Macrophytes as Key Element to Determine Ecological Quality Changes in Transitional Water Systems: The Venice Lagoon as Study Case
Abstract
1. Introduction
2. Materials and Methods
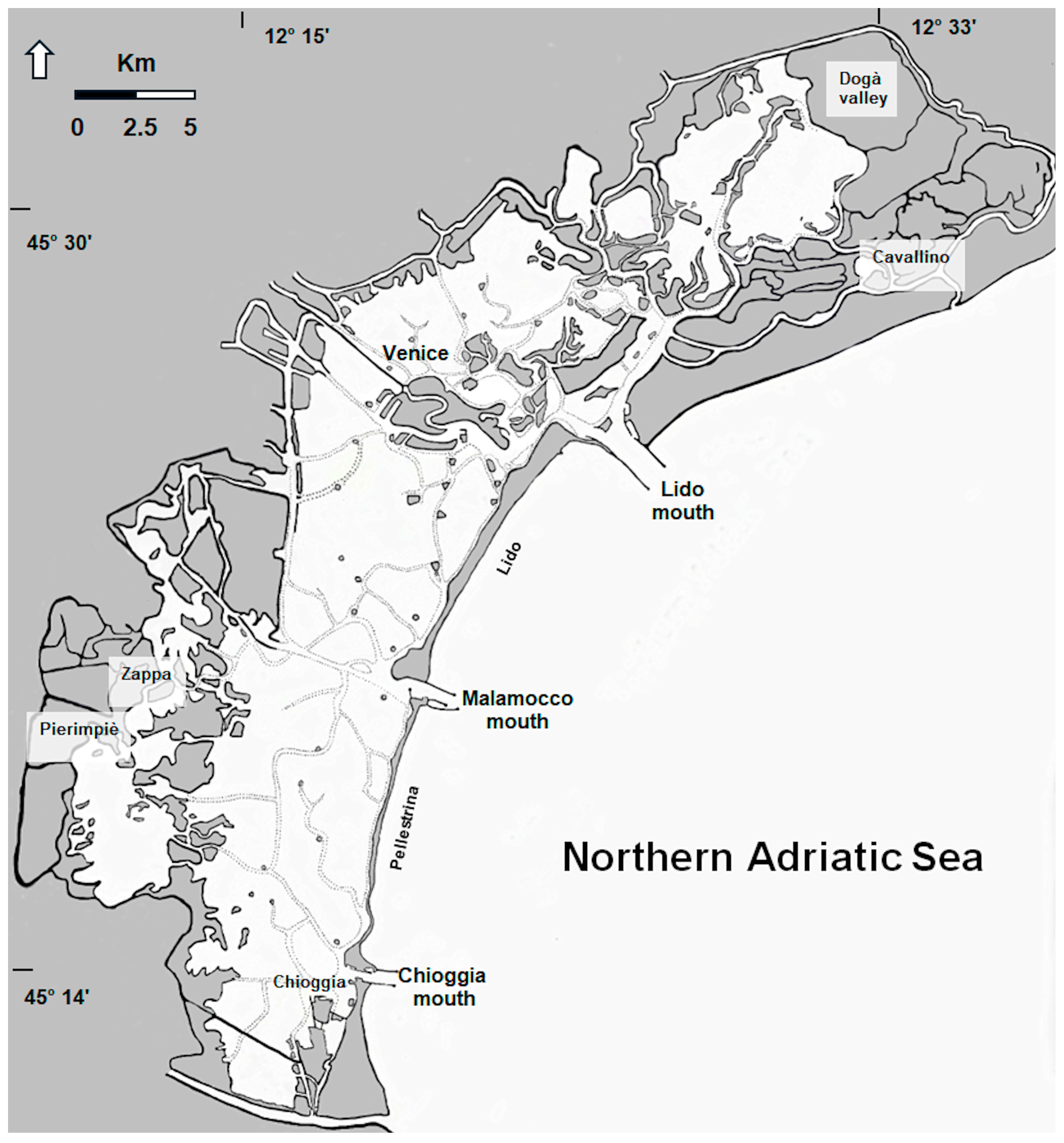
