/Municipal Wastewater | Synthetic Wastewater | Other Wastewater | ||||
| Mussel shells | PO43−, NH4+ (treated domestic wastewater effluent), | [63] | ||||
| PO43− (municipal) | PO43− | [64] | ||||
| P | [8] | |||||
| NH4+ | [18] | |||||
| PO43− | [34] | |||||
| PO43− | [37] | |||||
| PO43− | [65] | |||||
| PO43− | [66] | |||||
| Mediterranean mussel | Mytilus galloprovincialis Lamarck | P | [4] | |||
| P | PO43−-P (river water) | [11] | ||||
| PO43− (greywater) | [67] | |||||
| NH4+, PO43− (lake) | [68] | |||||
| PO43− | [69] | |||||
| Green-lipped mussels | Perna canaliculus | PO43− | [6] | |||
| Green-lipped mussels | Perna canaliculus | PO43− | [9] | |||
| Green mussel shells | Perna viridis | NH3-N (raw leachate) | [30] | |||
| Green mussel shells | Perna viridis | NH3-N (raw leachate) | [70] | |||
| Green mussels | NH3-N (raw leachate) | [71] | ||||
| Green mussels | Perna viridis | NH3-N (raw leachate) | [72] | |||
| Oyster shells | P | [7] | ||||
| PO43− | [19] | |||||
| P | [28] | |||||
| PO43− | [29] | |||||
| P | [61] | |||||
| P | [73] | |||||
| P | [74] | |||||
| P | [75] | |||||
| PO43− | [76] | |||||
| PO43− | [77] | |||||
| P | [78] | |||||
| NH4-N | [22] | |||||
| PO43− (lake water) | [38] | |||||
| NH3, NO3−, PO43− (landfill leachate) | [36] | |||||
| TP and TN (river water) | [40] | |||||
| TN (synthetic domestic wastewater) | [79] | |||||
| NH4-N (simulated wastewater) | [80] | |||||
| NH3+, NO3− (recirculating aquaponic) | [81] | |||||
| P (treated swine wastewater from constructed wetlands) | [82] | |||||
| NH4-N (domestic wastewater) | [83] | |||||
| NH3-N/TN (effluent from primary wastewater settling tank) | [84] | |||||
