Enhancing Swine Wastewater Treatment: A Sustainable and Systematic Approach through Optimized Chemical Oxygen Demand/Sulfate Mass Ratio in Attached-Growth Anaerobic Bioreactor
Abstract
:1. Introduction
2. Material and Methods
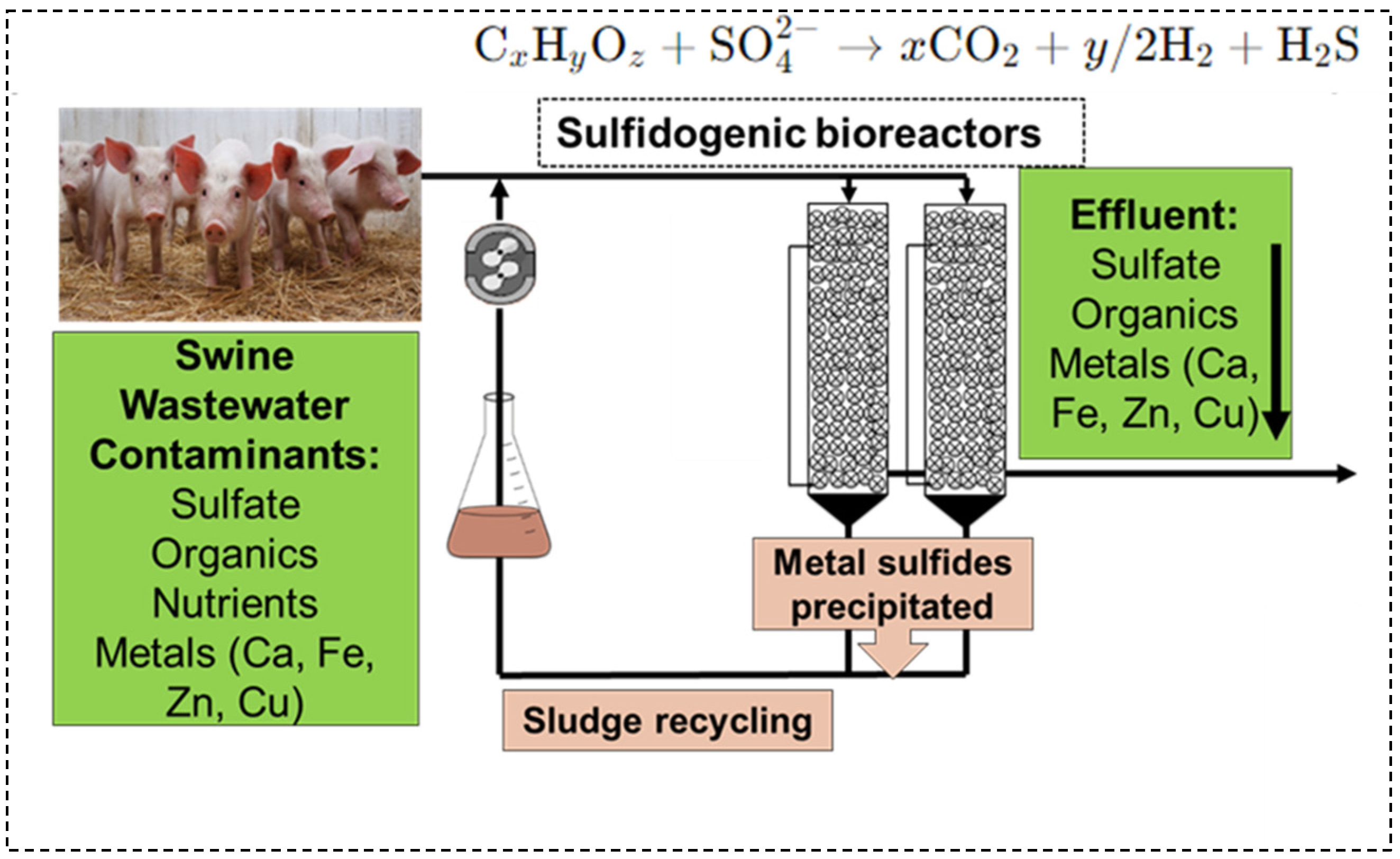
2.1. Anaerobic Bioreactors Setup
2.2. Operation
2.3. Measurement
3. Results and Discussion
3.1. Water Quality of Initial Swine Wastewater
3.2. Swine Wastewater Treatment
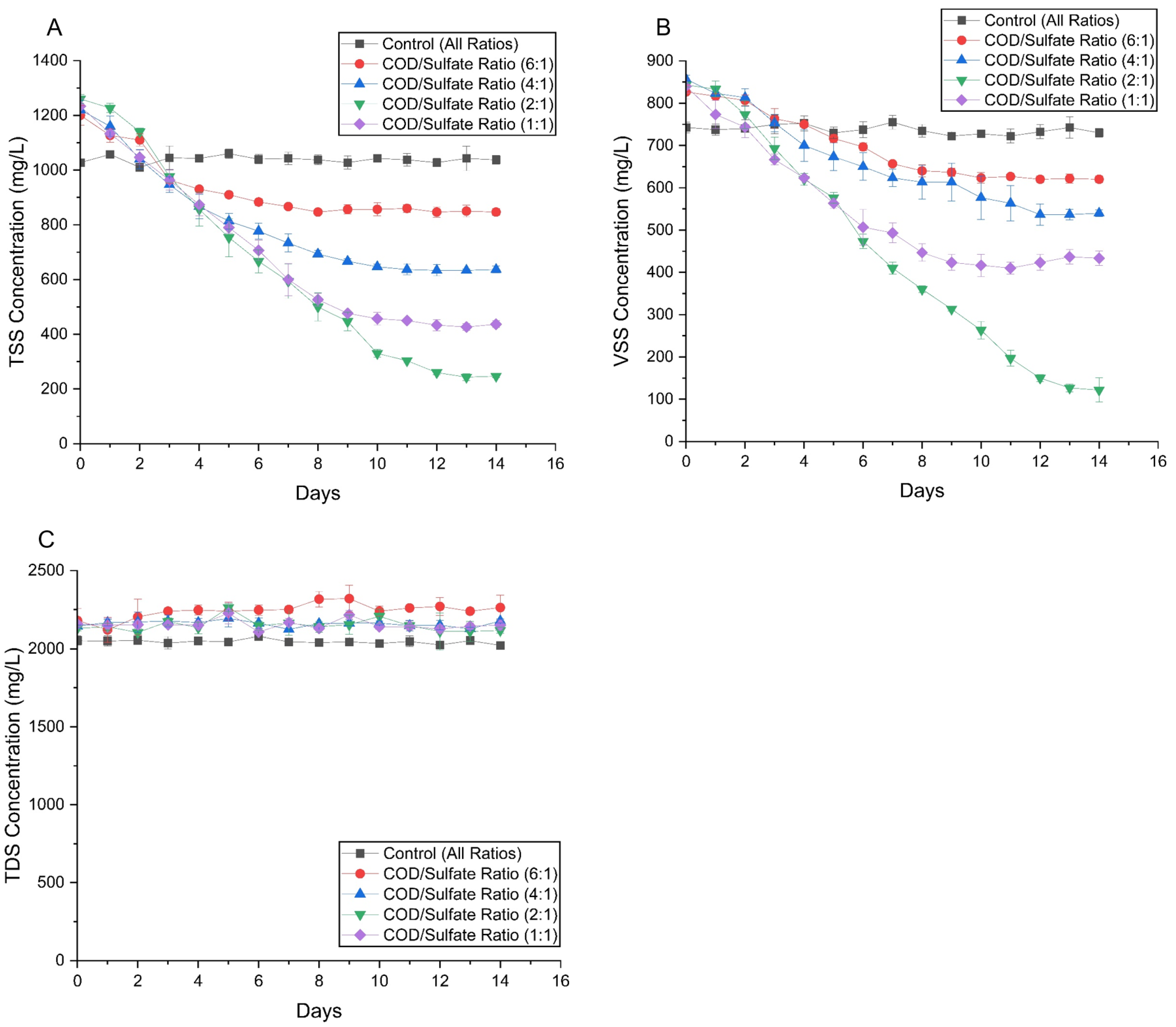
3.2.1. Solids Concentration (TSS, VSS, and TDS)
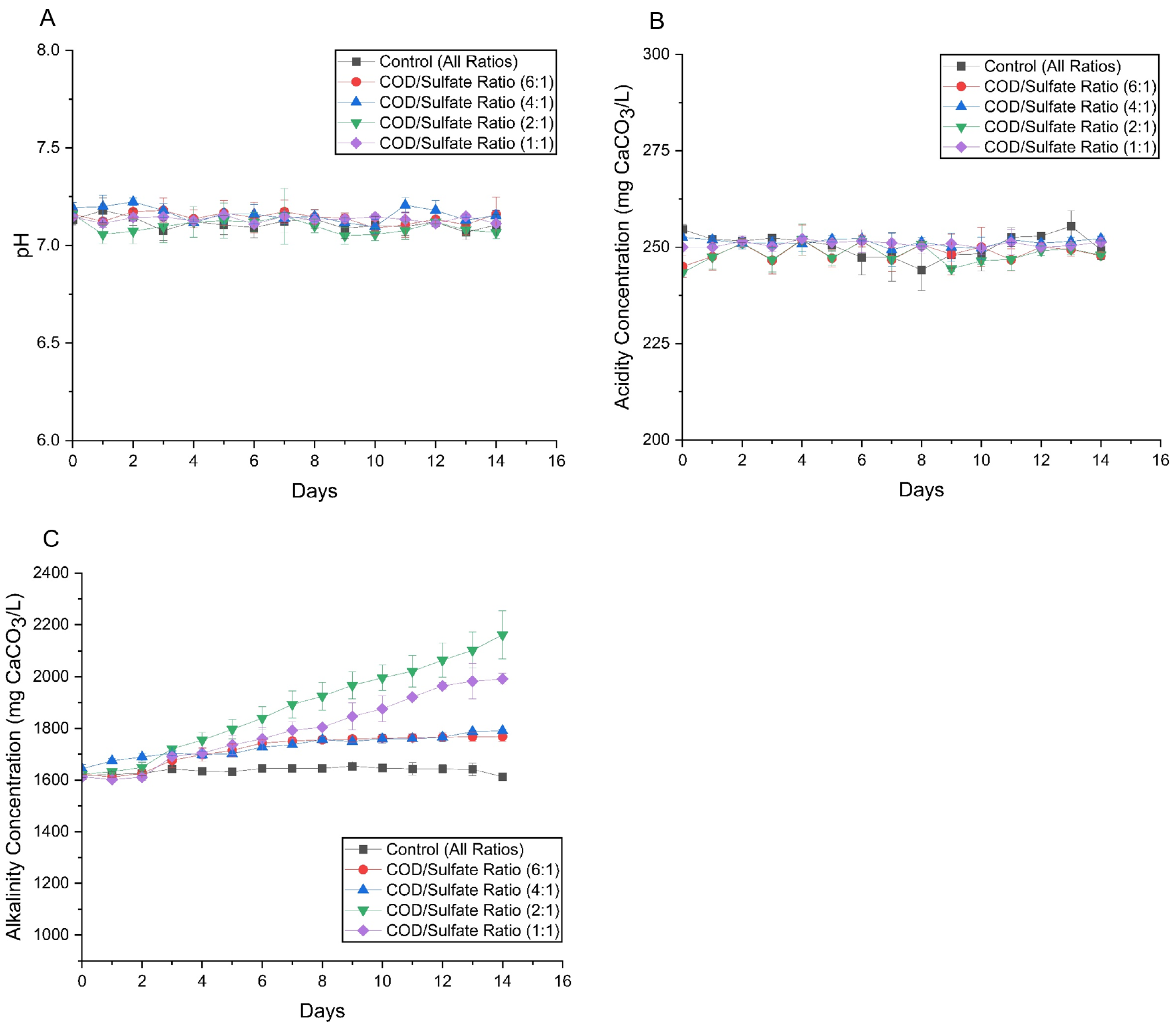
3.2.2. pH, Acidity, and Alkalinity
3.2.3. COD and Sulfate Removal
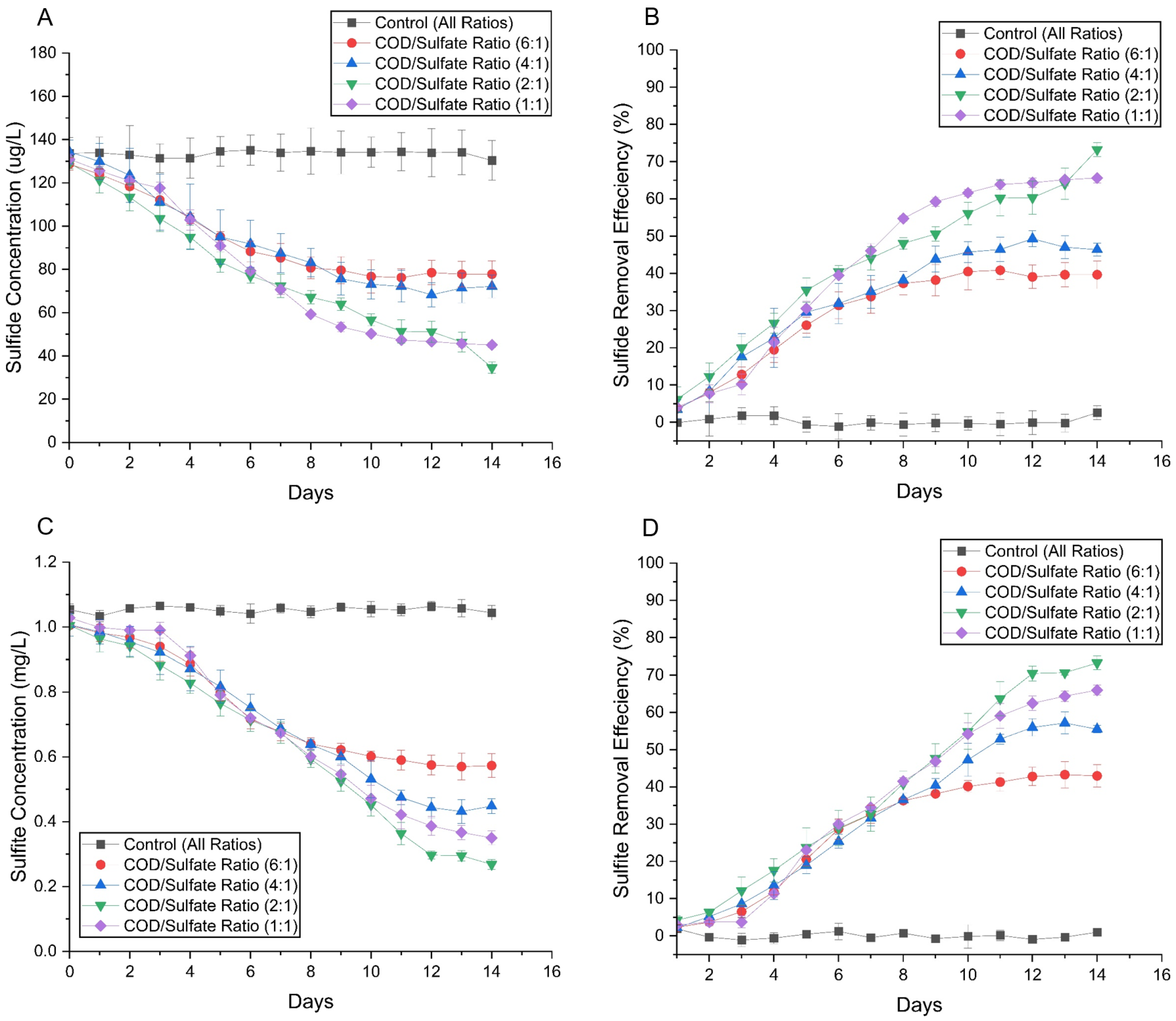
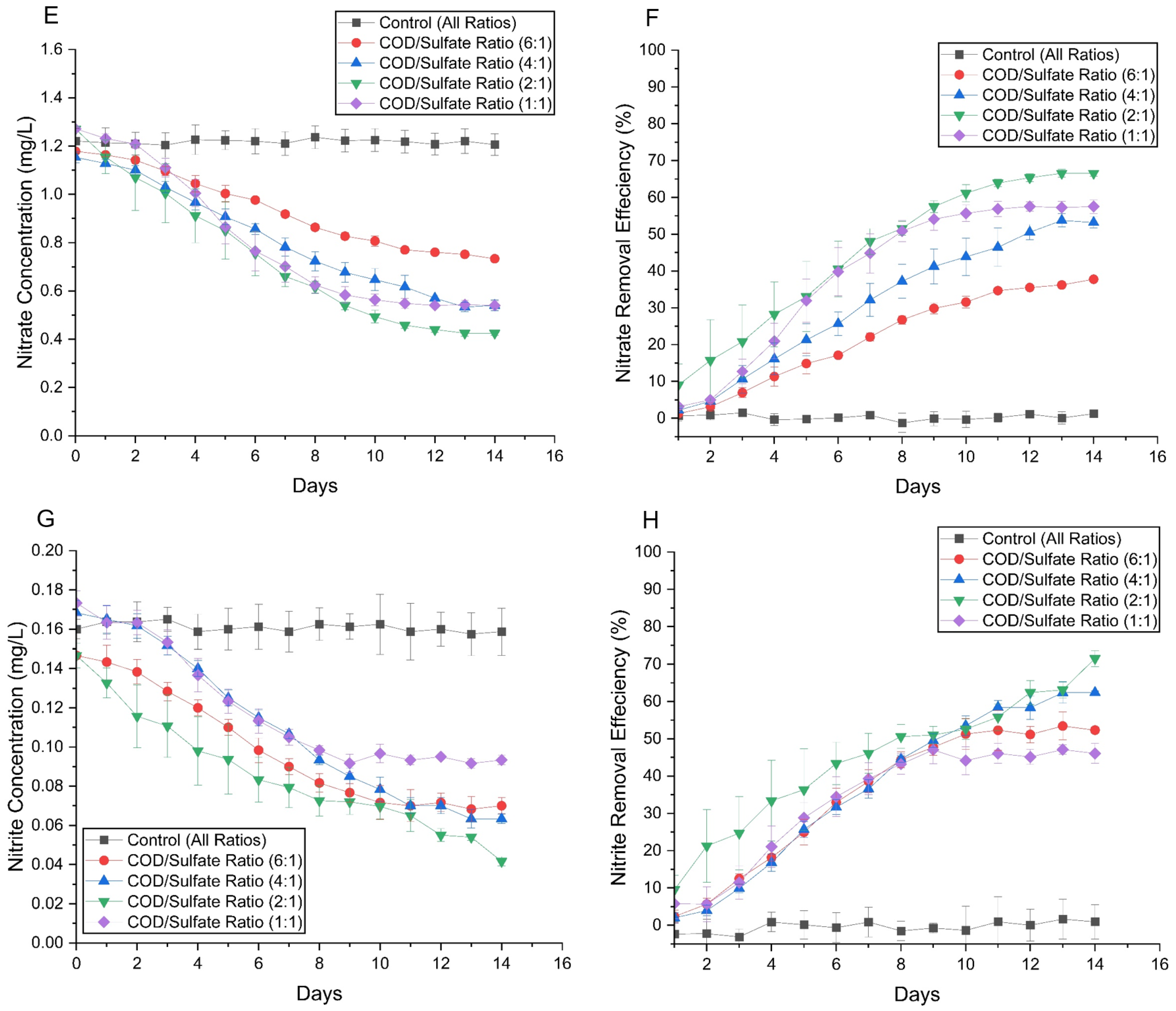
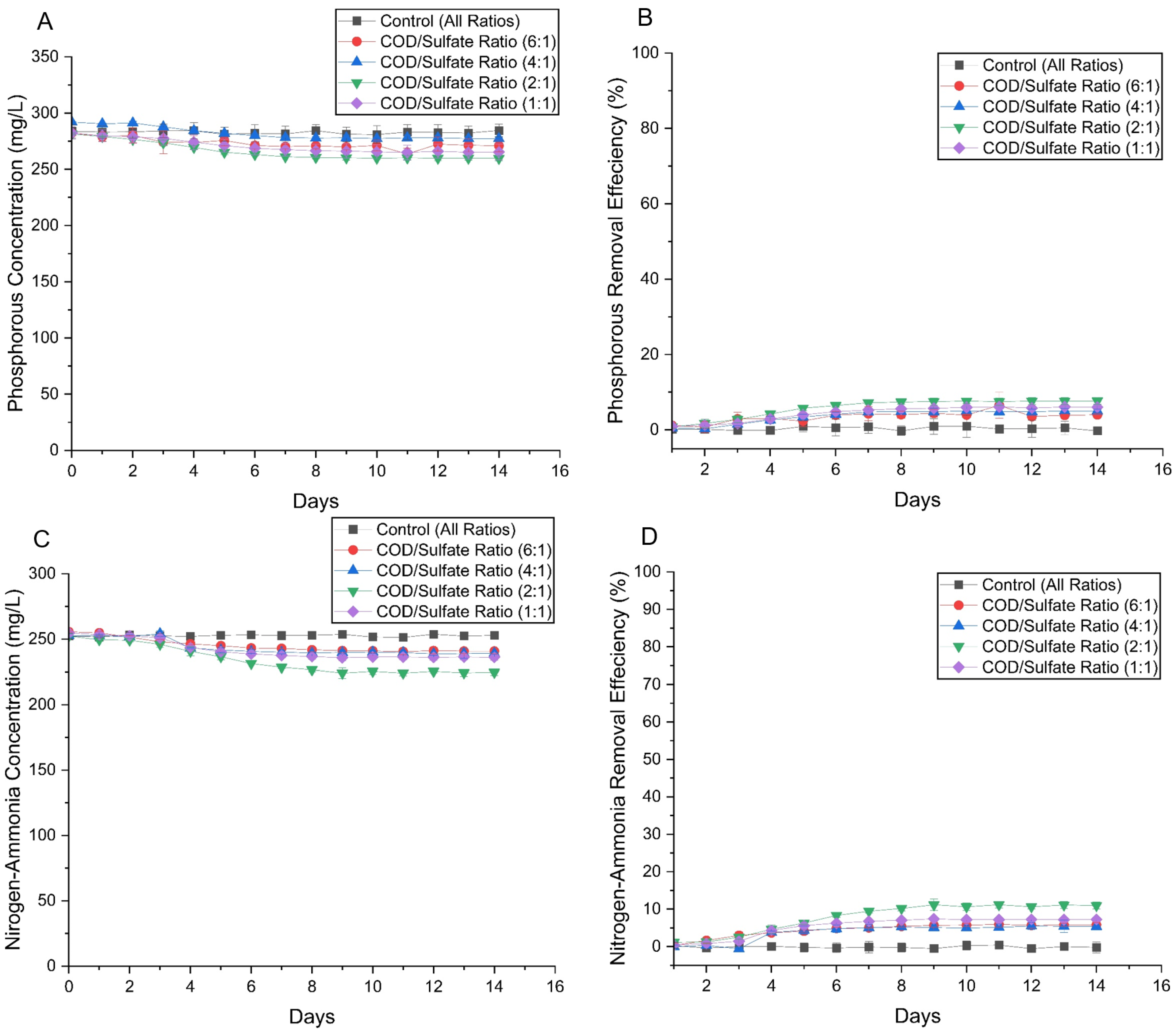
3.2.4. Nutrient Degradation and Reduced Sulfur Species
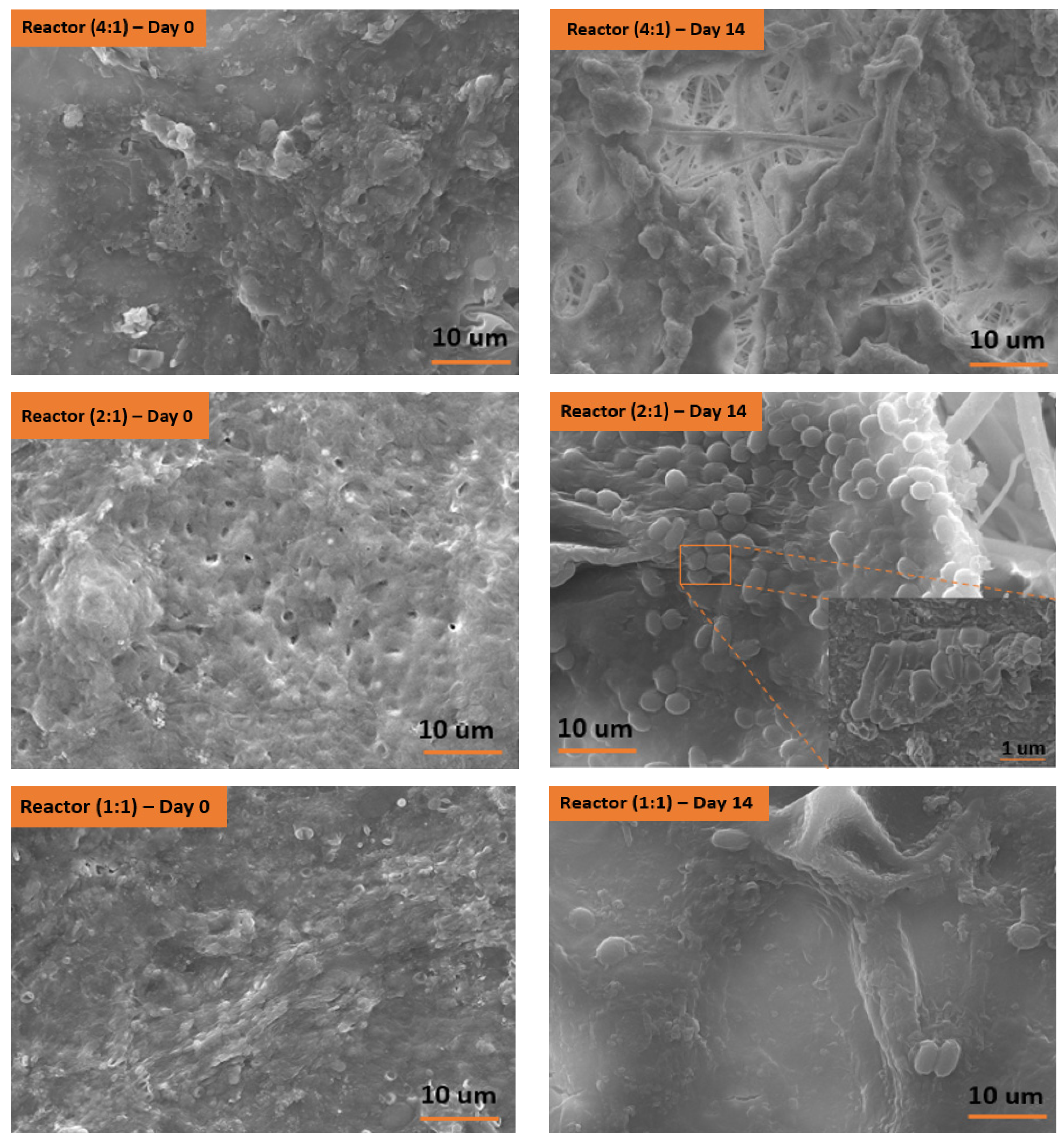
3.3. Solid Characterization by Scanning Electron Microscopy (SEM)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Venslauskas, K.; Navickas, K.; Rubežius, M.; Tilvikienė, V.; Supronienė, S.; Doyeni, M.O.; Barčauskaitė, K.; Bakšinskaitė, A.; Bunevičienė, K. Environmental impact assessment of sustainable pig farm via management of nutrient and co-product flows in the farm. Agronomy 2022, 12, 760. [Google Scholar] [CrossRef]