2.1. The Study Area
2.2. Macrophyte Sampling
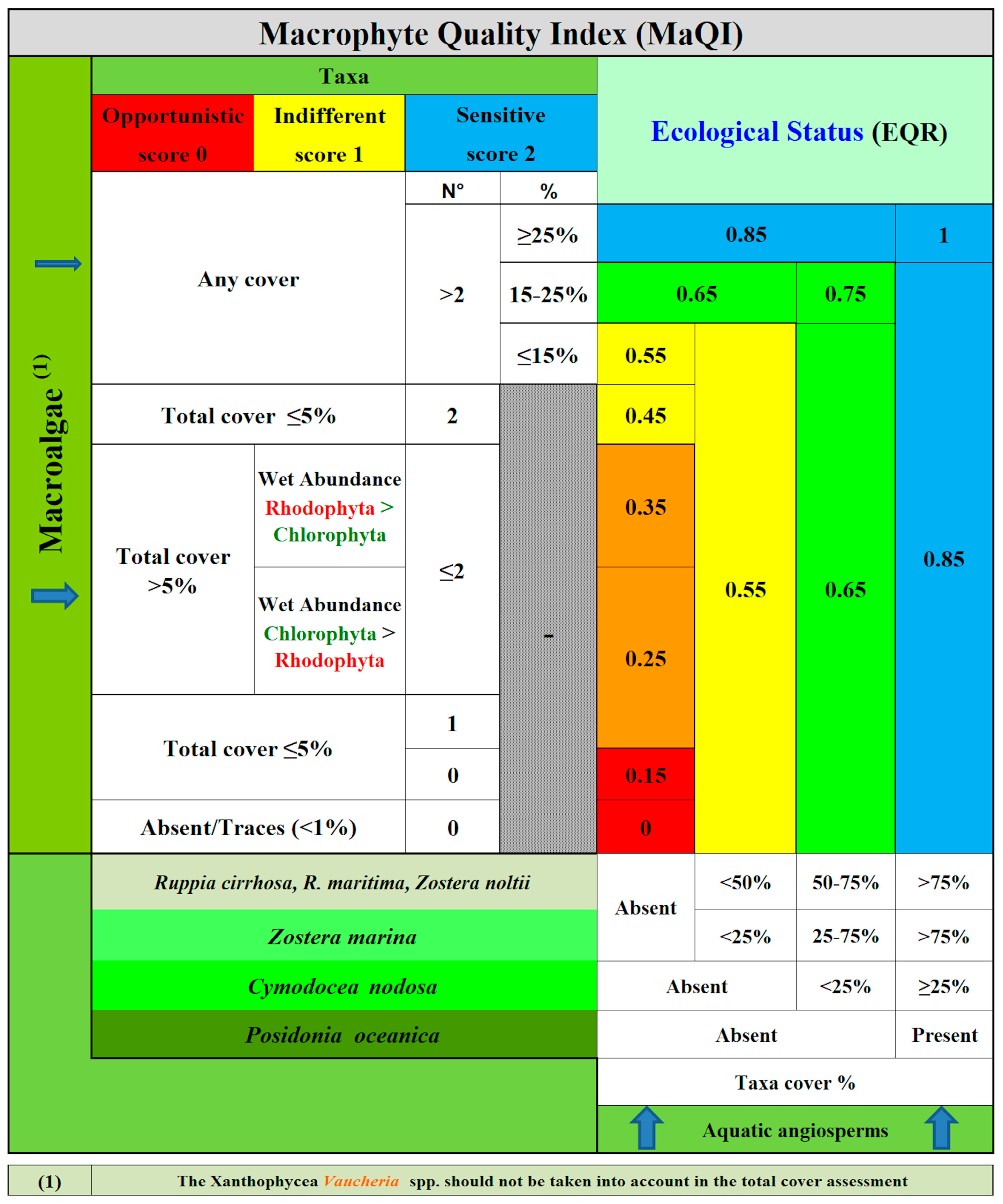
2.3. Macrophyte Quality Index (MaQI)
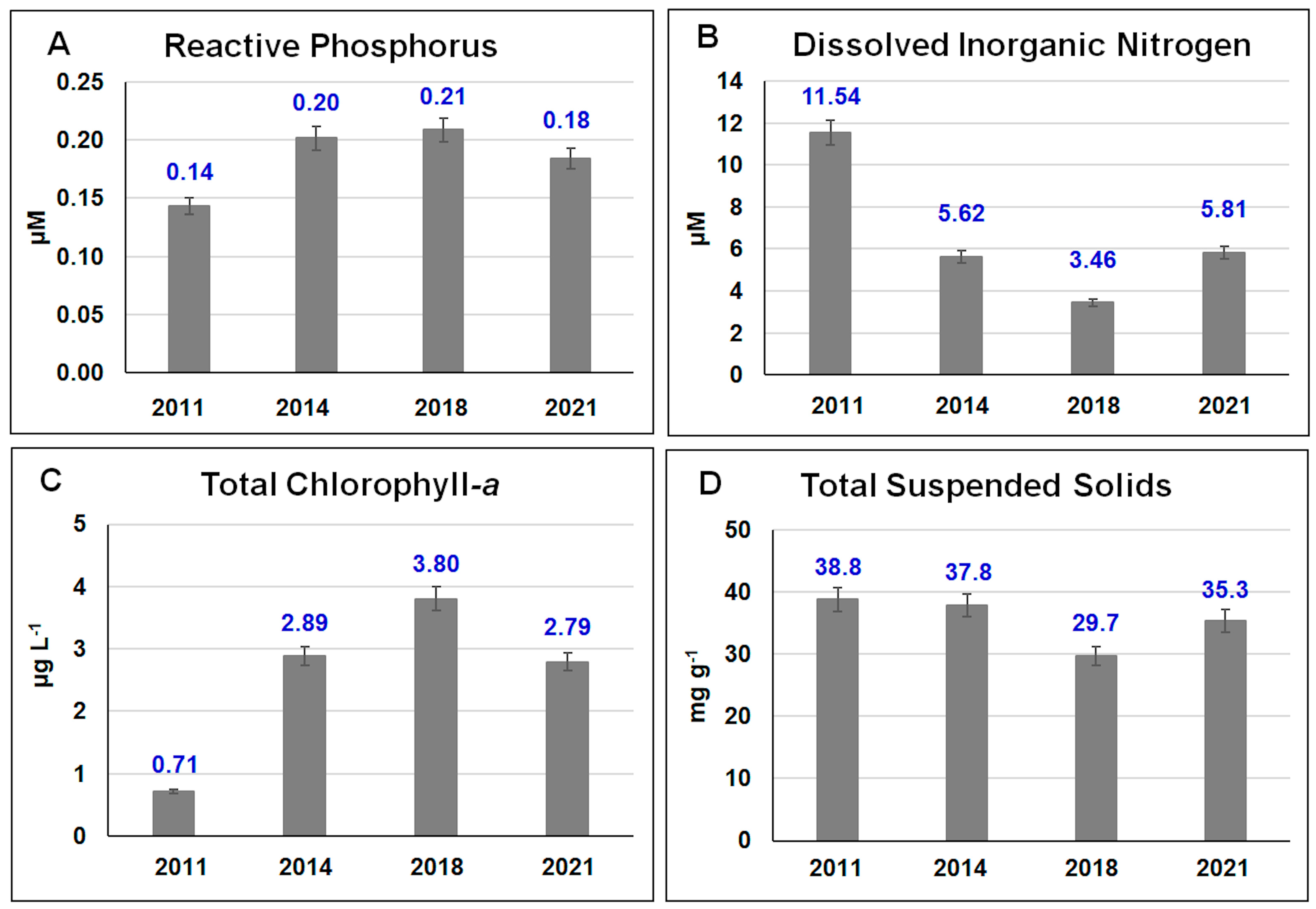
2.4. Environmental Parameters