| NO3-N, NH4-N, PO43−, and TP (domestic wastewater) | [85] | |||||
| NO3-N and TP (secondary clarifier effluent) | [86] | |||||
| P (swine wastewater) | P | [87] | ||||
| NH3+, TP (municipal) | [88] | |||||
| Egg shells | NO3-N and P | [10] | ||||
| NO3− | NO3− (groundwater) | [26] | ||||
| P | [27] | |||||
| NH4+ | [35] | |||||
| P | [62] | |||||
| NO3− | [89] | |||||
| P-PO4 | [90] | |||||
| P-PO4 | [91] | |||||
| P-PO4 | [92] | |||||
| P | [93] | |||||
| P (anaerobic digester effluent) P (dewatered anaerobic sludge) | P | [20] | ||||
| P (preliminary treatment b effluent and anaerobic digester supernatant) | P | [54] | ||||
| Bivalve shells | P, PO43− | [55] | ||||
| Clam shells | Anomalocardia brasiliana, Tagelus plebeius | P | [57] | |||
| Clam shells | Anomalocardia brasiliana, Tagelus plebeius | P | [58] | |||
| White hard clams | European flat shells | Meretrix lusoria | TP, PO43−-P (anaerobically digested swine) | P | [56] | |
| Crushed coral, oyster, and mussel-shells | PO43−-P (secondary effluent) | P | [5] | |||
| Marsh clam, mussel shells, and egg shells | PO43− | [94] | ||||
| Snail and clam shells | PO43− | [95] | ||||
| Zebra mussels | P (treated domestic wastewater) | P | [31] | |||
3.2. Wastewater Type and Form of Treated Nutrient
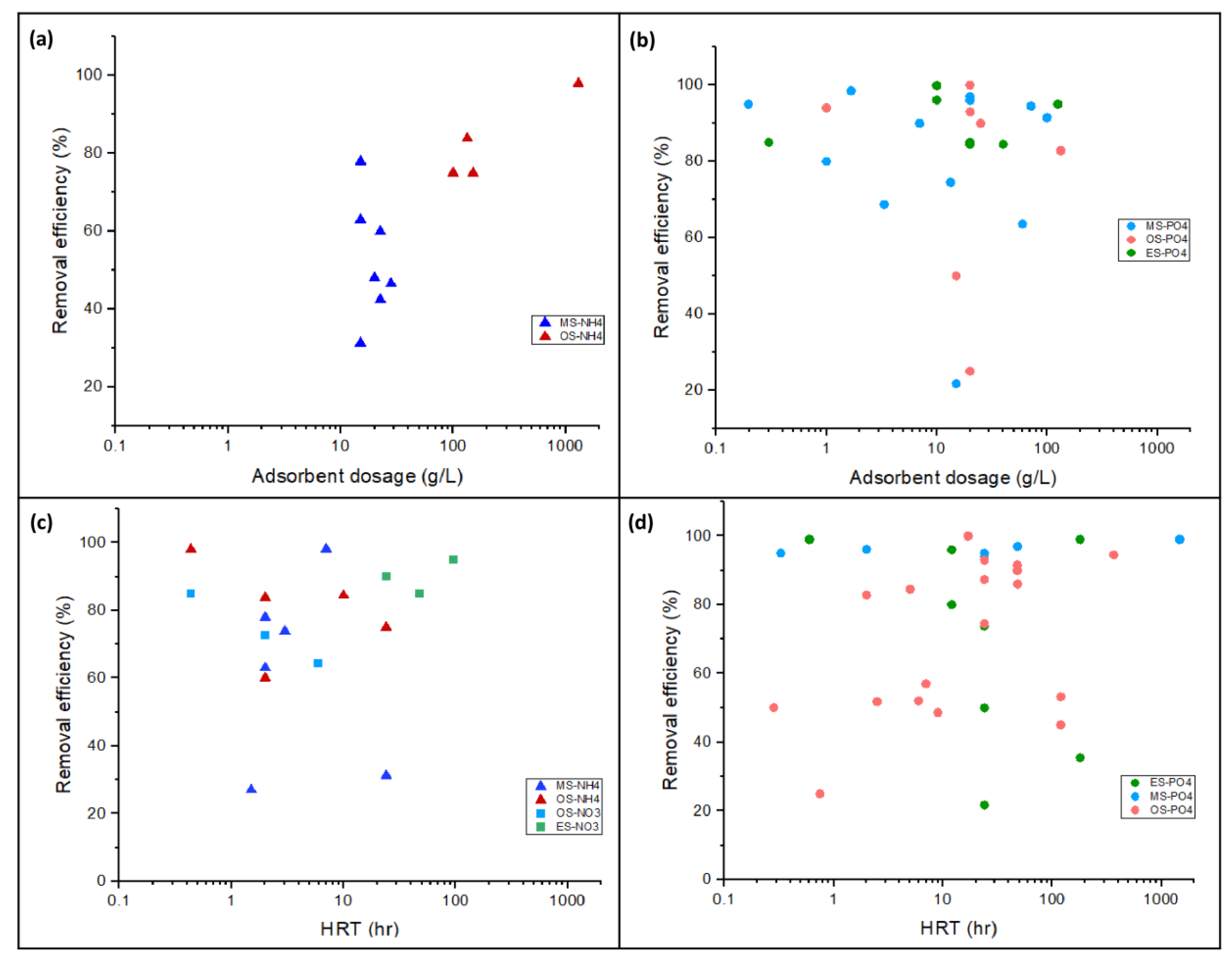
3.3. Effects of Experimental Conditions on Nutrient Removal
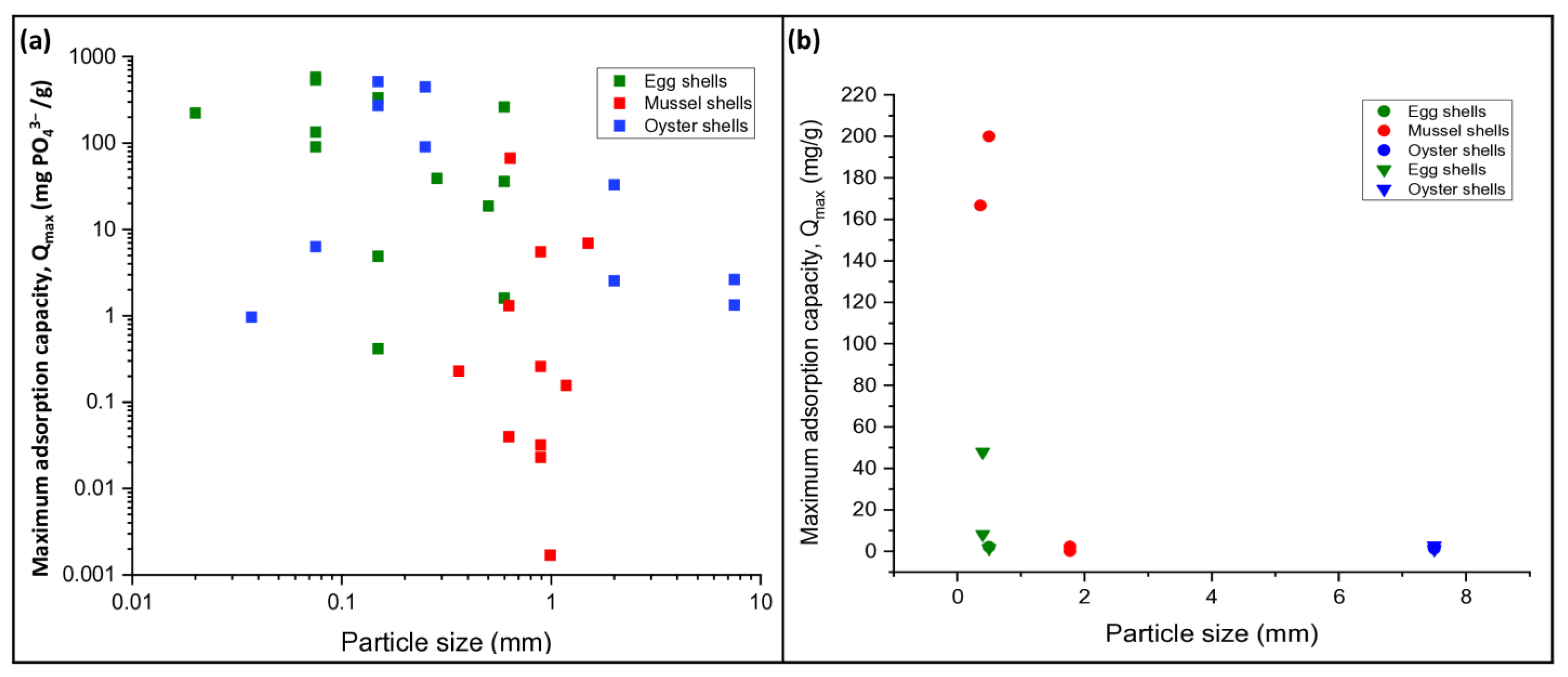
3.4. Isotherms and Kinetics
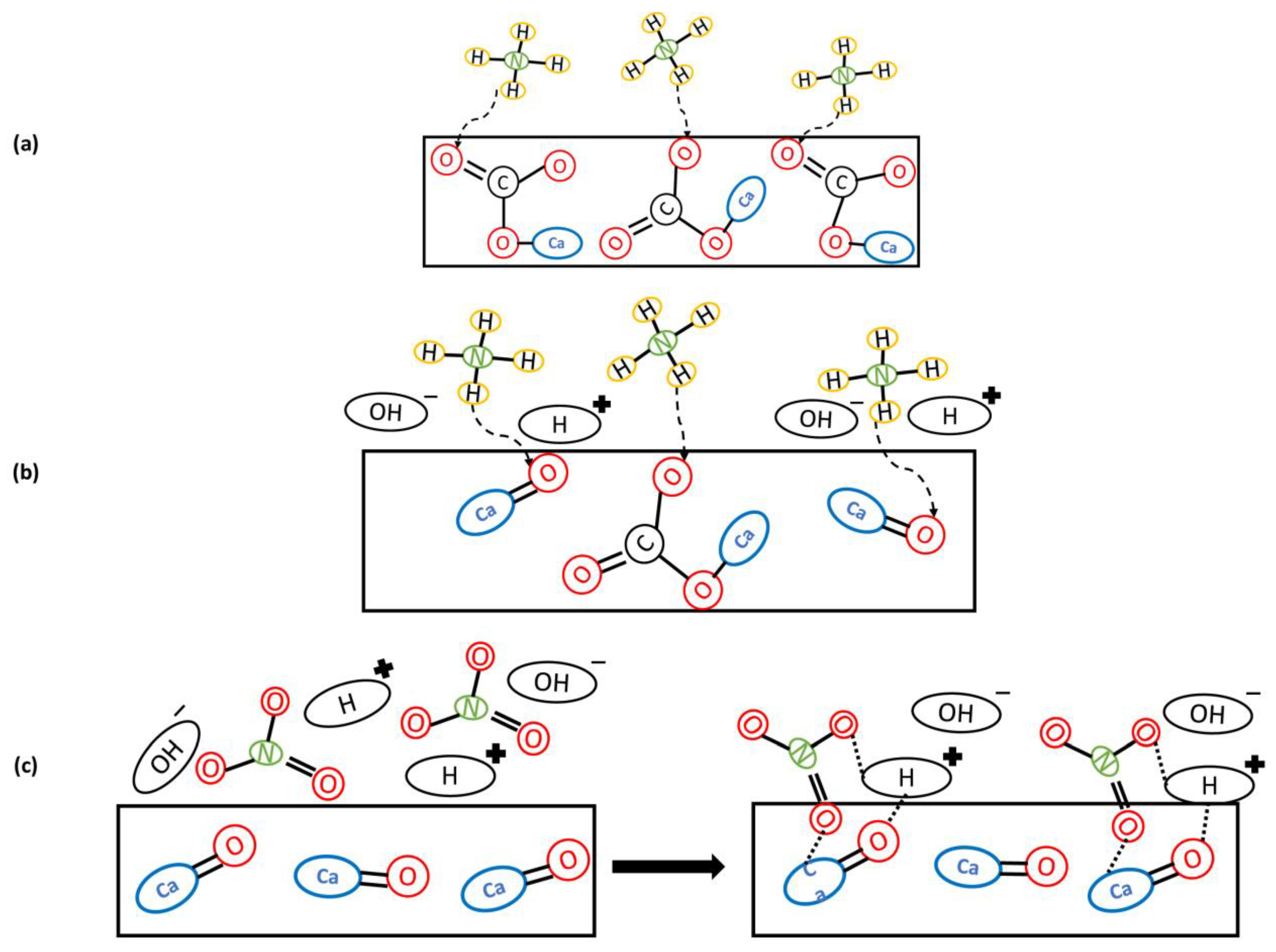
3.5. Removal Mechanisms
3.6. Effects of Experimental Variables on Nutrient Removal
3.7. Possible Reuse of Post-Treatment Adsorbents
4. Conclusions
- Does the CaCO3 content of different CWSs affect nutrient removal?