- Sharara, M.; Kim, D.; Sadaka, S.; Thoma, G. Consequential life cycle assessment of swine manure management within a thermal gasification scenario. Energies 2019, 12, 4081. [Google Scholar] [CrossRef]
- Deviney, A.; Classen, J.; Bruce, J.; Sharara, M. Sustainable swine manure management: A tale of two agreements. Sustainability 2020, 13, 15. [Google Scholar] [CrossRef]
- Burkholder, J.; Libra, B.; Weyer, P.; Heathcote, S.; Kolpin, D.; Thorne, P.S.; Wichman, M. Impacts of waste from concentrated animal feeding operations on water quality. Environ. Health Perspect. 2007, 115, 308–312. [Google Scholar] [CrossRef]
- Varma, V.S.; Parajuli, R.; Scott, E.; Canter, T.; Lim, T.T.; Popp, J.; Thoma, G. Dairy and swine manure management—Challenges and perspectives for sustainable treatment technology. Sci. Total Environ. 2021, 778, 146319. [Google Scholar] [CrossRef] [PubMed]
- Cole, D.; Todd, L.; Wing, S. Concentrated swine feeding operations and public health: A review of occupational and community health effects. Environ. Health Perspect. 2000, 108, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Rosa, D.M.; Sampaio, S.C.; Pereira, P.A.; Mauli, M.M.; Reis, R.R.D. Swine wastewater: Impacts on soil, plant, and leachate. Eng. Agrícola 2017, 37, 928–939. [Google Scholar] [CrossRef]
- Vymazal, J. Constructed wetlands for wastewater treatment: Five decades of experience. Environ. Sci. Technol. 2011, 45, 61–69. [Google Scholar] [CrossRef]