2.5. Statistical Analysis Methods
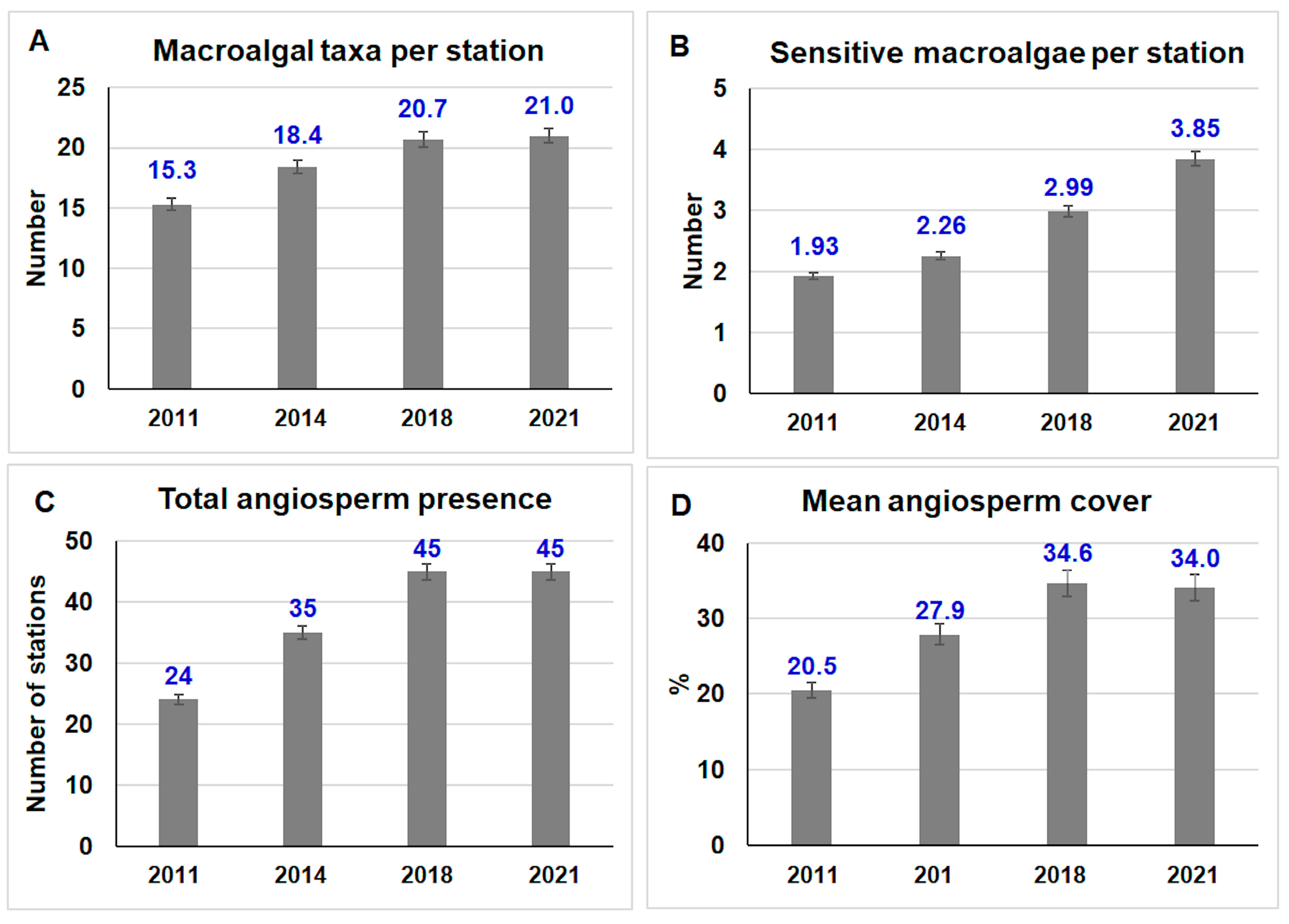
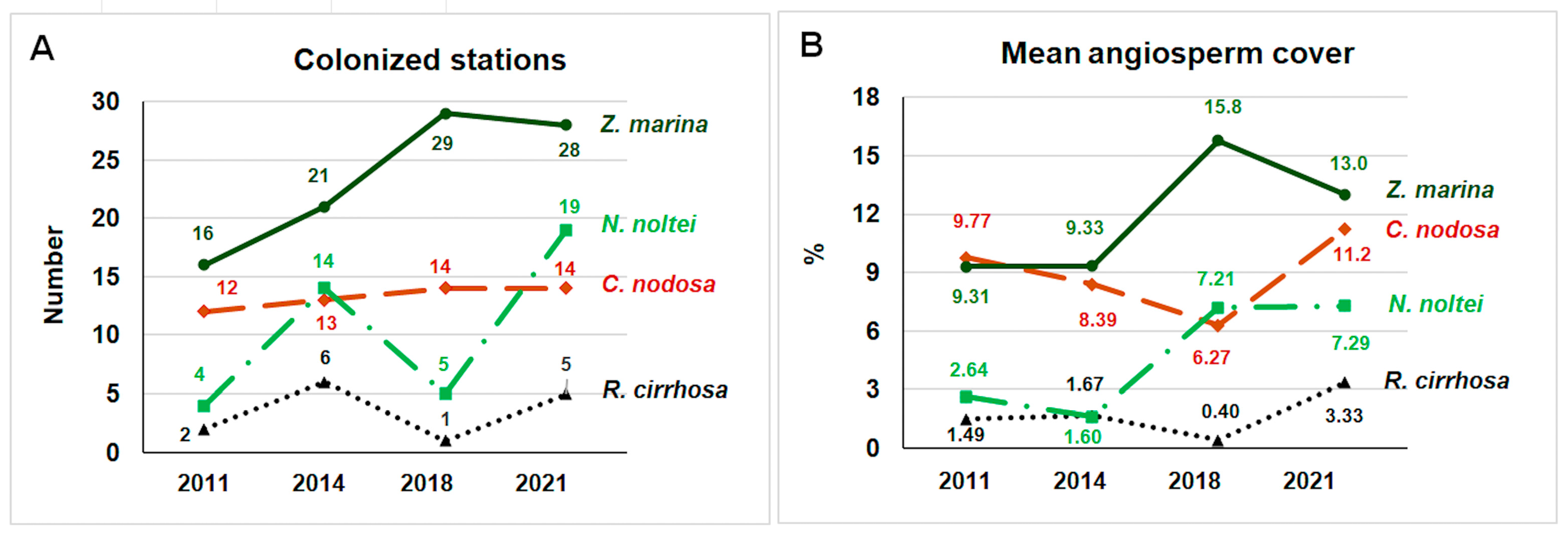
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Council Directive 92/43/EEC of 21 May 1992 on the Conservation of Natural Habitats and of Wild Fauna and Flora. Document 01992L0043-20130701. Available online: http://data.europa.eu/eli/dir/1992/43/2013-07-01 (accessed on 25 August 2024).
