Towards a Consensus Method for the Isolation of Microplastics from Freshwater Sediments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Reagents
2.3. Procedures
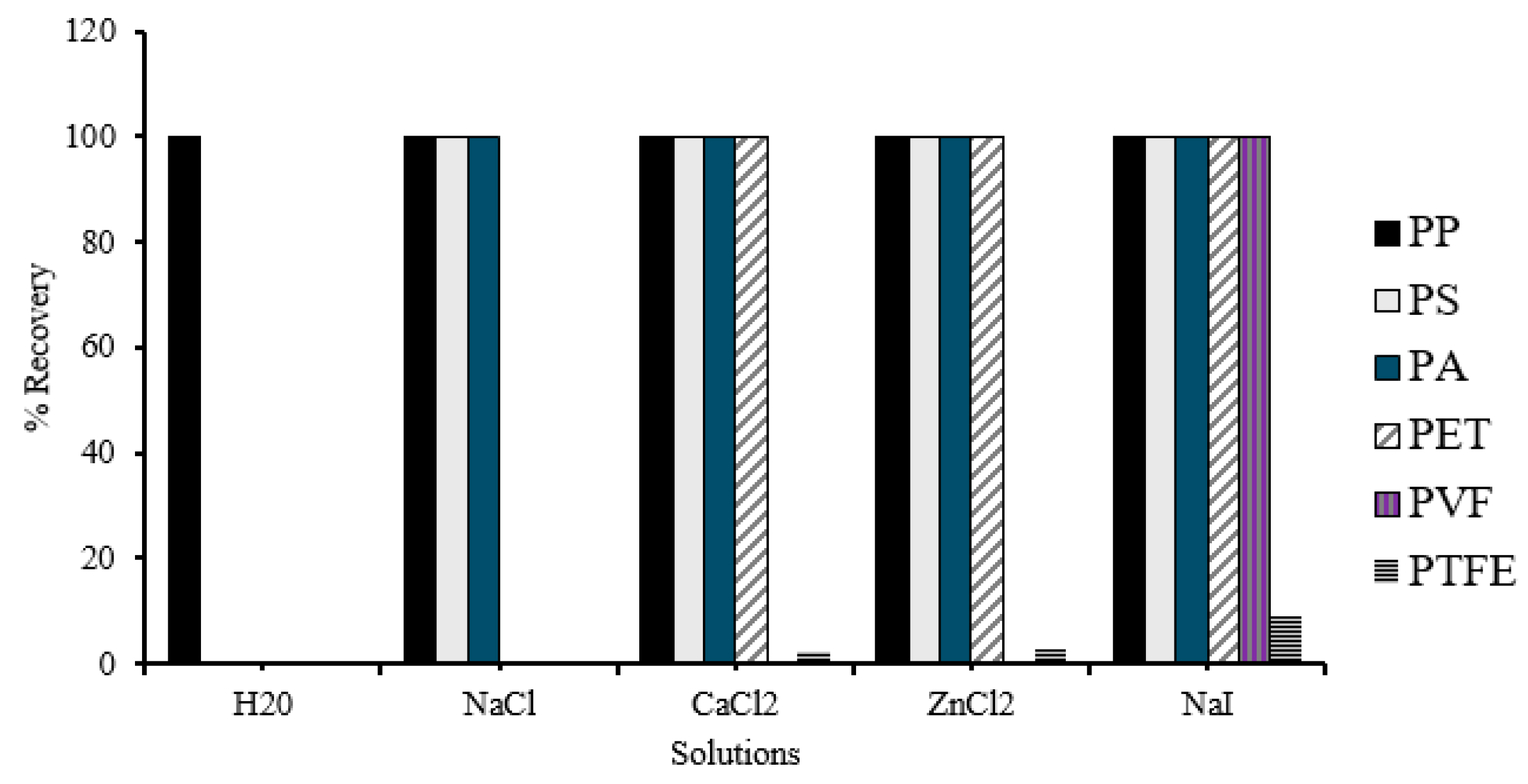
2.3.1. Comparison of Salt Solutions for Recovery of Microplastics by Density Flotation
2.3.2. Effect of Microplastic Size on Recovery by Density Flotation
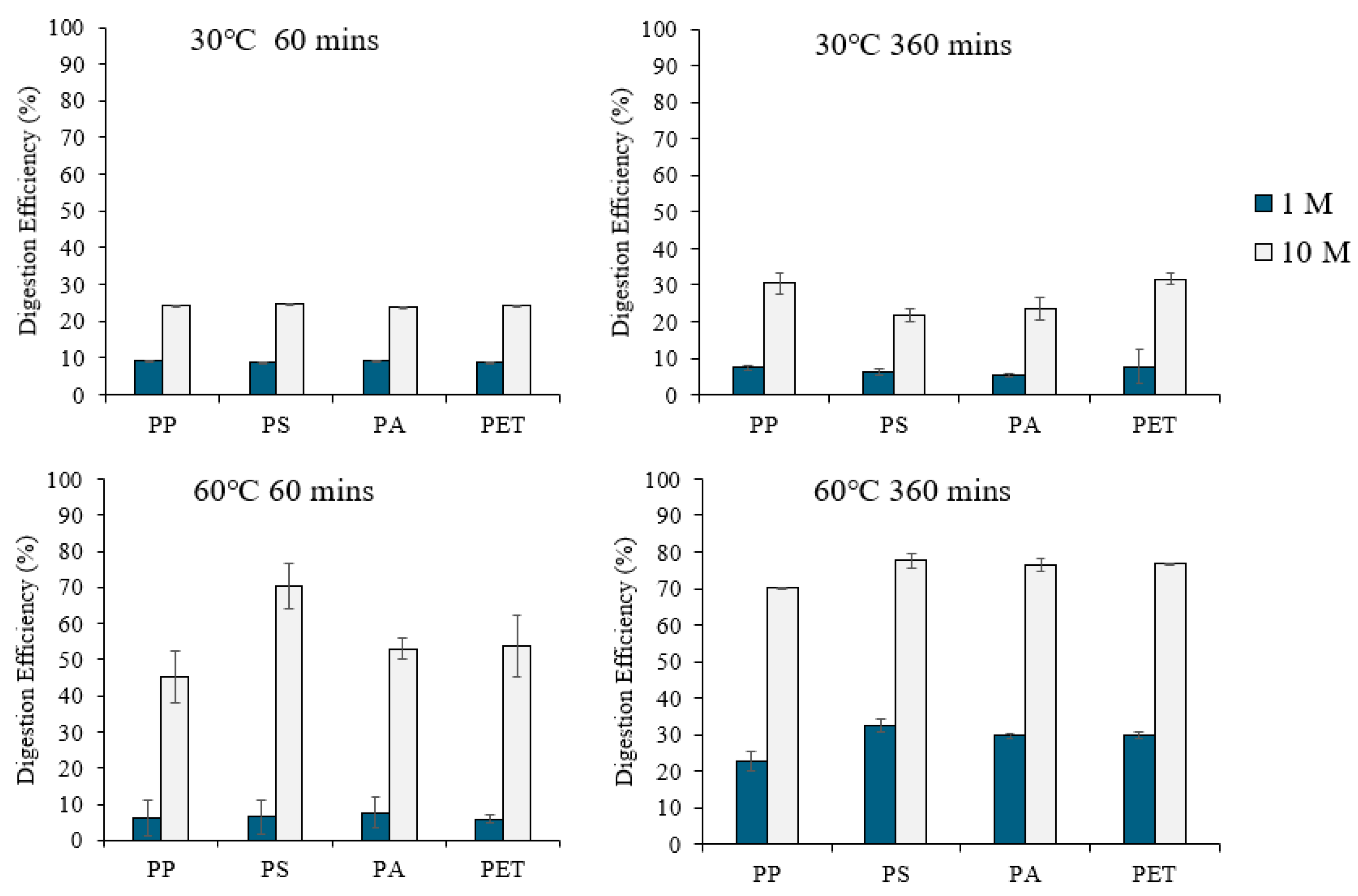
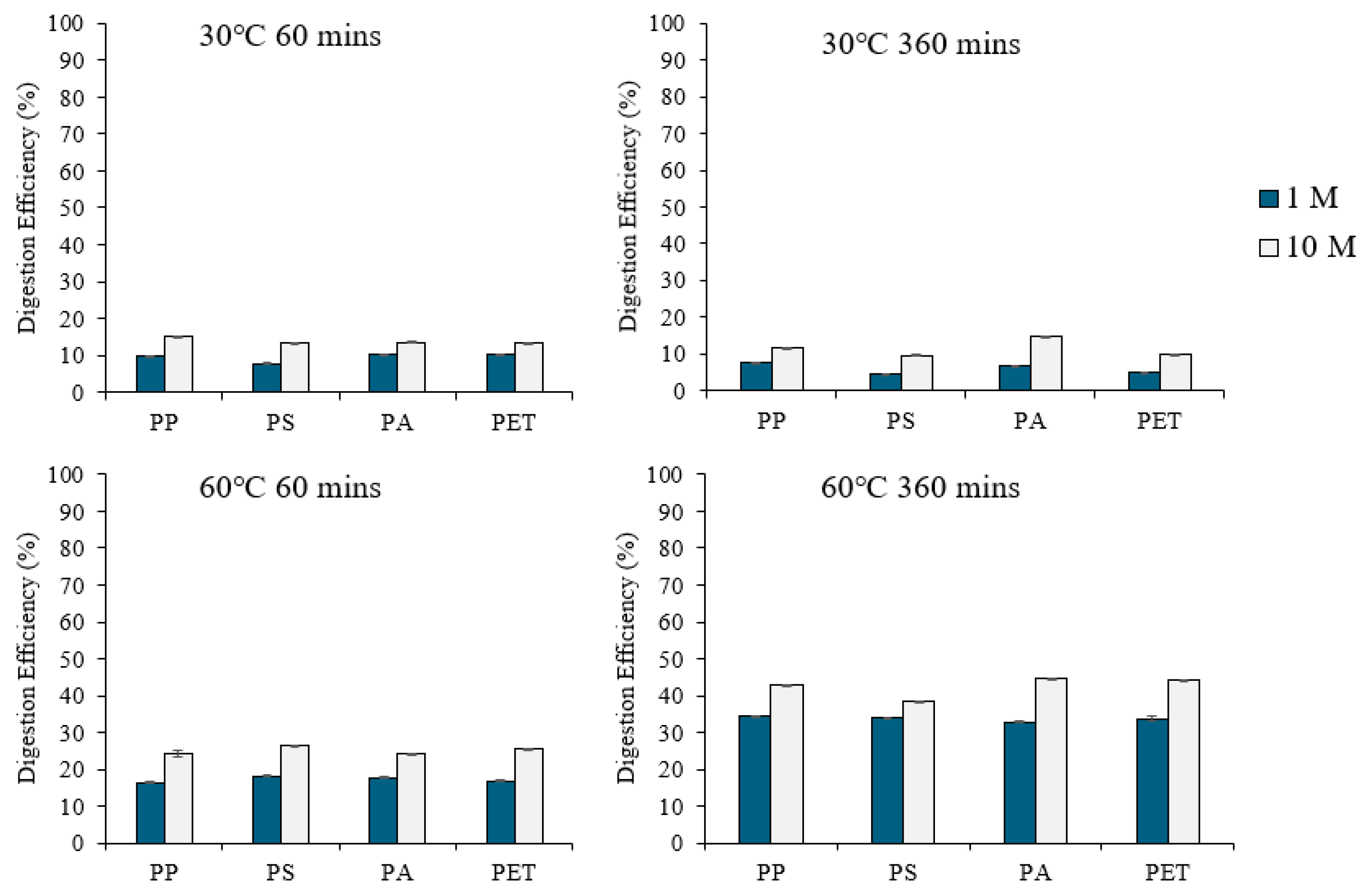
2.3.3. Comparison of Digestion Procedures for Organic Matter Removal
2.3.4. Analysis of Freshwater Sediment Sample
3. Results and Discussion
3.1. Comparison of Salt Solutions for Recovery of Microplastics by Density Flotation
3.2. Effect of Microplastic Size on Recovery by Density Flotation
3.3. Comparison of Digestion Procedures for Organic Matter Removal
3.3.1. Digestion of Organic Matter with HNO3
3.3.2. Digestion of Organic Matter with NaOH
3.3.3. Digestion of Organic Matter with Fenton’s Reagent
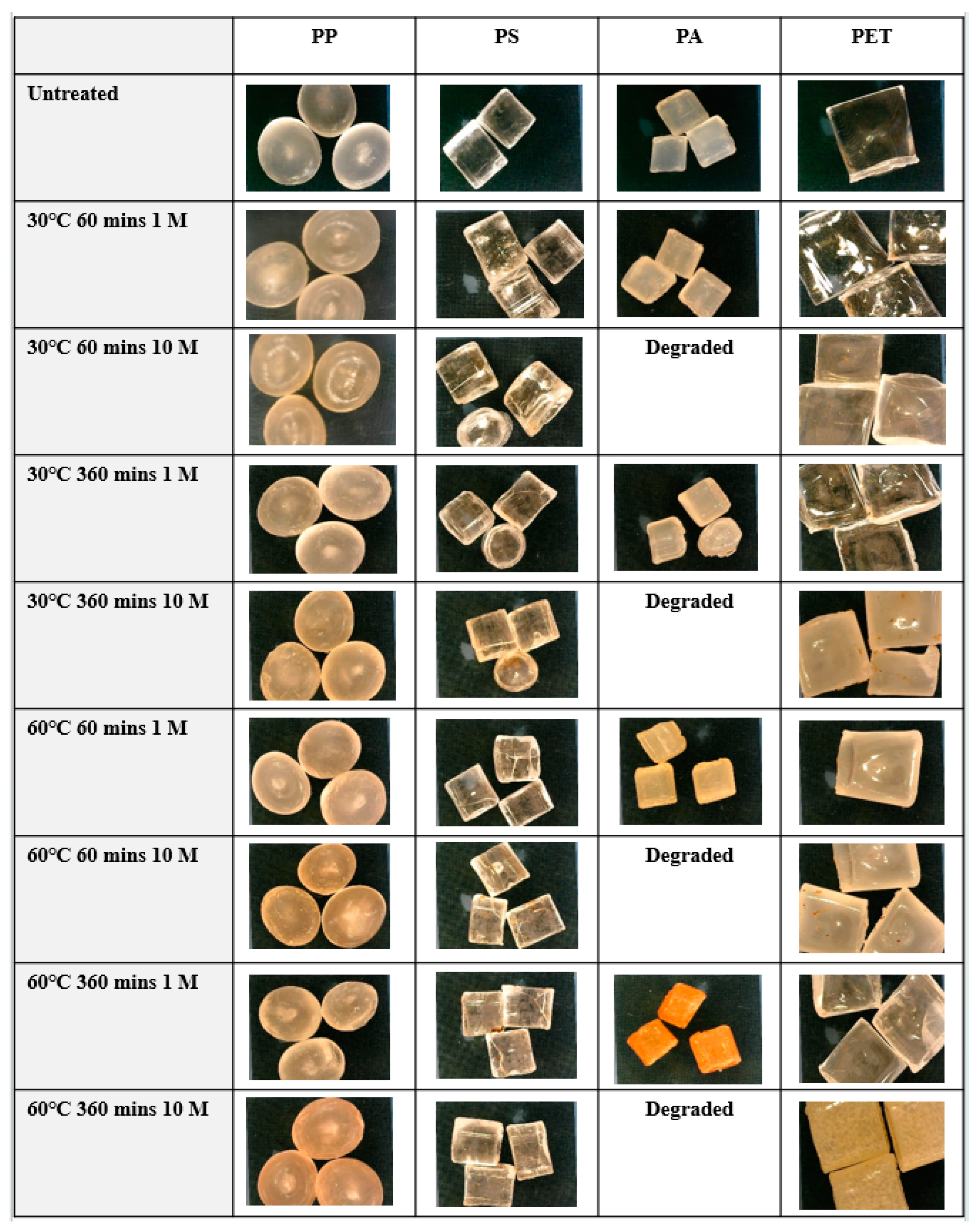
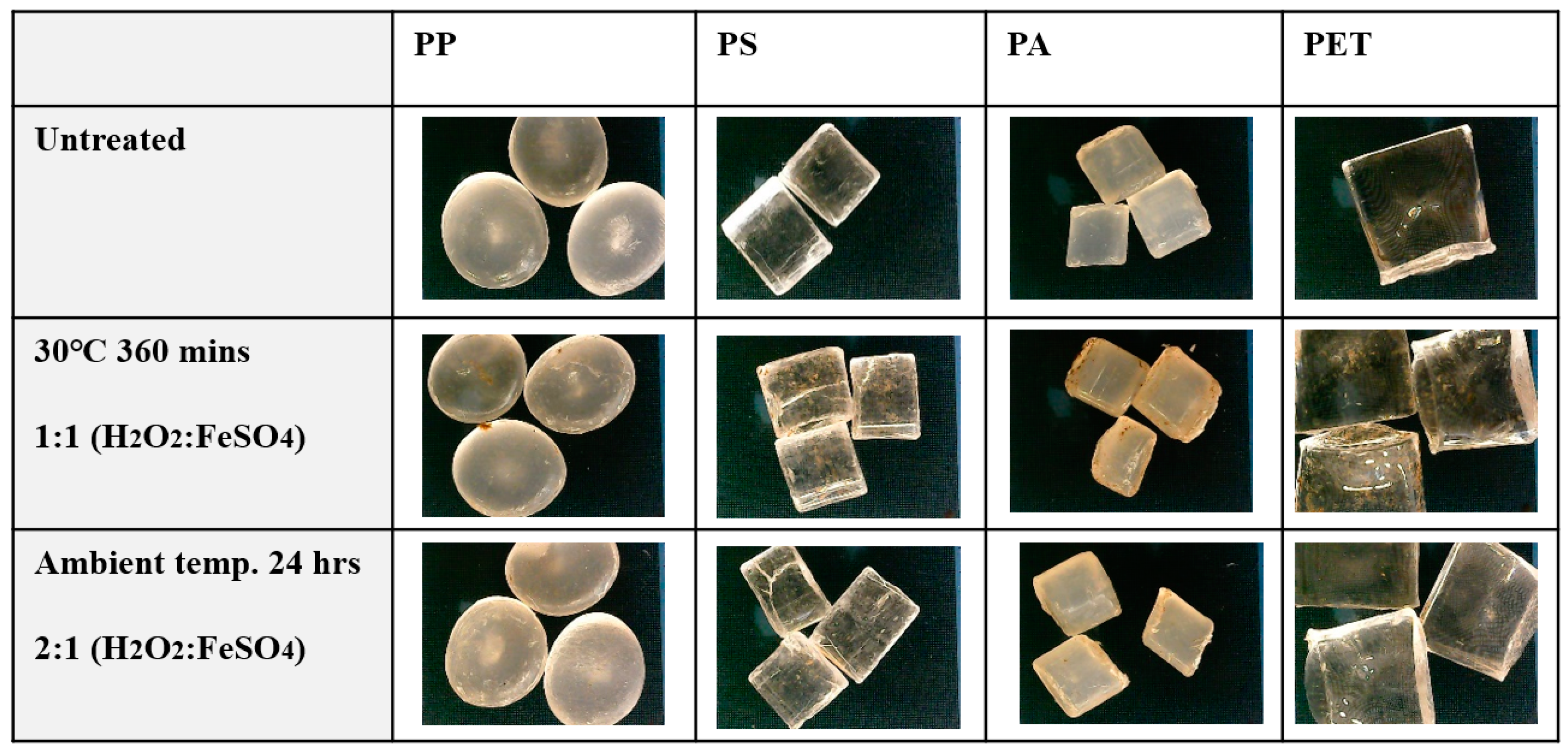
3.4. Effect of Digestion Procedures on Microplastics
3.4.1. Effect of Digestion with HNO3 on Microplastics
3.4.2. Effect of Digestion with HNO3 on Microplastics
3.4.3. Effect of Digestion with Fenton’s Reagent on Microplastics
3.5. Analysis of Freshwater Sediment Samples
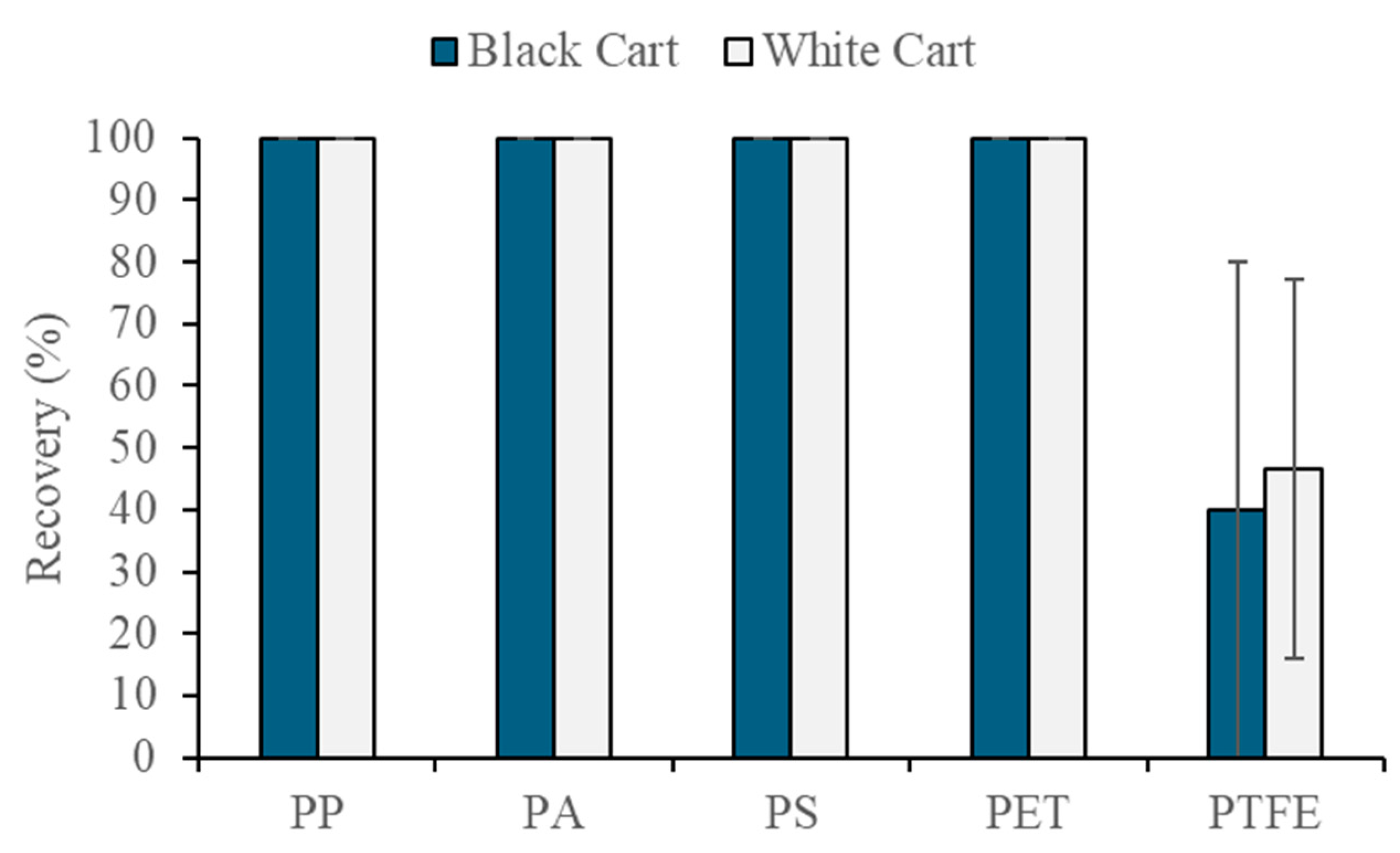
3.5.1. Recovery of Microplastics Added to the Sediment Samples
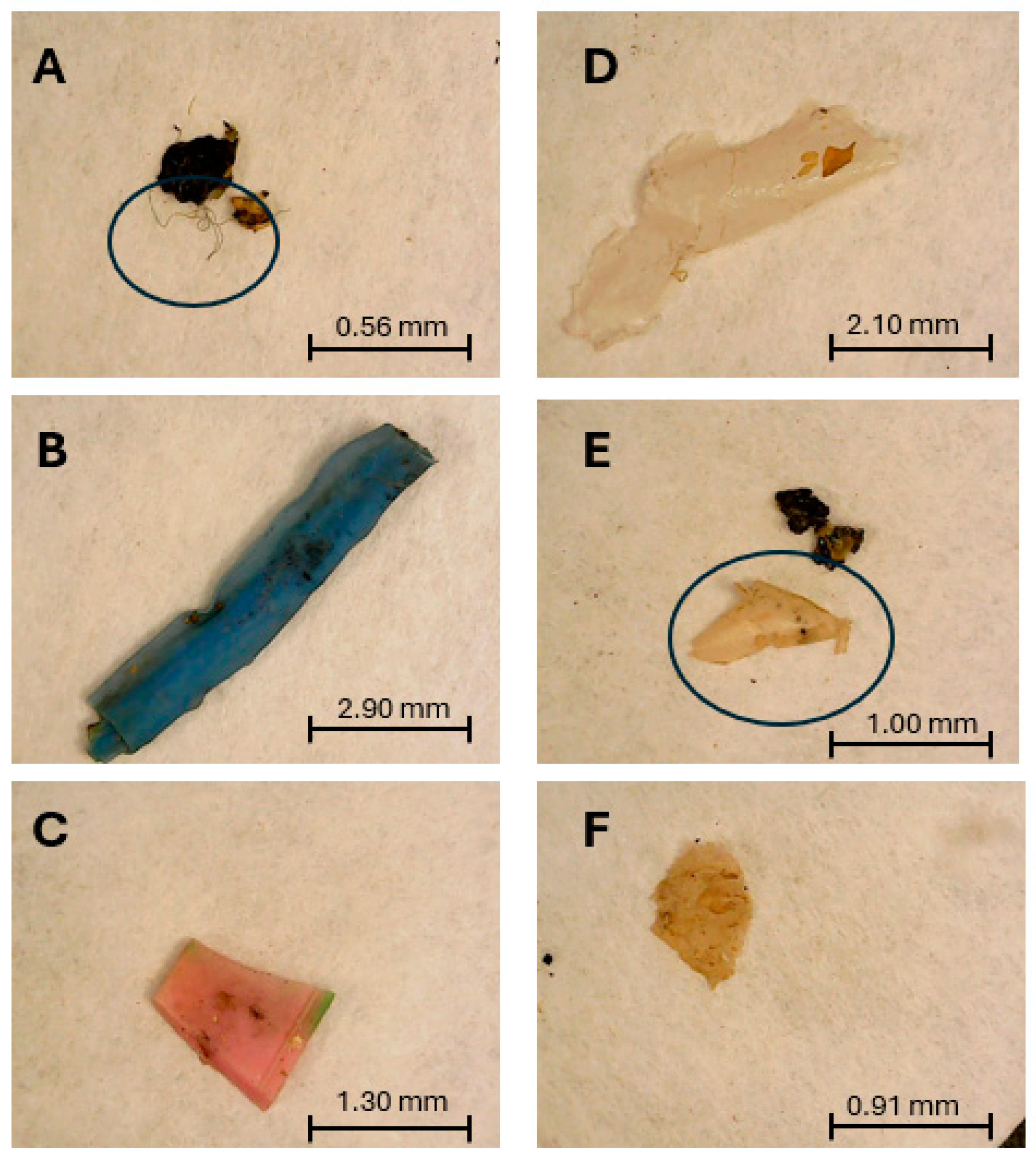
3.5.2. Study of Additional Microplastics Recovered from the Sediment Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- OECD. Global Plastics Outlook: Economic Drivers, Environmental Impacts and Policy Options; OECD Publishing: Paris, France, 2022. [Google Scholar] [CrossRef]
- Geyer, R. Chapter 2-Production, use, and fate of synthetic polymers. In Plastic Waste and Recycling; Letcher, T.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 13–32. [Google Scholar]
- Plastics Europe AISBL, Plastics—The Facts 2022. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/ (accessed on 11 June 2024).
- Lebreton, L.; Andrady, A. Future scenarios of global plastic waste generation and disposal. Palgrave Commun. 2019, 5, 6. [Google Scholar] [CrossRef]
- Robert, D.; Alle, P.H.; Keller, N.; Dzuila, M.-A.; Garcia-Muñoz, P. Chapter 18-Challenges and opportunities for microplastic and nanoplastic removal from industrial wastewater. In Current Developments in Biotechnology and Bioengineering; Tyagi, R.D., Pandey, A., Drogui, P., Yadav, B., Pilli, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 425–446. [Google Scholar]