- Oturan, M.A.; Aaron, J.-J. Advanced oxidation processes in water/wastewater treatment: Principles and applications. A review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Judd, S. The MBR Book: Principles and Applications of Membrane Bioreactors for Water and Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Lourinho, G.; Rodrigues, L.; Brito, P. Recent advances on anaerobic digestion of swine wastewater. Int. J. Environ. Sci. Technol. 2020, 17, 4917–4938. [Google Scholar] [CrossRef]
- Hao, O.J.; Chen, J.M.; Huang, L.; Buglass, R.L. Sulfate-reducing bacteria. Crit. Rev. Environ. Sci. Technol. 1996, 26, 155–187. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, C.; Yang, Y.; Zhang, Z.; Tang, Y.; Su, P.; Lin, Z. A review of sulfate-reducing bacteria: Metabolism, influencing factors and application in wastewater treatment. J. Clean. Prod. 2022, 376, 134109. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Uberoi, V.; Dronamraju, M. Interaction between acetate fed sulfate reducers and methanogens. Water Res. 1996, 30, 2239–2246. [Google Scholar] [CrossRef]
- Lens, P.; Kuenen, J.G. The biological sulfur cycle: Novel opportunities for environmental biotechnology. Water Sci. Technol. 2001, 44, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Muyzer, G.; Stams, A.J. The ecology and biotechnology of sulphate-reducing bacteria. Nat. Rev. Microbiol. 