- European Parliament and Council Water Framework Directive, 2000/60/EC. of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy. Document 32000L0060. Available online: http://data.europa.eu/eli/dir/2000/60/oj (accessed on 25 August 2024).
- Borja, Á. The European water framework directive: A challenge for nearshore, coastal and continental shelf research. Cont. Shelf Res. 2005, 25, 1768–1783. [Google Scholar] [CrossRef]
- Borja, Á.; Franco, J.; Valencia, V.; Bald, J.; Muxika, I.; Belzunce, M.J.; Solaun, O. Implementation of the European water framework directive from the Basque country (northern Spain): A methodological approach. Mar. Poll. Bull. 2004, 48, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Borja, Á.; Josefson, A.B.; Miles, A.; Muxika, I.; Olsgard, F.; Phillips, G.; Rodrıguez, J.G.; Rygg, B. An approach to the intercalibration of benthic ecological status assessment in the North Atlantic ecoregion, according to the European Water Framework Directive. Mar. Poll. Bull. 2007, 55, 42–52. [Google Scholar] [CrossRef]
- Derolez, V.; Oheix, J.; Gimard, A.; Laugier, T. Indicateur Macrophytes Exclame Pour les Lagunes Poly-et eu-halines (EXamination Tool for Coastal LAgoon Macrophyte Ecological Status). IFREMER, Agence Frąncaise pour la Biodiversité. Ministère de l’Environnement. 2006. Available online: https://envlit.ifremer.fr/content/download/122599/file/MED-MET-Macrophytes.pdf (accessed on 24 June 2024).
- Orfanidis, S.; Panayotidis, P.; Ugland, K.I. Ecological Evaluation Index continuous formula (EEI-c) application: A step forward for functional groups, the formula and reference condition values. Mediterr. Mar. Sci. 2011, 12, 199–231. [Google Scholar] [CrossRef]
- Sfriso, A.; Facca, C.; Bonometto, A.; Boscolo, R. Compliance of the Macrophyte Quality index (MaQI) with the WFD (2000/60/EC) and ecological status assessment in transitional areas: The Venice lagoon as study case. Ecol. Indic. 2014, 46, 536–547. [Google Scholar] [CrossRef]
- Regione del Veneto. Monitoring Plan for the Venice Lagoon Pursuant to Directive 2000/60/EC Aimed at Defining the Ecological Status (Legislative Decree N. 152/2006 s.m.i). Line 2: EQB: Macrophytes, Mo.V.Eco. I. Final Report, 2012; pp. 23 + Tables and Figures. Available online: https://bur.regione.veneto.it/BurvServices/Pubblica/DettaglioDecreto.aspx?id=294424&highlight=true (accessed on 25 June 2024).
- Regione del Veneto. Monitoring Plan for the Venice Lagoon Pursuant to Directive 2000/60/EC Aimed at Defining the Ecological Status (Legislative Decree N. 152/2006 s.m.i). EQB: Macrophytes, Mo.V.Eco. II. Final Report, 2015; pp. 27 + Tables and Figures. Available online: https://bur.regione.veneto.it/BurvServices/Pubblica/DettaglioDecreto.aspx?id=379241&highlight=true (accessed on 17 September 2024).
- Regione del Veneto. Monitoring Plan for the Venice Lagoon Pursuant to Directive 2000/60/EC Aimed at Defining the Ecological Status (Legislative Decree N. 152/2006 s.m.i). EQB: Macrophytes, Mo.V.Eco. III. Final Report, 2019; pp. 26 + Tables and Figures. Available online: https://bur.regione.veneto.it/BurvServices/Pubblica/DettaglioDecreto.aspx?id=367174 (accessed on 17 September 2024).
- Regione del Veneto. Monitoring Plan for the Venice Lagoon Pursuant to Directive 2000/60/EC Aimed at Defining the Ecological Status (Legislative Decree N. 152/2006 s.m.i). EQB: Macrophytes- Mo.V.Eco. IV, Final Report, 2022; pp. 19 + Tables and Figures. Available online: https://bur.regione.veneto.it/BurvServices/Pubblica/DettaglioDecreto.aspx?id=386914 (accessed on 17 September 2024).