- Bank, M.S.; Hansson, S.V. The Plastic Cycle: A Novel and Holistic Paradigm for the Anthropocene. Environ. Sci. Technol. 2019, 53, 7177–7179. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Gall, S.C.; Thompson, R.C. The impact of debris on marine life. Mar. Pollut. Bull. 2015, 92, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, M.S. Effects of Microplastics on Fish and in Human Health. Front. Environ. Sci. 2022, 10, 827289. [Google Scholar] [CrossRef]
- Bobori, D.; Dimitriadi, A.; Feidantsis, K.; Samiotaki, A.; Fafouti, D.; Sampsonidis, I.; Kalogiannis, S.; Kastrinaki, G.; Lambropoulou, D.; Kyzas, G.; et al. Toxic effects of polyethylene-microplastics on freshwater fish species: Implications for human health. Public Health Toxicol. 2022, 2, A108. [Google Scholar] [CrossRef]
- Corinaldesi, C.; Canensi, S.; Dell’Anno, A.; Tangherlini, M.; Di Capua, I.; Varrella, S.; Willis, T.J.; Cerrano, C.; Danovaro, R. Multiple impacts of microplastics can threaten marine habitat-forming species. Commun. Biol. 2021, 4, 431. [Google Scholar] [CrossRef] [PubMed]
- Thushari, G.G.N.; Senevirathna, J.D.M. Plastic pollution in the marine environment. Heliyon 2020, 6, e04709. [Google Scholar] [CrossRef]
- Garcia-Muñoz, P.; Robert, D.; Ruppert, A.M.; Keller, N. Chapter 1-Microplastics (MPs) and nanoplastics (NPs): Introduction. In Current Developments in Biotechnology and Bioengineering; Tyagi, R.D., Pandey, A., Drogui, P., Yadav, B., Pilli, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1–32. [Google Scholar]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- van Wezel, A.; Caris, I.; Kools, S.A.E. Release of primary microplastics from consumer products to wastewater in the Netherlands. Environ. Toxicol. Chem. 2016, 35, 1627–1631. [Google Scholar] [CrossRef]
- Rehse, S.; Kloas, W.; Zarfl, C. Short-term exposure with high concentrations of pristine microplastic particles leads to immobilisation of Daphnia magna. Chemosphere 2016, 153, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Nasser, F.; Lynch, I. Secreted protein eco-corona mediates uptake and impacts of polystyrene nanoparticles on Daphnia magna. J. Proteom. 2016, 137, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Kelpsiene, E.; Torstensson, O.; Ekvall, M.T.; Hansson, L.A.; Cedervall, T. Long-term exposure to nanoplastics reduces life-time in Daphnia magna. Sci. Rep. 2020, 10, 5979. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.L.; Hassan, H.U.; Khan, F.U.; Ghaffar, R.A.; Rafiq, N.; Bilal, M.; Khooharo, A.R.; Ullah, S.; Jafari, H.; Nadeem, K.; et al. Effects of microplastics in freshwater fishes health and the implications for human health. Braz. J. Biol. 2024, 84, e272524. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Hoh, E.; Hentschel, B.T.; Kaye, S. Long-Term Field Measurement of Sorption of Organic Contaminants to Five Types of Plastic Pellets: Implications for Plastic Marine Debris. Environ. Sci. Technol 2013, 47, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Kieu-Le, T.-C.; Thuong, Q.-T.; Truong, T.-N.-S.; Le, T.-M.-T.; Tran, Q.-V.; Strady, E. Baseline concentration of microplastics in surface water and sediment of the northern branches of the Mekong River Delta, Vietnam. Mar. Pollut. Bull. 2023, 187, 114605. [Google Scholar] [CrossRef] [PubMed]
- Shokunbi, O.S.; Idowu, G.A.; Aiyesanmi, A.F.; Davidson, C.M. Assessment of Microplastics and Potentially Toxic Elements in Surface Sediments of the River Kelvin, Central Scotland, United Kingdom. Environ. Manag. 2024, 73, 932–945. [Google Scholar] [CrossRef]
- Malla-Pradhan, R.; Phoungthong, K.; Suwunwong, T.; Joshi, T.P.; Pradhan, B.L. Microplastic pollution in lakeshore sediments: The first report on abundance and composition of Phewa Lake, Nepal. Environ. Sci. Pollut. Res. Int. 2023, 30, 70065–70075. [Google Scholar] [CrossRef]
- Oni, B.A.; Ayeni, A.O.; Agboola, O.; Oguntade, T.; Obanla, O. Comparing microplastics contaminants in (dry and raining) seasons for Ox-Bow Lake in Yenagoa, Nigeria. Ecotoxicol. Environ. Safe 2020, 198, 110656. [Google Scholar] [CrossRef]
- Eerkes-Medrano, D.; Thompson, R.C.; Aldridge, D.C. Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res. 2015, 75, 63–82. [Google Scholar] [CrossRef]
- Tornero, Q.; Dzuila, M.-A.; Robert, D.; Keller, N.; Rodríguez-Chueca, J.; Garcia-Muñoz, P. Chapter 4-Methods of sampling and sample preparation for detection of microplastics and nanoplastics in the environment. In Current Developments in Biotechnology and Bioengineering; Tyagi, R.D., Pandey, A., Drogui, P., Yadav, B., Pilli, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 79–97. [Google Scholar]
- Grbić, J.; Helm, P.; Athey, S.; Rochman, C.M. Microplastics entering northwestern Lake Ontario are diverse and linked to urban sources. Water Resour. 2020, 174, 115623. [Google Scholar] [CrossRef]