2008, 6, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Zinder, S.H. Physiological ecology of methanogens. In Methanogenesis: Ecology, Physiology, Biochemistry & Genetics; Ferry, J.G., Ed.; Springer: Boston, MA, USA, 1993; pp. 128–206. [Google Scholar]
- Omil, F.; Lens, P.; Visser, A.; Pol, L.H.; Lettinga, G. Long-term competition between sulfate reducing and methanogenic bacteria in UASB reactors treating volatile fatty acids. Biotechnol. Bioeng. 1998, 57, 676–685. [Google Scholar] [CrossRef]
- Xu, Y.-N.; Chen, Y. Advances in heavy metal removal by sulfate-reducing bacteria. Water Sci. Technol. 2020, 81, 1797–1827. [Google Scholar] [CrossRef] [PubMed]
- Kushkevych, I. Isolation and purification of sulfate-reducing bacteria. In Microorganisms; Blumenberg, M., Shaaban, M., Elgaml, A., Eds.; IntechOpen: London, UK, 2020. [Google Scholar]
- Lens, P.; Visser, A.; Janssen, A.; Pol, L.H.; Lettinga, G. Biotechnological treatment of sulfate-rich wastewaters. Crit. Rev. Environ. Sci. Technol. 1998, 28, 41–88. [Google Scholar] [CrossRef]
- Rabus, R.; Hansen, T.A.; Widdel, F. Dissimilatory sulfate-and sulfur-reducing prokaryotes. Prokaryotes 2006, 2, 659–768. [Google Scholar]