- Sfriso, A.; Buosi, A.; Tomio, Y.; Juhmani, A.-S.; Facca, C.; Wolf, M.; Sfriso, A.A.; Franzoi, P.; Scapin, L.; Bonometto, A.; et al. Environmental restoration by aquatic angiosperm transplants in transitional water systems: The Venice Lagoon as a case study. Sci. Total Environ. 2021, 795, 148859. [Google Scholar] [CrossRef]
- Sfriso, A.; Buosi, A.; Facca, C.; Sfriso, A.A. Role of environmental factors in affecting macrophyte dominance in transitional environments: The Italian Lagoons as a study case. Mar. Ecol. 2017, 38, e12414. [Google Scholar] [CrossRef]
- Short, F. World Atlas of Seagrasses; University of California Press: Berkeley, CA, USA, 2003; p. 332. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, University of Galway. Available online: https://www.algaebase.org (accessed on 16 August 2024).
- ISPRA (Istituto Superiore per la Protezione e la Ricerca Ambientale). Implementazione della Direttiva 2000/60/EC. Linee Guida per L’applicazione del Macrophyte Quality Index (MaQI); TW—Sistema di Classificazione Ecologica—Macrofite; ISPRA: Roma, Italy, 2014; p. 30. [Google Scholar]
- Mellors, J.E. An evaluation of a rapid visual technique for estimating seagrass biomass. Aquat. Bot. 1991, 42, 67–73. [Google Scholar]
- Hebert, P.D.; Cywinska, A.; Ball, S.L.; deWaard, J.R. Biological identifications through DNA barcodes. Proc. Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef]
- Strickland, J.D.H.; Parsons, T.R. A Practical Handbook of Seawater Analyses, 2nd ed.; Bulletin of Fishery Research Board of Canada: Ottawa, Canada, 1984; pp. 1–310. [Google Scholar]
- Lorenzen, C.J. Determination of Chlorophyll and Phaeopigments: Spectrophotometric Equations. Limnol. Oceanogr. 1967, 12, 343–346. [Google Scholar] [CrossRef]
- Chapman, A.R.O.; Craigie, J.S. Seasonal growth in Laminaria longicruris: Relations with dissolved inorganic nutrients and internal reserves of nitrogen. Mar. Biol. 1977, 40, 197–205. [Google Scholar] [CrossRef]
- Bellucci, L.G.; Frignani, M.; Paolucci, D.; Ravanelli, M. Distribution of heavy metals in sediments of the Venice Lagoon: The role of the industrial area. Mar. Pollut. Bull. 2002, 40, 65–76. [Google Scholar] [CrossRef]
- Bernardello, V.; Bonometto, A.; Boscolo Brusà, R.; Cacciatore, F.; Oselladore, F.; Ponis, E. Transitional Waters—Biological Quality Element Macrophytes MaQI-TW (Acque di Transizione-Elemento Qualità Biologica Macrofite MaQI-TW. ISPRA). On line Data Sheet Update 2023. Available online: https://indicatoriambientali.isprambiente.it/it/acque-marino-costiere-e-transizione/acque-di-transizione-elemento-di-qualita-biologica-macrofite-maqi-tw (accessed on 4 September 2024).
- Cecere, E.; Petrocelli, A.; Izzo, G.; Sfriso, A. Flora and Vegetation of the Italian Transitional Water Systems; CoRiLa, Multigraf: Spinea, Italy, 2009; p. 278. [Google Scholar]
- Sfriso, A.; Buosi, A.; Wolf, M.A.; Sciuto, K.; Molinaroli, E.; Mistri, M.; Munari, C.; Moro, I.; Sfriso, A.A. Microcalcareus seaweeds a sentinel of trophic changes and CO2 trapping in transitional waters. Ecol. Indic. 2020, 118, 1–10. [Google Scholar] [CrossRef]
- Le, B.; Shin, J.A.; Kang, M.-G.; Sun, S.; Yang, S.H.; Chung, G. Enhanced growth rate and ulvan yield of Ulva pertusa using light-emitting diodes (LEDs). Aquacult. Int. 2018, 26, 937–946. [Google Scholar] [CrossRef]
- Sfriso, A.; Buosi, A.; Sciuto, K.; Wolf, M.; Tomio, Y.; Juhmani, A.-S.; Sfriso, A.A. Effect of ecological recovery on macrophyte dominance and production in the Venice Lagoon. Front. Mar. Sci. 2022, 9, 882463. [Google Scholar] [CrossRef]
- Life SERESTO. Habitat 1150* (Coastal Lagoon) Recovery by SEagrass RESTOration. A New Strategic Approach to Meet HD & WFD Objectives. LIFE12-NAT-IT-000331. Available online: https://una.city/nbs/venezia/life-seagrass-restoration (accessed on 25 June 2024).
- Life LAGOON REFRESH. Coastal Lagoon Habitat (1150*) and Species Recovery by Restoring the Salt Gradient Increasing Fresh Water Input. LIFE16-NAT-IT-000663. Available online: www.lifelagoonrefresh.eu (accessed on 25 June 2024).