- Chaisanguansuk, P.; Phantuwongraj, S.; Jirapinyakul, A.; Assawincharoenkij, T. Preliminary study on microplastic abundance in mangrove sediment cores at Mae Klong River, upper Gulf of Thailand. Front. Environ. Sci. 2023, 11, 34988. [Google Scholar] [CrossRef]
- Semmouri, I.; Vercauteren, M.; Van Acker, E.; Pequeur, E.; Asselman, J.; Janssen, C. Distribution of microplastics in freshwater systems in an urbanized region: A case study in Flanders (Belgium). Sci. Total Environ. 2023, 872, 162192. [Google Scholar] [CrossRef]
- Stock, F.; Kochleus, C.; Bänsch-Baltruschat, B.; Brennholt, N.; Reifferscheid, G. Sampling techniques and preparation methods for microplastic analyses in the aquatic environment—A review. TrAC Trends Anal. Chem. 2019, 113, 84–92. [Google Scholar] [CrossRef]
- Turner, S.; Horton, A.A.; Rose, N.L.; Hall, C. A temporal sediment record of microplastics in an urban lake, London, UK. J. Paleolimnol. 2019, 61, 449–462. [Google Scholar] [CrossRef]
- Gohla, J.; Bracun, S.; Gretschel, G.; Koblmuller, S.; Wagner, M.; Pacher, C. Potassium carbonate (K2CO3)—A cheap, non-toxic and high-density floating solution for microplastic isolation from beach sediments. Mar. Pollut. Bull. 2021, 170, 112618. [Google Scholar] [CrossRef]
- Li, W.X.; Li, X.; Tong, J.; Xiong, W.P.; Zhu, Z.Q.; Gao, X.; Li, S.; Jia, M.Y.; Yang, Z.H.; Liang, J. Effects of environmental and anthropogenic factors on the distribution and abundance of microplastics in freshwater ecosystems. Sci. Total Environ. 2023, 856, 159030. [Google Scholar] [CrossRef]
- Onink, V.; Kaandorp, M.L.A.; van Sebille, E.; Laufkötter, C. Influence of Particle Size and Fragmentation on Large-Scale Microplastic Transport in the Mediterranean Sea. Environ. Sci. Technol. 2022, 56, 15528–15540. [Google Scholar] [CrossRef]
- Bermúdez, J.R.; Swarzenski, P.W. A microplastic size classification scheme aligned with universal plankton survey methods. MethodsX 2021, 8, 101516. [Google Scholar] [CrossRef]
- Yu, F.; Pei, Y.; Zhang, X.; Wu, X.; Zhang, G.; Ma, J. Occurrence and distribution characteristics of aged microplastics in the surface water, sediment, and crabs of the aquaculture pond in the Yangtze River Delta of China. Sci. Total Environ. 2023, 871, 162039. [Google Scholar] [CrossRef]
- Duan, J.; Han, J.; Zhou, H.; Lau, Y.L.; An, W.; Wei, P.; Cheung, S.G.; Yang, Y.; Tam, N.F.-y. Development of a digestion method for determining microplastic pollution in vegetal-rich clayey mangrove sediments. Sci. Total Environ. 2020, 707, 136030. [Google Scholar] [CrossRef]