- Li, J.; Li, A.; Li, Y.; Cai, M.; Luo, G.; Wu, Y.; Tian, Y.; Xing, L.; Zhang, Q. PICRUSt2 functionally predicts organic compounds degradation and sulfate reduction pathways in an acidogenic bioreactor. Front. Environ. Sci. Eng. 2022, 16, 47. [Google Scholar] [CrossRef]
- Damianovic, M.H.R.Z.; Foresti, E. Anaerobic degradation of synthetic wastewaters at different levels of sulfate and COD/sulfate ratios in horizontal-flow anaerobic reactors (HAIB). Environ. Eng. Sci. 2007, 24, 383–393. [Google Scholar] [CrossRef]
- Barbosa, L.d.P.; Bertolino, S.; Freitas, P.; Oliveira, V.; Pina, P.D.; Leão, V.; Teixeira, M. Effects of different COD/sulfate ratios on the growth of metal tolerant sulfate reducing bacteria (SRB). Adv. Mater. Res. 2009, 71, 569–572. [Google Scholar] [CrossRef]
- Kushkevych, I.; Dordević, D.; Vítězová, M. Toxicity of hydrogen sulfide toward sulfate-reducing bacteria Desulfovibrio piger Vib-7. Arch. Microbiol. 2019, 201, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Lin, L.-S. Continuous sulfidogenic wastewater treatment with iron sulfide sludge oxidation and recycle. Water Res. 2017, 114, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Villamar, C.A.; Cañuta, T.; Belmonte, M.; Vidal, G. Characterization of swine wastewater by toxicity identification evaluation methodology (TIE). Water Air Soil Pollut. 2012, 223, 363–369. [Google Scholar] [CrossRef]