- Which experimental variable (HRT, adsorption dosage, particle size, or wastewater strength) is most influential on nutrient removal capacity?
- To what extent would climatic variables influence the adsorption capacity of CWS removing nutrients if experiments were performed under field conditions?
- Would the isotherms and kinetic studies change if studies were conducted for longer under field conditions?
- Can CWSs be modified (functionalised) differently to improve nitrate adsorption?
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rauh, E.; Hughes, S. Collaboration for source water protection in the United States: Community water systems engagement in nitrate pollution reduction. WIREs Water 2023, 11, e1682. [Google Scholar] [CrossRef]
- Larned, S.; Booker, D.; Dudley, B.; Moores, J.M.R.; Baillie, B.; Schallenberg, M.; Moriarty, E.; Zeldis, J.; Short, K. Land-Use Impacts on Freshwater and Marine Environments in New Zealand; National Institute of Water & Atmospheric Research Ltd. (NIWA): Christchurch, New Zealand, 2018; p. 291. Available online: https://environment.govt.nz/assets/Publications/Files/land-use-impacts-on-freshwater-and-marine-environments-.pdf (accessed on 22 October 2023).
- Reddy, K.R.; Dastgheibi, S.; Cameselle, C. Mixed versus layered multi-media filter for simultaneous removal of nutrients and heavy metals from urban stormwater runoff. Environ. Sci. Pollut. Res. 2021, 28, 7574–7585. [Google Scholar] [CrossRef]
- Paradelo, R.; Conde-Cid, M.; Cutillas-Barreiro, L.; Arias-Estévez, M.; Nóvoa-Muñoz, J.; Álvarez-Rodríguez, E.; Fernández-Sanjurjo, M.; Núñez-Delgado, A. Phosphorus removal from wastewater using mussel shell: Investigation on retention mechanisms. Ecol. Eng. 2016, 97, 558–566. [Google Scholar] [CrossRef]
- Zapater-Pereyra, M.; Malloci, E.; van Bruggen, J.; Lens, P. Use of marine and engineered materials for the removal of phosphorus from secondary effluent. Ecol. Eng. 2014, 73, 635–642. [Google Scholar] [CrossRef]
- Abeynaike, A.; Wang, L.; Jones, M.I.; Patterson, D.A. Pyrolysed powdered mussel shells for eutrophication control: Effect of particle size and powder concentration on the mechanism and extent of phosphate removal. Asia-Pac. J. Chem. Eng. 2011, 6, 231–243. [Google Scholar] [CrossRef]
- Xu, Y.; Liao, H.; Zhang, J.; Lu, H.; He, X.; Zhang, Y.; Wu, Z.; Wang, H.; Lu, M. A Novel Ca-Modified Biochar for Efficient Recovery of Phosphorus from Aqueous Solution and Its Application as a Phosphorus Biofertilizer. Nanomaterials 2022, 12, 2755. [Google Scholar] [CrossRef]
- Lee, J.-I.; Oh, J.-S.; Yoo, S.-C.; Jho, E.H.; Lee, C.-G.; Park, S.-J. Removal of phosphorus from water using calcium-rich organic waste and its potential as a fertilizer for rice growth. J. Environ. Chem. Eng. 2022, 10, 107367. [Google Scholar] [CrossRef]
- Jones, M.I.; Wang, L.Y.; Abeynaike, A.; Patterson, D.A. Utilisation of waste material for environmental applications: Calcination of mussel shells for waste water treatment. Adv. Appl. Ceram. 2011, 110, 280–286. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, D.; Li, Y.; Pan, Q.; Wang, J.; Xue, L.; Howard, A. Phosphorus and Nitrogen Adsorption Capacities of Biochars Derived from Feedstocks at Different Pyrolysis Temperatures. Water 2019, 11, 1559. [Google Scholar] [CrossRef]
- Yin, H.; Liu, L.; Lv, M.; Feng, L.; Zhou, J. Metal-Modified Mussel Shell for Efficient Binding of Phosphorus in Eutrophic Waters. Int. J. Environ. Res. 2020, 14, 135–143. [Google Scholar] [CrossRef]
- Yousefi, H.; Douna, B.K. Risk of Nitrate Residues in Food Products and Drinking Water. Asian Pac. J. Environ. Cancer 2023, 6, 69–79. [Google Scholar] [CrossRef]
- USEPA. Aquatic Life Ambient Water Quality Criteria for Ammonia—Freshwater (2013); USEPA: Washington, DC, USA, August 2013; p. 3. Available online: https://www.epa.gov/sites/default/files/2015-08/documents/fact_sheet_aquatic-life-ambient-water-quality-criteria-for-ammonia-freshwater-2013.pdf (accessed on 27 September 2023).