- Zou, Y.-d.; Xu, Q.-q.; Zhang, G.; Li, F.-y.; Li, F.-m. Influence of Six Digestion Methods on the Determination of Polystyrene Microplastics in Organisms Using the Fluorescence Intensity. Huanjing Kexue 2019, 40, 496–503. [Google Scholar] [CrossRef]
- Pfeiffer, F.; Fischer, E.K. Various Digestion Protocols Within Microplastic Sample Processing—Evaluating the Resistance of Different Synthetic Polymers and the Efficiency of Biogenic Organic Matter Destruction. Front. Environ. Sci. 2020, 8, 572424. [Google Scholar] [CrossRef]
- Karami, A.; Golieskardi, A.; Choo, C.K.; Romano, N.; Ho, Y.B.; Salamatinia, B. A high-performance protocol for extraction of microplastics in fish. Sci. Total Environ. 2017, 578, 485–494. [Google Scholar] [CrossRef]
- Hurley, R.R.; Lusher, A.L.; Olsen, M.; Nizzetto, L. Validation of a Method for Extracting Microplastics from Complex, Organic-Rich, Environmental Matrices. Environ. Sci. Technol. 2018, 52, 7409–7417. [Google Scholar] [CrossRef]
- Herrera, A.; Garrido-Amador, P.; Martínez, I.; Samper, M.D.; López-Martínez, J.; Gómez, M.; Packard, T.T. Novel methodology to isolate microplastics from vegetal-rich samples. Mar. Pollut. Bull. 2018, 129, 61–69. [Google Scholar] [CrossRef]
- Schrank, I.; Möller, J.N.; Imhof, H.K.; Hauenstein, O.; Zielke, F.; Agarwal, S.; Löder, M.G.J.; Greiner, A.; Laforsch, C. Microplastic sample purification methods-Assessing detrimental effects of purification procedures on specific plastic types. Sci. Total Environ. 2022, 833, 154824. [Google Scholar] [CrossRef]
- Cole, M.; Webb, H.; Lindeque, P.K.; Fileman, E.S.; Halsband, C.; Galloway, T.S. Isolation of microplastics in biota-rich seawater samples and marine organisms. Sci. Rep. 2014, 4, 4528. [Google Scholar] [CrossRef]
- Xu, Q.; Deng, T.; LeBlanc, G.A.; An, L. An effective method for evaluation of microplastic contaminant in gastropod from Taihu Lake, China. Environ. Sci. Pollut. Res. 2020, 27, 22878–22887. [Google Scholar] [CrossRef]
- Redondo-Hasselerharm, P.E.; Rico, A.; Koelmans, A.A. Risk assessment of microplastics in freshwater sediments guided by strict quality criteria and data alignment methods. J. Hazard. Mater. 2023, 441, 129814. [Google Scholar] [CrossRef]