- Deng, D.; Weidhaas, J.L.; Lin, L.-S. Kinetics and microbial ecology of batch sulfidogenic bioreactors for co-treatment of municipal wastewater and acid mine drainage. J. Hazard. Mater. 2016, 305, 200–208. [Google Scholar] [CrossRef]
- Deng, D.; Lin, L.-S. Two-stage combined treatment of acid mine drainage and municipal wastewater. Water Sci. Technol. 2013, 67, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Barton, L.L.; Hamilton, W.A. Sulphate-Reducing Bacteria: Environmental and Engineered Systems; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Hu, B.; Wheatley, A.; Ishtchenko, V.; Huddersman, K. Performance linked to residence time distribution by a novel wool-based bioreactor for tertiary sewage treatment. Appl. Microbiol. Biotechnol. 2012, 94, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Cordone, L.; Carlson, C.; Plaehn, W.; Shangraw, T.; Wilmoth, D. Case Study and Retrospective: Aerobic Fixed Film Biological Treatment Process for 1, 4-Dioxane at the Lowry Landfill Superfund Site. Remediat. J. 2016, 27, 159–172. [Google Scholar] [CrossRef]
- Rice, E.W.; Bridgewater, L.; Association, A.P.H. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012; Volume 10. [Google Scholar]
- ASTM D1252; Standard Test Methods for Chemical Oxygen Demand (Dichromate Oxygen Demand) of Water. ASTM International: West Conshohocken, PA, USA, 2012.