- Keshvardoostchokami, M.; Majidi, M.; Zamani, A.; Liu, B. A review on the use of chitosan and chitosan derivatives as the bio-adsorbents for the water treatment: Removal of nitrogen-containing pollutants. Carbohydr. Polym. 2021, 273, 118625. [Google Scholar] [CrossRef]
- Zhan, J.; Lu, J.; Wang, D. Review of shell waste reutilization to promote sustainable shellfish aquaculture. Rev. Aquac. 2022, 14, 477–488. [Google Scholar] [CrossRef]
- Heasman, K.G.; Scott, N.; Ericson, J.A.; Taylor, D.I.; Buck, B.H. Extending New Zealand’s Marine Shellfish Aquaculture Into Exposed Environments—Adapting to Modern Anthropogenic Challenges. Front. Mar. Sci. 2020, 7, 565686. [Google Scholar] [CrossRef]
- MPI. Grinding Mussel Shells into Calcium Carbonate Offers High-Value Waste Solution. 7 February 2023. Available online: https://www.mpi.govt.nz/news/media-releases/grinding-mussel-shells-into-calcium-carbonate-offers-high-value-waste-solution/#:~:text=%22Currently%20New%20Zealand%20mussel%20shells,biowaste%2C%22%20says%20Dr%20Gobindlal (accessed on 10 August 2023).
- Nguyen, V.Q.; Van, H.T.; Le Sy, H.; Nguyen, T.M.L.; Nguyen, D.K. Application of Mussell-derived biosorbent to remove NH4+ from aqueous solution: Equilibrium and Kinetics. SN Appl. Sci. 2021, 3, 496. [Google Scholar] [CrossRef]
- Tran, T.-T.; Tran, N.-N.T.; Sugiyama, S.; Liu, J.-C. Enhanced phosphate removal by thermally pretreated waste oyster shells. J. Mater. Cycles Waste Manag. 2021, 23, 177–185. [Google Scholar] [CrossRef]
- Panagiotou, E.; Kafa, N.; Koutsokeras, L.; Kouis, P.; Nikolaou, P.; Constantinides, G.; Vyrides, I. Turning calcined waste egg shells and wastewater to Brushite: Phosphorus adsorption from aqua media and anaerobic sludge leach water. J. Clean. Prod. 2018, 178, 419–428. [Google Scholar] [CrossRef]
- Hart, A. Mini-review of waste shell-derived materials’ applications. Waste Manag. Res. J. A Sustain. Circ. Econ. 2020, 38, 514–527. [Google Scholar] [CrossRef]
- Li, C.; Lin, X.; Duan, L.; Liu, D.; Han, Z. Ammonia nitrogen removal in a biological nitrifying system using oyster shells as alkalinity-releasing filling materials. In Proceedings of the 2016 ASABE Annual International Meeting, Orlando, FL, USA, 17–20 July 2016. [Google Scholar] [CrossRef]
- Onoda, H.; Kawade, H.; Takenaka, A. Preparation of calcium phosphates from artificial phosphorus wastewater and sea urchin shells. J. Ecotechnology Res. 2010, 15, 107–111. [Google Scholar] [CrossRef]
- Anand, M.; Rangesh, K.; Maruthupandy, M.; Jayanthi, G.; Rajeswari, B.; Priya, R.J. Effect of CO2 driven ocean acidification on calcification, physiology and ovarian cells of tropical sea urchin Salmacis virgulata—A microcosm approach. Heliyon 2021, 7, e05970. [Google Scholar] [CrossRef]
- Lee, M.; Tsai, W.-S.; Chen, S.-T. Reusing shell waste as a soil conditioner alternative? A comparative study of eggshell and oyster shell using a life cycle assessment approach. J. Clean. Prod. 2020, 265, 121845. [Google Scholar] [CrossRef]
- Jendia, A.H.; Hamzah, S.; Abuhabib, A.A.; El-Ashgar, N.M. Removal of nitrate from groundwater by eggshell biowaste. Water Supply 2020, 20, 2514–2529. [Google Scholar] [CrossRef]
- Quisperima, A.; Pérez, S.; Flórez, E.; Acelas, N. Valorization of potato peels and eggshells wastes: Ca-biocomposite to remove and recover phosphorus from domestic wastewater. Bioresour. Technol. 2022, 343, 126106. [Google Scholar] [CrossRef]
- Ohno, M.; Kobayashi, Y.; Sohma, R.; Suzuki, M.; Kose, T.; Asada, T.; Kawata, K. Examination and Evaluation of Oyster Shell Utilization with Rice Husk Biochar for Phosphorus Adsorption. J. Water Environ. Technol. 2022, 20, 71–83. [Google Scholar] [CrossRef]
- Qiu, S.; Zhao, D.; Feng, Y.; Li, M.; Liang, X.; Zhang, L.; Luo, Y.; Zhang, K.; Wang, F. Adsorption performance and mechanism of Ca–Al-LDHs prepared by oyster shell and pop can for phosphate from aqueous solutions. J. Environ. Manag. 2022, 303, 114235. [Google Scholar] [CrossRef]
- Detho, A.; Daud, Z.; Almohana, A.I.; Almojil, S.F.; Alali, A.; Din, M.F.M.; Rosli, M.A.; Memon, A.A.; Awang, H.; Ridzuan, M.B. Adsorption of chemical oxygen demand and ammoniacal nitrogen removal from leachate using seafood waste (green mussel shell) as low-cost adsorbent. Desalination Water Treat. 2022, 260, 102–110. [Google Scholar] [CrossRef]
- McCorquodale-Bauer, K.; Cicek, N. Zebra mussel shells as an alternative mineral resource for lime production as a phosphorus precipitant. Environ. Technol. 2022, 43, 1446–1457. [Google Scholar] [CrossRef]
- Amarowicz, R.; Synowiecki, J.; Shahidi, F. Chemical composition of shells from red (Strongylocentrotus franciscanus) and green (Strongylocentrotus droebachiensis) sea urchin. Food Chem. 2012, 133, 822–826. [Google Scholar] [CrossRef]
- Morris, J.P.; Thierry, B.; Gauthier, C. Shells from aquaculture: A valuable biomaterial, not a nuisance waste product. Rev. Aquac. 2019, 11, 42–57. [Google Scholar] [CrossRef]
- Salim, N.A.A.; Fulazzaky, M.A.; Puteh, M.H.; Khamidun, M.H.; Yusoff, A.R.M.; Abdullah, N.H.; Ahmad, N.; Lazim, Z.M.; Nuid, M. Adsorption of Phosphate from Aqueous Solution onto Iron-coated Waste Mussel Shell: Physicochemical Characteristics, Kinetic, and Isotherm Studies. Biointerface Res. Appl. Chem. 2021, 11, 12831–12842. [Google Scholar] [CrossRef]
- Xu, D.; Cao, J.; Li, Y.; Howard, A.; Yu, K. Effect of pyrolysis temperature on characteristics of biochars derived from different feedstocks: A case study on ammonium adsorption capacity. Waste Manag. 2019, 87, 652–660. [Google Scholar] [CrossRef]
- Morris, S.; Garcia-Cabellos, G.; Ryan, D.; Enright, D.; Enright, A.-M. Low-cost physicochemical treatment for removal of ammonia, phosphate and nitrate contaminants from landfill leachate. J. Environ. Sci. Health Part A 2019, 54, 1233–1244. [Google Scholar] [CrossRef]
- Abdullah, N.H.; Liom, S.L.; Zainudin, A.H.; Huzil, M.A.I.; Yaacob, M.S.S.; Salim, N.A.A.; Kaamin, M.; Talaiekhozani, A. Waste Mussel Shells as an Adsorbent for Phosphate Removal in Solution: Kinetic and Isotherm Model. Int. J. Nanoelectron. Mater. 2022, 15, 25–36. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85129915856&partnerID=40&md5=136177ce9516a540537543ec95eaabeb (accessed on 30 January 2023).