- Life TRANSFER. Seagrass Transplantation for Transitional Ecosystem Recovery. LIFE19-NAT-IT-000264. Available online: www.lifetransfer.eu (accessed on 25 June 2024).
- Boström, C.; Jackson, E.L.; Simenstad, C.A. Seagrass landscapes and their effects on associated fauna: A review. Estuar. Coast. Shelf Sci. 2006, 68, 383–403. [Google Scholar] [CrossRef]
- Bos, A.R.; Bouma, T.J.; de Kort, G.L.J.; van Katwijk, M.M. Ecosystem engineering by annual intertidal seagrass beds: Sediment accretion and modification. Estuar. Coast. Shelf Sci. 2007, 74, 344–348. [Google Scholar] [CrossRef]
- Duarte, C.M.; Sintes, T.; Marbà, N. Assessing the CO2 capture potential of seagrass restoration projects. J. Appl. Ecol. 2013, 50, 1341–1349. [Google Scholar] [CrossRef]
- Ondiviela, B.; Losada, I.J.; Lara, J.L.; Maza, M.; Galván, C.; Bouma, T.J.; van Belzen, J. The role of seagrasses in coastal protection in a changing climate. Coast. Eng. 2014, 87, 158–168. Available online: https://www.sciencedirect.com/journal/coastal-engineering (accessed on 25 June 2024). [CrossRef]
- Arévalo, R.; Pinedo, S.; Ballesteros, E. Changes in the composition and structure of Mediterranean rocky-shore communities following a gradient of nutrient enrichment: Descriptive study and test of proposed methods to assess water quality regarding macroalgae. Mar. Pollut. Bull. 2007, 55, 104–113. [Google Scholar] [CrossRef]
- Ramos, J.B.E.; Schulz, K.G.; Voss, M.; Narciso, A.; Müller, M.N.; Reis, F.V.; Cachão, M.; Azeve, E.B. Nutrient-specific responses of a phytoplankton community: A case study of the North Atlantic Gyre. J. Plankton Res. 2017, 39, 744–761. [Google Scholar] [CrossRef]
- Kautsky, L.; Kautsky, H. Algal species diversity and dominance along gradients of stress and disturbance in marine environments. Vegetatio 1989, 83, 259–267. [Google Scholar] [CrossRef]
- Kornfeldt, R.A. Relation between nitrogen and phosphorus content of macroalgae and the waters of northern Oresund. Bot. Mar. 1982, 25, 197–201. [Google Scholar]
- Rijstenbil, J.W.; Haritonidis, S. Macroalgae, Eutrophication and Trace Metal Cycling in Estuaries and Lagoons; Thessaloniki, Greece. Symposium Proceedings Sub Group III, Action COST-48 (Aquatic Primary Biomass-Marine Macroalgae. Commission of the European Communities; BRIDGE—DG XII; Brussels, Belgium NIOPO-CEMO, Yerseke, The Netherlands, 1993; p. 167.
- Schramm, W.; Nienhuis, P.H. Marine Benthic Vegetation: Recent Changes and the Effects of Eutrophication; Springer: Berlin, Germany, 1996; Volume 123, p. 470. [Google Scholar]
- Valiela, I.; McClelland, J.; Hauxwell, J.; Behr, P.J.; Hersh, D.; Foreman, K. Macroalgal blooms in shallow estuaries: Controls and ecophysiological and ecosystem consequences. Limnol. Oceanogr. 1997, 42, 1105–1118. [Google Scholar] [CrossRef]
- Cassin, D.; Dominik, J.; Botter, M.; Zonta, R. PAH and PCB contamination in the sediments of the Venice Lagoon (Italy) before the installation of the MOSE flood defence works. Environ. Sci. Pollut. Res. Int. 2018, 25, 24951–24964. [Google Scholar] [CrossRef]
- Mannino, A.M.; Menéndez, M.; Obrador, B.; Sfriso, A.; Triest, L. The genus Ruppia L. (Ruppiaceae) in the Mediterranean region: An overview. Aquat. Bot. 2015, 124, 1–9. [Google Scholar] [CrossRef]
- Wang, H.; Wang, G.; Gu, W. Macroalgal blooms caused by marine nutrient changes resulting from human activities. J. Appl. Ecol. 2020, 57, 766–776. [Google Scholar] [CrossRef]