- Karimi Estahbanati, M.R.; Feilizadeh, M.; Avazpour, S.; Kavand, M.; Drogui, P.; Tyagi, R.D. Chapter 11-Physical and physicochemical separation of microplastics and nanoplastics from water. In Current Developments in Biotechnology and Bioengineering; Tyagi, R.D., Pandey, A., Drogui, P., Yadav, B., Pilli, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 269–292. [Google Scholar]
- Cashman, M.A.; Ho, K.T.; Boving, T.B.; Russo, S.; Robinson, S.; Burgess, R.M. Comparison of microplastic isolation and extraction procedures from marine sediments. Mar. Pollut. Bull. 2020, 159, 111507. [Google Scholar] [CrossRef]
- Duong, T.T.; Le, P.T.; Nguyen, T.N.H.; Hoang, T.Q.; Ngo, H.M.; Doan, T.O.; Le, T.P.Q.; Bui, H.T.; Bui, M.H.; Trinh, V.T.; et al. Selection of a density separation solution to study microplastics in tropical riverine sediment. Environ. Monit. Assess. 2022, 194, 65. [Google Scholar] [CrossRef]
- Radford, F.; Zapata-Restrepo, L.M.; Horton, A.A.; Hudson, M.D.; Shaw, P.J.; Williams, I.D. Developing a systematic method for extraction of microplastics in soils. Anal. Methods 2021, 13, 1695–1705. [Google Scholar] [CrossRef]
- Han, X.; Lu, X.; Vogt, R.D. An optimized density-based approach for extracting microplastics from soil and sediment samples. Environ. Pollut. 2019, 254, 113009. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Girao, A.V.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Identifying a quick and efficient method of removing organic matter without damaging microplastic samples. Sci. Total Environ. 2019, 686, 131–139. [Google Scholar] [CrossRef]
- Lohmann, R.; Cousins, I.T.; Dewitt, J.C.; Glüge, J.; Goldenman, G.; Herzke, D.; Lindstrom, A.B.; Miller, M.F.; Ng, C.A.; Patton, S.; et al. Are Fluoropolymers Really of Low Concern for Human and Environmental Health and Separate from Other PFAS? Environ. Sci. Technol. 2020, 54, 12820–12828. [Google Scholar] [CrossRef]
- Pansu, M. Handbook of Soil Analysis: Mineralogical, Organic and Inorganic Methods/[Internet Resource], 1st ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2006. [Google Scholar]
- Zhang, S.; Yang, X.; Gertsen, H.; Peters, P.; Salánki, T.; Geissen, V. A simple method for the extraction and identification of light density microplastics from soil. Sci. Total Environ. 2018, 616–617, 1056–1065. [Google Scholar] [CrossRef]
- Keene, J.; Turner, A. Microplastics in coastal urban sediments: Discrepancies in concentration and character revealed by different approaches to sample processing. Sci. Total Environ. 2023, 865, 161140. [Google Scholar] [CrossRef]