- Huh, J.-H.; Choi, Y.-H.; Lee, H.-J.; Choi, W.J.; Ramakrishna, C.; Lee, H.-W.; Lee, S.-H.; Ahn, J.-W. The Use of Oyster Shell Powders for Water Quality Improvement of Lakes by Algal Blooms Removal. J. Korean Ceram. Soc. 2016, 53, 1–6. [Google Scholar] [CrossRef]
- Popović, N.T.; Lorencin, V.; Strunjak-Perović, I.; Čož-Rakovac, R. Shell Waste Management and Utilization: Mitigating Organic Pollution and Enhancing Sustainability. Appl. Sci. 2023, 13, 623. [Google Scholar] [CrossRef]
- Nam, G.; Choi, Y.-H.; Lee, N.; Ahn, J.W. Effect by Alkaline Flocculation of Algae and Phosphorous from Water Using a Calcined Waste Oyster Shell. Water 2017, 9, 661. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. A review of emerging adsorbents for nitrate removal from water. Chem. Eng. J. 2011, 168, 493–504. [Google Scholar] [CrossRef]
- Nhung, N.T.H.; Long, V.D.; Fujita, T. A Critical Review of Snail Shell Material Modification for Applications in Wastewater Treatment. Materials 2023, 16, 1095. [Google Scholar] [CrossRef]
- Sharma, A.; Devi, I.; Singh, R. A review on role of Molluscan shells in the removal of pollutants from aquatic water bodies. Ecol. Environ. Conserv. 2020, 27, 615–621. [Google Scholar]
- Summa, D.; Lanzoni, M.; Castaldelli, G.; Fano, E.A.; Tamburini, E. Trends and Opportunities of Bivalve Shells’ Waste Valorization in a Prospect of Circular Blue Bioeconomy. Resources 2022, 11, 48. [Google Scholar] [CrossRef]
- Vandeginste, V. Food waste eggshell valorization through development of new composites: A review. Sustain. Mater. Technol. 2021, 29, e00317. [Google Scholar] [CrossRef]
- Mahari, W.A.W.; Waiho, K.; Azwar, E.; Fazhan, H.; Peng, W.; Ishak, S.D.; Tabatabaei, M.; Yek, P.N.Y.; Almomani, F.; Aghbashlo, M.; et al. A state-of-the-art review on producing engineered biochar from shellfish waste and its application in aquaculture wastewater treatment. Chemosphere 2022, 288 Pt 2, 132559. [Google Scholar] [CrossRef]
- Thürer, M.; Tomašević, I.; Stevenson, M.; Qu, T.; Huisingh, D. A systematic review of the literature on integrating sustainability into engineering curricula. J. Clean. Prod. 2018, 181, 608–617. [Google Scholar] [CrossRef]
- Bellei, P.; Torres, I.; Solstad, R.; Flores-Colen, I. Potential Use of Oyster Shell Waste in the Composition of Construction Composites: A Review. Buildings 2023, 13, 1546. [Google Scholar] [CrossRef]
- Koviessen, S.; O’Sullivan, A.; Gholami, M.; Vining, M.; de Vries, T. Physical and chemical parameters of various waste materials for living roof systems: A critical review. Ecol. Eng. 2023, 194, 107013. [Google Scholar] [CrossRef]
- Gholami, M.; O’Sullivan, A.D.; Mackey, H.R. Nutrient treatment of greywater in green wall systems: A critical review of removal mechanisms, performance efficiencies and system design parameters. J. Environ. Manag. 2023, 345, 118917. [Google Scholar] [CrossRef]
- Begum, S.; Yuhana, N.Y.; Saleh, N.M.; Kamarudin, N.H.N.; Sulong, A.B. Review of chitosan composite as a heavy metal adsorbent: Material preparation and properties. Carbohydr. Polym. 2021, 259, 117613. [Google Scholar] [CrossRef]
- Burnham, J.F. Scopus database: A review. Biomed. Digit. Libr. 2006, 3, 1. [Google Scholar] [CrossRef]
- Pranckutė, R. Web of Science (WoS) and Scopus: The Titans of Bibliographic Information in Today’s Academic World. Publications 2021, 9, 12. [Google Scholar] [CrossRef]
- Fritzen, R.R.; Benetti, A.D. Phosphorus removal in domestic wastewater treatment plant by calcined eggshell. Water Sci. Technol. 2021, 84, 995–1010. [Google Scholar] [CrossRef]
- Den, C.; Mariquit, E.G.; Kurniawan, W.; Hinode, H. Phosphate removal from wastewater using calcium silicate hydrate syn-thesized from lake sediment and bivalve shell. J. Chem. Eng. Jpn. 2020, 53, 287–295. [Google Scholar] [CrossRef]
- Nguyen, T.A.H.; Ngo, H.H.; Guo, W.S.; Nguyen, T.T.; Vu, N.D.; Soda, S.; Nguyen, T.H.H.; Nguyen, M.K.; Tran, T.V.H.; Dang, T.T.; et al. White hard clam (Meretrix lyrata) shells as novel filter media to augment the phosphorus removal from wastewater. Sci. Total. Environ. 2020, 741, 140483. [Google Scholar] [CrossRef]
- de Almeida, M.L.S.; Lima, A.C.; Nagahama, K.d.J.; Santos, T.S. Application of the Southwell Plot method to determine equilibrium time in phosphate adsorption. J. Contam. Hydrol. 2021, 242, 103841. [Google Scholar] [CrossRef]
- Souza, T.A.; Mascarenhas, A.J.S.; Andrade, H.M.C.; Santos, T.S.M. Combining Sewage Sludge and Clam Shell Waste to Prepare Adsorbents for Efficient Phosphorous Removal. Water Air Soil Pollut. 2018, 229, 383. [Google Scholar] [CrossRef]
- Hyland, K. Enter the dragon: China and global academic publishing. Learn. Publ. 2023, 36, 394–403. [Google Scholar] [CrossRef]
- Daudzai, Z.; Dolphen, R.; Thiravetyan, P. Simultaneous Removal of Phosphate and Nitrate from Synthetic and Real Wastewater by Meretrix lusoria as an Efficient and Novel Material. Water Air Soil Pollut. 2021, 232, 186. [Google Scholar] [CrossRef]
- Feng, M.; Li, M.; Zhang, L.; Luo, Y.; Zhao, D.; Yuan, M.; Zhang, K.; Wang, F. Oyster Shell Modified Tobacco Straw Biochar: Efficient Phosphate Adsorption at Wide Range of pH Values. Int. J. Environ. Res. Public Health 2022, 19, 7227. [Google Scholar] [CrossRef]