- Mohammed, A.; Abdullah, A. Scanning electron microscopy (SEM): A review. In Proceedings of the 2018 International Conference on Hydraulics and Pneumatics—HERVEX, Băile Govora, Romania, 7–9 November 2018; pp. 7–9. [Google Scholar]
- Omil, F.; Lens, P.; Pol, L.H.; Lettinga, G. Effect of upward velocity and sulphide concentration on volatile fatty acid degradation in a sulphidogenic granular sludge reactor. Process Biochem. 1996, 31, 699–710. [Google Scholar] [CrossRef]
- Rinzema, A.; Boone, M.; van Knippenberg, K.; Lettinga, G. Bactericidal effect of long chain fatty acids in anaerobic digestion. Water Environ. Res. 1994, 66, 40–49. [Google Scholar] [CrossRef]
- Ahmed, M.; Lin, L.-S. Ferric reduction in organic matter oxidation and its applicability for anaerobic wastewater treatment: A review and future aspects. Rev. Environ. Sci. BioTechnol. 2017, 16, 273–287. [Google Scholar] [CrossRef]
- Shrestha, S.; Pandey, R.; Aryal, N.; Lohani, S.P. Recent advances in co-digestion conjugates for anaerobic digestion of food waste. J. Environ. Manag. 2023, 345, 118785. [Google Scholar] [CrossRef] [PubMed]
- Gerardi, M.H. The Microbiology of Anaerobic Digesters; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Postgate, J.R. The Sulphate-Reducing Bacteria; CUP Archive: Cambridge, UK, 1979. [Google Scholar]
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Bella, K.; Rao, P.V. Anaerobic digestion of dairy wastewater: Effect of different parameters and co-digestion options—A review. Biomass Convers. Biorefinery 2023, 13, 2527–2552. [Google Scholar] [CrossRef]
- Diao, C.; Ye, W.; Yan, J.; Hao, T.; Huang, L.; Chen, Y.; Long, J.; Xiao, T.; Zhang, H. Application of microbial sulfate-reduction process for sulfate-laden wastewater treatment: A review. J. Water Process Eng. 2023, 52, 103537. [Google Scholar] [CrossRef]
- Lens, P.; Pol, L.H. Environmental Technologies to Treat Sulfur Pollution; IWA publishing: London, UK, 2000. [Google Scholar]
- Stefanie, J.O.E.; Visser, A.; Pol, L.W.H.; Stams, A.J. Sulfate reduction in methanogenic bioreactors. FEMS Microbiol. Rev. 1994, 15, 119–136. [Google Scholar]
- O’Flaherty, V.; Mahony, T.; O’Kennedy, R.; Colleran, E. Effect of pH on growth kinetics and sulphide toxicity thresholds of a range of methanogenic, syntrophic and sulphate-reducing bacteria. Process Biochem. 1998, 33, 555–569. [Google Scholar] [CrossRef]
- Spanjers, H.; Weijma, J.; Abusam, A. Modelling the competition between sulphate reducers and methanogens in a thermophilic methanol-fed bioreactor. Water Sci. Technol. 2002, 45, 93–98. [Google Scholar] [CrossRef]
- Reis, M.; Almeida, J.; Lemos, P.; Carrondo, M. Effect of hydrogen sulfide on growth of sulfate reducing bacteria. Biotechnol. Bioeng. 1992, 40, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Poh, P.; Gouwanda, D.; Mohan, Y.; Gopalai, A.; Tan, H. Optimization of wastewater anaerobic digestion using mechanistic and meta-heuristic methods: Current limitations and future opportunities. Water Conserv. Sci. Eng. 2016, 1, 1–20. [Google Scholar] [CrossRef]
- Zieliński, M.; Kazimierowicz, J.; Dębowski, M. Advantages and limitations of anaerobic wastewater treatment—Technological basics, development directions, and technological innovations. Energies 2022, 16, 83. [Google Scholar] [CrossRef]
- Rahimi, S.; Modin, O.; Mijakovic, I. Technologies for biological removal and recovery of nitrogen from wastewater. Biotechnol. Adv. 2020, 43, 107570. [Google Scholar] [CrossRef]
- Di Costanzo, N.; Cesaro, A.; Di Capua, F.; Esposito, G. Exploiting the nutrient potential of anaerobically digested sewage sludge: A review. Energies 2021, 14, 8149. [Google Scholar] [CrossRef]
- Mao, Y.; Xiong, R.; Gao, X.; Jiang, L.; Peng, Y.; Xue, Y. Analysis of the status and improvement of microalgal phosphorus removal from municipal wastewater. Processes 2021, 9, 1486. [Google Scholar] [CrossRef]
- Dar, S.A.; Kleerebezem, R.; Stams, A.J.; Kuenen, J.G.; Muyzer, G. Competition and coexistence of sulfate-reducing bacteria, acetogens and methanogens in a lab-scale anaerobic bioreactor as affected by changing substrate to sulfate ratio. Appl. Microbiol. Biotechnol. 2008, 78, 1045–1055. [Google Scholar] [CrossRef]