- Gao, B.; Chen, Y.; Xu, D.; Sun, K.; Xing, B. Substantial burial of terrestrial microplastics in the Three Gorges Reservoir, China. Commun. Earth Environ. 2023, 4, 32. [Google Scholar] [CrossRef]
- Kniggendorf, A.-K.; Nogueira, R.; Lorey, C.; Roth, B. Calcium carbonate deposits and microbial assemblages on microplastics in oligotrophic freshwaters. Chemosphere 2021, 266, 128942. [Google Scholar] [CrossRef]
- Yu, Z.; Peng, B.; Liu, L.Y.; Wong, C.S.; Zeng, E.Y. Development and Validation of an Efficient Method for Processing Microplastics in Biota Samples. Environ. Toxicol. Chem. 2019, 38, 1400–1408. [Google Scholar] [CrossRef]
- Tagg, A.S.; Sapp, M.; Harrison, J.P.; Ojeda, J.J. Identification and Quantification of Microplastics in Wastewater Using Focal Plane Array-Based Reflectance Micro-FT-IR Imaging. Anal. Chem. 2015, 87, 6032–6040. [Google Scholar] [CrossRef]
- Ge, A.; Zhao, S.; Sun, C.; Yuan, Z.; Liu, L.; Chen, L.; Li, F. Comparison of three digestion methods for microplastic extraction from aquaculture feeds. Sci. Total Environ. 2024, 912, 168919. [Google Scholar] [CrossRef]
- Rendell-Bhatti, F.; Bull, C.; Cross, R.; Cox, R.; Adediran, G.A.; Lahive, E. From the environment into the biomass: Microplastic uptake in a protected lamprey species. Environ. Pollut. 2023, 323, 121267. [Google Scholar] [CrossRef]
- Reina Maricela Blair, E. Microplastics in Wastewater Treatment Systems and Receiving Waters. Ph.D. Thesis, University of Glasgow, Scotland, UK, 2019. [Google Scholar]
- Dai, J.M.; Liu, P.; Wang, C.Y.; Li, H.; Qiang, H.; Yang, Z.Y.; Guo, X.T.; Gao, S.X. Which factors mainly drive the photoaging of microplastics in freshwater? Sci. Total Environ. 2023, 858, 159845. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.L.; Kang, S.C.; Wang, Z.Q.; Wu, C.X. Microplastics in freshwater sediment: A review on methods, occurrence, and sources. Sci. Total Environ. 2021, 754, 141948. [Google Scholar] [CrossRef]
- Qiao, R.; Deng, Y.; Zhang, S.; Wolosker, M.B.; Zhu, Q.; Ren, H.; Zhang, Y. Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere 2019, 236, 124334. [Google Scholar] [CrossRef]
- Blašković, A.; Fastelli, P.; Čižmek, H.; Guerranti, C.; Renzi, M. Plastic litter in sediments from the Croatian marine protected area of the natural park of Telaščica bay (Adriatic Sea). Mar. Pollut. Bull. 2017, 114, 583–586. [Google Scholar] [CrossRef]



| Plastic | Origin | Reported Density (g/cm3) * | Particle Size (mm) | Shape |
|---|---|---|---|---|