- Pérez, S.; Muñoz-Saldaña, J.; Garcia-Nunez, J.A.; Acelas, N.; Flórez, E. Unraveling the Ca–P species produced over the time during phosphorus removal from aqueous solution using biocomposite of eggshell-palm mesocarp fiber. Chemosphere 2022, 287 Pt 3, 132333. [Google Scholar] [CrossRef]
- Salim, N.A.A.; Fulazzaky, M.A.; Puteh, M.H.; Khamidun, M.H.; Yusoff, A.R.M.; Abdullah, N.H.; Fulazzaky, M.; Zaini, M.A.A. Mass Transfer Kinetics and Mechanisms of Phosphate Adsorbed on Waste Mussel Shell. Water Air Soil Pollut. 2022, 233, 223. [Google Scholar] [CrossRef]
- Salim, N.A.A.; Fulazzaky, M.A.; Zaini, M.A.A.; Puteh, M.H.; Khamidun, M.H.; Yusoff, A.R.M.; Abdullah, N.H.; Ahmad, N.; Lazim, Z.M.; Nuid, M. Phosphate Removal from Wastewater in Batch System Using Waste Mussel Shell. Biointerface Res. Appl. Chem. 2020, 11, 11473–11486. [Google Scholar] [CrossRef]
- Abdullah, N.H.; Yaacob, M.S.S.; Rani, N.A.I.; Azman, T.M.F.H.T.; Sumawan, M.N.I.; Hamid, N.B.; Salim, N.A.A.; Kaamin, M.; Kadir, M.A.A.; Ahmad, N.; et al. Phosphate Adsorption from Synthetic Aqueous Solutions by Waste Mussel Shell—Kinetics and Isotherms Studies. Int. J. Nanoelectron. Mater. 2021, 14, 1–10. [Google Scholar]
- Salim, N.A.A.; Puteh, M.H.; Yusoff, A.R.M.; Abdullah, N.H.; Fulazzaky, M.A.; Arman, M.A.Z.R.; Khamidun, M.H.; Zaini, M.A.A.; Syafiuddin, A.; Ahmad, N.; et al. Adsorption isotherms and kinetics of phosphate on waste mussel shell. Malays. J. Fundam. Appl. Sci. 2020, 16, 393–399. [Google Scholar] [CrossRef]
- Khamidun, M.H.; Ali, U.F.M.; Abdullah, S. Calculation of Filter Lifetime Using Empirical Model Applied to Hydrodynamic Column for Phosphate Removal from Greywater. Int. J. Eng. Technol. 2018, 7, 11–14. [Google Scholar] [CrossRef]
- Zukri, N.I.; Khamidun, M.H.; Sapiren, M.S.; Abdullah, S.; Rahman, M.A.A. Lake Water Quality Improvement by Using Waste Mussel Shell Powder as an Adsorbent. IOP Conf. Ser. Earth Environ. Sci. 2017, 140, 012057. [Google Scholar] [CrossRef]
- Xiong, J.; Qin, Y.; Islam, E.; Yue, M.; Wang, W. Phosphate removal from solution using powdered freshwater mussel shells. Desalination 2011, 276, 317–321. [Google Scholar] [CrossRef]
- Detho, A.; Daud, Z.; Rosli, M.A.; Awang, H.; Bin Ridzuan, M.B.; Halim, A.A.; Bin Tajarudin, H.A. Comparison study of COD and ammoniacal nitrogen adsorption on activated coconut shell carbon, green mussel (Perna viridis), zeolite and composite material in stabilized landfill leachate treatment. Desalination Water Treat. 2021, 220, 101–108. [Google Scholar] [CrossRef]
- Detho, A.; Daud, Z.; Rosli, M.A.; Awang, H. Reduction of COD and ammoniacal nitrogen from stabilized landfill leachate by using green mussel and zeolite as composite adsorbent. J. Air Waste Manag. Assoc. 2022, 72, 69–75. [Google Scholar] [CrossRef]
- Daud, Z.; Detho, A.; Rosli, M.A.; Abubakar, M.H.; Samo, K.A.; Rais, N.F.M.; Halim, A.A.; Tajarudin, H.A. Ammoniacal nitrogen and COD removal from stabilized landfill leachate using granular activated carbon and green mussel (Perna viridis) shell powder as a composite adsorbent. Desalination Water Treat. 2020, 192, 111–117. [Google Scholar] [CrossRef]
- Feng, Y.; Luo, Y.; He, Q.; Zhao, D.; Zhang, K.; Shen, S.; Wang, F. Performance and mechanism of a biochar-based Ca-La composite for the adsorption of phosphate from water. J. Environ. Chem. Eng. 2021, 9, 105267. [Google Scholar] [CrossRef]
- Feng, Y.; Zhao, D.; Qiu, S.; He, Q.; Luo, Y.; Zhang, K.; Shen, S.; Wang, F. Adsorption of Phosphate in Aqueous Phase by Biochar Prepared from Sheep Manure and Modified by Oyster Shells. ACS Omega 2021, 6, 33046–33056. [Google Scholar] [CrossRef]
- Adhikari, R.A.; Krishna, K.B.; Sarukkalige, R. Evaluation of phosphorus adsorption capacity of various filter materials from aqueous solution. Adsorpt. Sci. Technol. 2016, 34, 320–330. [Google Scholar] [CrossRef]
- Chen, J.; Cai, Y.; Clark, M.; Yu, Y. Equilibrium and kinetic studies of phosphate removal from solution onto a hydrothermally modified oyster shell material. PLoS ONE 2013, 8, e60243. [Google Scholar] [CrossRef]
- Chen, W.-T.; Lin, C.-W.; Shih, P.-K.; Chang, W.-L. Adsorption of phosphate into waste oyster shell: Thermodynamic parameters and reaction kinetics. Desalination Water Treat. 2012, 47, 86–95. [Google Scholar] [CrossRef]
- Lai, S.-L. Structure characterization and dephosphorization effect analysis of oyster shell-silica micropowder waste water dephosphorization materials. Chin. J. Struct. Chem. 2010, 29, 33–38. [Google Scholar]
- Hwang, C.-C.; Weng, C.-H. Key factors contributing to simultaneous nitrification-denitrification in a biological aerated filter system using oyster shell medium. Environ. Prot. Eng. 2017, 43, 75–86. [Google Scholar] [CrossRef]
- Han, Z.; Li, C.; Fan, R.; Lin, X.; Liu, D.; Wang, S.; Zhu, S.; Ye, Z. Ammonia Nitrogen Removal Using Oyster Shell as Alkalinity-Releasing Media in a Biological Nitrification System. Trans. ASABE 2017, 60, 1721–1728. [Google Scholar] [CrossRef]
- Yen, H.Y.; Chou, J.H. Water purification by oyster shell bio-medium in a recirculating aquaponic system. Ecol. Eng. 2016, 95, 229–236. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, J.; Liu, L.; Zhu, G.; Liu, C. Study of oyster shell as a potential substrate for constructed wetlands. Water Sci. Technol. 2013, 67, 2265–2272. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Chen, S. Building a low-cost domestic wastewater reclamation system using local agricultural waste in Kinmen islands, Taiwan. Paddy Water Environ. 2017, 15, 809–819. [Google Scholar] [CrossRef]