- Saia, F.T.; Souza, T.S.; Duarte, R.T.D.; Pozzi, E.; Fonseca, D.; Foresti, E. Microbial community in a pilot-scale bioreactor promoting anaerobic digestion and sulfur-driven denitrification for domestic sewage treatment. Bioprocess Biosyst. Eng. 2016, 39, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Harirchi, S.; Wainaina, S.; Sar, T.; Nojoumi, S.A.; Parchami, M.; Parchami, M.; Varjani, S.; Khanal, S.K.; Wong, J.; Awasthi, M.K. Microbiological insights into anaerobic digestion for biogas, hydrogen or volatile fatty acids (VFAs): A review. Bioengineered 2022, 13, 6521–6557. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.H. Biofilm dynamics and kinetics during high-rate sulfate reduction under anaerobic conditions. Appl. Environ. Microbiol. 1987, 53, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Gagliano, M.; Ismail, S.B.; Stams, A.J.; Plugge, C.M.; Temmink, H.; Van Lier, J. Biofilm formation and granule properties in anaerobic digestion at high salinity. Water Res. 2017, 121, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Cayetano, R.D.A.; Kim, G.-B.; Park, J.; Yang, Y.-H.; Jeon, B.-H.; Jang, M.; Kim, S.-H. Biofilm formation as a method of improved treatment during anaerobic digestion of organic matter for biogas recovery. Bioresour. Technol. 2022, 344, 126309. [Google Scholar] [CrossRef] [PubMed]
- Sarti, A.; Pozzi, E.; Chinalia, F.A.; Ono, A.; Foresti, E. Microbial processes and bacterial populations associated to anaerobic treatment of sulfate-rich wastewater. Process Biochem. 2010, 45, 164–170. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Nguyen, A.Q.; Nghiem, L.D. Microbial community in anaerobic digestion system: Progression in microbial ecology. Water Wastewater Treat. Technol. 2019, 331–355. [Google Scholar]
- Nakasaki, K.; Nguyen, K.K.; Ballesteros Jr, F.C.; Maekawa, T.; Koyama, M. Characterizing the microbial community involved in anaerobic digestion of lipid-rich wastewater to produce methane gas. Anaerobe 2020, 61, 102082. [Google Scholar] [CrossRef] [PubMed]
- El Houari, A.; Ranchou-Peyruse, M.; Ranchou-Peyruse, A.; Bennisse, R.; Bouterfas, R.; Goni Urriza, M.S.; Qatibi, A.-I.; Guyoneaud, R. Microbial Communities and Sulfate-Reducing Microorganisms Abundance and Diversity in Municipal Anaerobic Sewage Sludge Digesters from a Wastewater Treatment Plant (Marrakech, Morocco). Processes 2020, 8, 1284. [Google Scholar] [CrossRef]

| Parameter | Average ± SD |
|---|---|
| pH | 7.1 ± 0.04 |
| Alkalinity (mg/L) | 1620.3 ± 9.4 |
| Acidity (mg/L) | 245 ± 6.2 |
| Nitrite NO2−–N (mg/L) | 0.14 ± 0.005 |
| Nitrate NO3−–N (mg/L) | 1.3 ± 0.10 |
| Phosphorus PO43− (mg/L) | 284.6 ± 7.3 |
| Nitrogen, Ammonia NH3–N (mg/L) | 255.4 ± 1.5 |
| Sulfide S2− (μg/L) | 137 ± 2.7 |
| Sulfite SO32− (mg/L) | 1.0 ± 0.004 |
| Sulfate SO42− (mg/L) | 133.1 ± 1.9 |
| COD (mg/L) | 953.9 ± 0.7 |
| TSS (mg/L) | 1195 ± 8.5 |
| TDS (mg/L) | 2011.8 ± 5.1 |
| VSS (mg/L) | 804.5 ± 3.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamssali, M.; Mantripragada, S.; Deng, D.; Zhang, L. Enhancing Swine Wastewater Treatment: A Sustainable and Systematic Approach through Optimized Chemical Oxygen Demand/Sulfate Mass Ratio in Attached-Growth Anaerobic Bioreactor. Environments 2024, 11, 162. https://doi.org/10.3390/environments11080162
Lamssali M, Mantripragada S, Deng D, Zhang L. Enhancing Swine Wastewater Treatment: A Sustainable and Systematic Approach through Optimized Chemical Oxygen Demand/Sulfate Mass Ratio in Attached-Growth Anaerobic Bioreactor. Environments. 2024; 11(8):162. https://doi.org/10.3390/environments11080162
Chicago/Turabian StyleLamssali, Mehdi, Shobha Mantripragada, Dongyang Deng, and Lifeng Zhang. 2024. "Enhancing Swine Wastewater Treatment: A Sustainable and Systematic Approach through Optimized Chemical Oxygen Demand/Sulfate Mass Ratio in Attached-Growth Anaerobic Bioreactor" Environments 11, no. 8: 162. https://doi.org/10.3390/environments11080162
APA StyleLamssali, M., Mantripragada, S., Deng, D., & Zhang, L. (2024). Enhancing Swine Wastewater Treatment: A Sustainable and Systematic Approach through Optimized Chemical Oxygen Demand/Sulfate Mass Ratio in Attached-Growth Anaerobic Bioreactor. Environments, 11(8), 162. https://doi.org/10.3390/environments11080162