| PP | Virgin (Merck) | 0.85–0.93 | 4.4–4.6 | Flat oval |
| PS | Virgin (Merck) | 1.04–1.08 | 2.6–3.0 | Cylindrical |
| PA | Virgin (Merck) | 1.13–1.16 | 2.4–2.7 | Cylindrical |
| PET | Virgin (Merck) | 1.38–1.41 | 3.6–4.4 | Flat squared |
| PVF | Virgin (Merck) | 1.78 | 4.2–4.8 | Spherical |
| PTFE | Post-consumer | 2.2 | Variable | Irregular |
| Salt | Mass of Salt Weighed (g) | Vol. of Solution Prepared (mL) | Density of Solution (g/cm3) |
|---|---|---|---|
| NaCl | 180 | 500 | 1.195 |
| CaCl2 | 300 | 500 | 1.350 |
| ZnCl2 | 450 | 500 | 1.450 |
| NaI | 500 | 500 | 1.800 |
| PS (%) | PA (%) | PET (%) | PTFE (%) | ||
|---|---|---|---|---|---|
| H2O | <1 mm | 67.0 ± 1.4 | 100 ± 0.0 | 40.6 ± 0.9 | 28.2 ± 11.4 |
| 1–2 mm | 59.0 ± 1.4 | 92.8 ± 3.7 | 46.2 ± 2.3 | 38.4 ± 8.4 | |
| >2 mm | 54.5 ± 2.1 | 0 | 0 | 4.00 ± 1.4 | |
| Unground | 0 | 0 | 0 | 0 | |
| NaCl | <1 mm | - | - | 90.2 ± 4.5 | 11.2 ± 2.5 |
| 1–2 mm | - | - | 64.6 ± 0.7 | 35.6 ± 14.8 | |
| >2 mm | - | - | 13.5 ± 2.8 | 8.60 ± 5.10 | |
| Unground | 100 | 100 | 0 | 0 | |
| CaCl2 | <1 mm | - | - | - | 80.2 ± 2.9 |
| 1–2 mm | - | - | - | 30.2 ± 27.3 | |
| >2 mm | - | - | - | 12.1 ± 5.7 | |
| Unground | 100 | 100 | 100 | 2.50 ± 2.10 | |
| ZnCl2 | <1 mm | - | - | - | 60.7 ± 17.4 |
| 1–2 mm | - | - | - | 65.7 ± 3.3 | |
| >2 mm | - | - | - | 11.0 ± 1.5 | |
| Unground | 100 | 100 | 100 | 3.0 ± 2.0 | |
| NaI | <1 mm | - | - | - | 60.2 ± 12.9 |
| 1–2 mm | - | - | - | 10.9 ± 3.0 | |
| >2 mm | - | - | - | 7.65 ± 5.20 | |
| Unground | 100 | 100 | 100 | 9.00 ± 1.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enenche, D.E.; Davidson, C.M.; Liggat, J.J. Towards a Consensus Method for the Isolation of Microplastics from Freshwater Sediments. Environments 2024, 11, 146. https://doi.org/10.3390/environments11070146
Enenche DE, Davidson CM, Liggat JJ. Towards a Consensus Method for the Isolation of Microplastics from Freshwater Sediments. Environments. 2024; 11(7):146. https://doi.org/10.3390/environments11070146
Chicago/Turabian StyleEnenche, Daniel E., Christine M. Davidson, and John J. Liggat. 2024. "Towards a Consensus Method for the Isolation of Microplastics from Freshwater Sediments" Environments 11, no. 7: 146. https://doi.org/10.3390/environments11070146
APA StyleEnenche, D. E., Davidson, C. M., & Liggat, J. J. (2024). Towards a Consensus Method for the Isolation of Microplastics from Freshwater Sediments. Environments, 11(7), 146. https://doi.org/10.3390/environments11070146