- Feng, Y.; Li, X.; Yu, Y.; Qi, J.; Jia, X.; Wang, J.; Li, X. Production of unburned calcium silicon filter material (UCSFM) from oyster shell and its performance investigation in an A/O integrated biological aerated filter reactor (A/O-BAF). RSC Adv. 2016, 6, 85324–85332. [Google Scholar] [CrossRef]
- Shih, P.-K.; Chang, W.-L. The effect of water purification by oyster shell contact bed. Ecol. Eng. 2015, 77, 382–390. [Google Scholar] [CrossRef]
- Azimi, S.M.; Malakootian, M.; Yaghmaeian, K.; Ahmadian, M.; Rahimi, S. Studying the efficiency of a biological aerated filter (BAF) with oyster Media on improving the quality of effluent produced by treatment plants. Fresenius Environ. Bull. 2014, 23, 2401–2406. [Google Scholar]
- Wang, Z.; Dong, J.; Liu, L.; Zhu, G.; Liu, C. Screening of phosphate-removing substrates for use in constructed wetlands treating swine wastewater. Ecol. Eng. 2013, 54, 57–65. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Yang, T.O.; Yuan, D.-X.; Wu, X.-Y. Study of municipal wastewater treatment with oyster shell as biological aerated filter medium. Desalination 2010, 254, 149–153. [Google Scholar] [CrossRef]
- Chen, J.; Zuo, K.; Li, Y.; Huang, X.; Hu, J.; Yang, Y.; Wang, W.; Chen, L.; Jain, A.; Verduzco, R.; et al. Eggshell membrane derived nitrogen rich porous carbon for selective electrosorption of nitrate from water. Water Res. 2022, 216, 118351. [Google Scholar] [CrossRef]
- Cao, H.; Wu, X.; Syed-Hassan, S.S.A.; Zhang, S.; Mood, S.H.; Milan, Y.J.; Garcia-Perez, M. Characteristics and mechanisms of phosphorous adsorption by rape straw-derived biochar functionalized with calcium from eggshell. Bioresour. Technol. 2020, 318, 124063. [Google Scholar] [CrossRef]
- Bus, A.; Karczmarczyk, A.; Baryła, A. Calcined Eggshell as a P Reactive Media Filter—Batch Tests and Column Sorption Experiment. Water Air Soil Pollut. 2019, 230, 20. [Google Scholar] [CrossRef]
- Santos, A.F.; Arim, A.L.; Lopes, D.V.; Gando-Ferreira, L.M.; Quina, M.J. Recovery of phosphate from aqueous solutions using calcined eggshell as an eco-friendly adsorbent. J. Environ. Manag. 2019, 238, 451–459. [Google Scholar] [CrossRef]
- Guo, Z.; Li, J.; Guo, Z.; Guo, Q.; Zhu, B. Phosphorus removal from aqueous solution in parent and aluminum-modified eggshells: Thermodynamics and kinetics, adsorption mechanism, and diffusion process. Environ. Sci. Pollut. Res. 2017, 24, 14525–14536. [Google Scholar] [CrossRef]
- Abdullah, N.H.; Adnan, N.A.; Rashidi, N.F.N.M.; Yaacob, M.S.S.; Salim, N.A.A. Comparing the adsorption isotherms and kinetics of phosphate adsorption on various waste shells as adsorbent. Water Pr. Technol. 2022, 17, 974–985. [Google Scholar] [CrossRef]
- Xiong, J.B.; Qin, Y.; Islam, E. Adsorptive removal of phosphate from aqueous solutions by waste snail and clam shells. Environ. Eng. Manag. J. 2015, 14, 1053–1058. [Google Scholar] [CrossRef]
- Suwannasingha, N.; Kantavong, A.; Tunkijjanukij, S.; Aenglong, C.; Liu, H.-B.; Klaypradit, W. Effect of calcination temperature on structure and characteristics of calcium oxide powder derived from marine shell waste. J. Saudi Chem. Soc. 2022, 26, 101441. [Google Scholar] [CrossRef]
- Majd, M.M.; Kordzadeh-Kermani, V.; Ghalandari, V.; Askari, A.; Sillanpää, M. Adsorption isotherm models: A comprehensive and systematic review (2010–2020). Sci. Total Environ. 2022, 812, 151334. [Google Scholar] [CrossRef]
- Tsai, W.-T. Microstructural Characterization of Calcite-Based Powder Materials Prepared by Planetary Ball Milling. Materials 2013, 6, 3361–3372. [Google Scholar] [CrossRef]
- Fulazzaky, M.A.; Salim, N.A.A.; Khamidun, M.H.; Puteh, M.H.; Yusoff, A.R.M.; Abdullah, N.H.; Syafiuddin, A.; Zaini, M.A.A. The mechanisms and kinetics of phosphate adsorption onto iron-coated waste mussel shell observed from hydrodynamic column. Int. J. Environ. Sci. Technol. 2021, 19, 6345–6358. [Google Scholar] [CrossRef]
- Alidoust, D.; Kawahigashi, M.; Yoshizawa, S.; Sumida, H.; Watanabe, M. Mechanism of cadmium biosorption from aqueous solutions using calcined oyster shells. J. Environ. Manag. 2015, 150, 103–110. [Google Scholar] [CrossRef]
- Ji, L.; Song, W.; Wei, D.; Jiang, D.; Cai, L.; Wang, Y.; Guo, J.; Zhang, H. Modified mussel shell powder for microalgae immobilization to remove N and P from eutrophic wastewater. Bioresour. Technol. 2019, 284, 36–42. [Google Scholar] [CrossRef]
- Pai, S.; Kini, S.M.; Selvaraj, R.; Pugazhendhi, A. A review on the synthesis of hydroxyapatite, its composites and adsorptive removal of pollutants from wastewater. J. Water Process. Eng. 2020, 38, 101574. [Google Scholar] [CrossRef]
- Azizian, S.; Eris, S. Adsorption isotherms and kinetics. In Adsorption: Fundamental Processes and Applications; Ghaedi, M., Ed.; Academic Press: Cambridge, MA, USA, 2021; Volume 33, pp. 445–509. [Google Scholar]
- Pap, S.; Gaffney, P.P.; Bremner, B.; Sekulic, M.T.; Maletic, S.; Gibb, S.W.; Taggart, M.A. Enhanced phosphate removal and potential recovery from wastewater by thermo-chemically calcinated shell adsorbents. Sci. Total. Environ. 2022, 814, 152794. [Google Scholar] [CrossRef]

 median line; □ mean; * outlier; ■ extreme values; Comb. of shells—combination of shells. Data points within boxplots are optimum values reported or mid-value if values are reported as ranges.
median line; □ mean; * outlier; ■ extreme values; Comb. of shells—combination of shells. Data points within boxplots are optimum values reported or mid-value if values are reported as ranges.
 median line; □ mean; * outlier; ■ extreme values; Comb. of shells—combination of shells. Data points within boxplots are optimum values reported or mid-value if values are reported as ranges.
median line; □ mean; * outlier; ■ extreme values; Comb. of shells—combination of shells. Data points within boxplots are optimum values reported or mid-value if values are reported as ranges.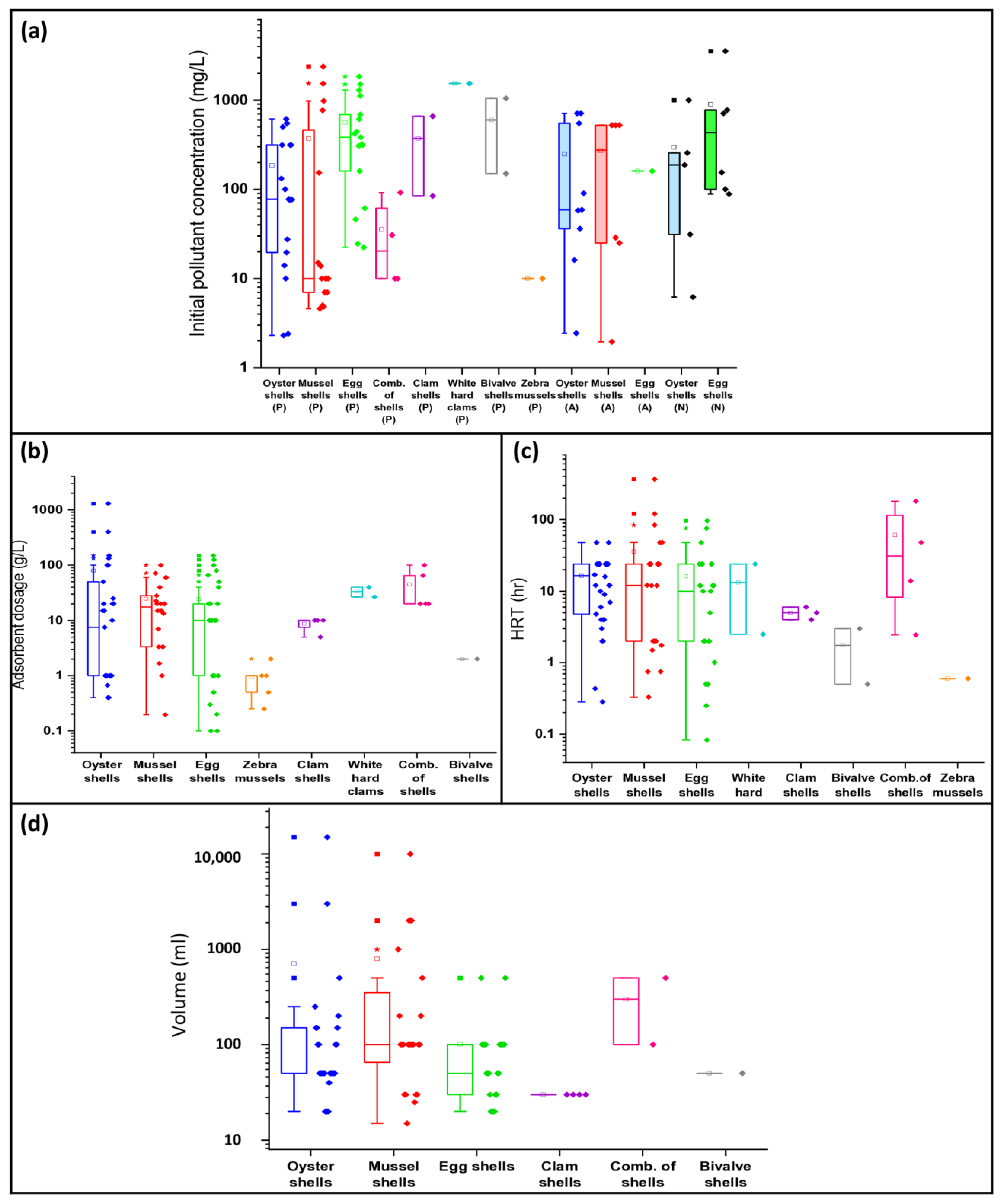

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tat Wai, K.; O’Sullivan, A.D.; Bello-Mendoza, R. Nitrogen and Phosphorus Removal from Wastewater Using Calcareous Waste Shells—A Systematic Literature Review. Environments 2024, 11, 119. https://doi.org/10.3390/environments11060119
Tat Wai K, O’Sullivan AD, Bello-Mendoza R. Nitrogen and Phosphorus Removal from Wastewater Using Calcareous Waste Shells—A Systematic Literature Review. Environments. 2024; 11(6):119. https://doi.org/10.3390/environments11060119
Chicago/Turabian StyleTat Wai, Kien, Aisling D. O’Sullivan, and Ricardo Bello-Mendoza. 2024. "Nitrogen and Phosphorus Removal from Wastewater Using Calcareous Waste Shells—A Systematic Literature Review" Environments 11, no. 6: 119. https://doi.org/10.3390/environments11060119
APA StyleTat Wai, K., O’Sullivan, A. D., & Bello-Mendoza, R. (2024). Nitrogen and Phosphorus Removal from Wastewater Using Calcareous Waste Shells—A Systematic Literature Review. Environments, 11(6), 119. https://doi.org/10.3390/environments11060119