- Kautsky, N.; Kautsky, H.; Kautsky, U.; Waern, M. Decreased depth penetration of Fucus vesiculosus (L.) since the 1940’s indicates eutrophication of the Baltic Sea. Mar. Ecol. Prog. Ser. 1986, 28, 1–8. [Google Scholar] [CrossRef]
- Ji, Y.; Xu, Z.; Zou, D.; Gao, K. Ecophysiological responses of marine macroalgae to climate change factors. J. Appl. Phycol. 2016, 28, 2953–2967. [Google Scholar] [CrossRef]
- Mota, C.F.; Engelen, A.H.; Serrao, E.A.; Pearson, G.A. Some don’t like it hot: Microhabitat-dependent thermal and water stresses in a trailing edge population. Funct. Ecol. 2015, 29, 640–649. [Google Scholar] [CrossRef]
- Wilson, K.L.; Kay, L.M.; Schmidt, A.L.; Lotze, H.K. Effects of increasing water temperatures on survival and growth of ecologically and economically important seaweeds in Atlantic Canada: Implications for climate change. Mar. Biol. 2015, 162, 2431–2444. [Google Scholar] [CrossRef]

| MaQI EQR Changes | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ecological Quality Ratio (EQR) | |||||||||
| Water Bodies | Station Number | 2011 | 2014 | 2018 | 2021 | Difference 2021–2011 | Kruskal–Wallis Test 2011–2021 | ||
| Unmodified | Euryhaline Restricted | ER | 13 | 0.408 | 0.631 | 0.854 | 0.812 | 0.404 | p < 0.001, H = 0.516 |
| Euryhaline Non-Restricted 1 | ENR1 | 21 | 0.721 | 0.769 | 0.769 | 0.871 | 0.150 | NS: p = 0.082, H = 0.051 | |
| Euryhaline Non-Restricted 2 | ENR2 | 7 | 0.479 | 0.629 | 0.714 | 0.671 | 0.192 | NS: p = 0.235, F = 1.560 | |
| Euryhaline Non-Restricted 3 | ENR3 | 3 | 0.417 | 0.483 | 0.750 | 0.783 | 0.366 | NS: p = 0.072. H = 0.058 | |
| Euryhaline Non-Restricted 4 | ENR4 | 10 | 0.520 | 0.490 | 0.530 | 0.615 | 0.095 | NS: p = 0.263, H = 0.014 | |
| Polyhaline Restricted 1 | PR1 | 6 | 0.317 | 0.292 | 0.533 | 0.467 | 0.150 | NS: p = 0.295, H = 0.010 | |
| Polyhaline Restricted 2 | PR2 | 4 | 0.325 | 0.350 | 0.375 | 0.625 | 0.300 | p < 0.05, H = 0.816 | |
| Polyhaline Restricted 3 | PR3 | 3 | 0.317 | 0.317 | 0.350 | 0.550 | 0.233 | NS: p = 0.369, H = 0.048 | |
| Polyhaline Restricted 4 | PR4 | 3 | 0.317 | 0.317 | 0.317 | 0.283 | −0.033 | NS: p = 0.456, H = 0.111 | |
| Polyhaline Non-Restricted 1 | PNR1 | 5 | 0.330 | 0.350 | 0.330 | 0.350 | 0.020 | NS: p = 0.317, H < 0.001 | |
| Polyhaline Non-Restricted 2 | PNR2 | 9 | 0.261 | 0.350 | 0.394 | 0.406 | 0.144 | NS: p = 0.266, H = 0.015 | |
| Modified | Zappa Valley | ZV | 1 | 0.250 | 0.250 | 0.250 | 0.250 | 0 | NS: p = 0.317, H < 0.001 |
| Dogà Valley, Cavallino Valley | DV-CV | 2 | 0.925 | 0.925 | 0.925 | 0.925 | 0 | NS: p = 0.266, H = 0.015 | |
| Means of all stations | 87 | 0.459 | 0.542 | 0.620 | 0.658 | 0.199 | p < 0.001, H = 0.100 | ||
| Means of the 11 unmodified water bodies | 0.401 | 0.453 | 0.538 | 0.585 | 0.184 | NS: p = 0.077, H = 0.089 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sfriso, A.; Buosi, A.; Tomio, Y.; Silan, G.; Wolf, M.A.; Sciuto, K.; Sfriso, A.A. Macrophytes as Key Element to Determine Ecological Quality Changes in Transitional Water Systems: The Venice Lagoon as Study Case. Environments 2024, 11, 209. https://doi.org/10.3390/environments11090209
Sfriso A, Buosi A, Tomio Y, Silan G, Wolf MA, Sciuto K, Sfriso AA. Macrophytes as Key Element to Determine Ecological Quality Changes in Transitional Water Systems: The Venice Lagoon as Study Case. Environments. 2024; 11(9):209. https://doi.org/10.3390/environments11090209
Chicago/Turabian StyleSfriso, Adriano, Alessandro Buosi, Yari Tomio, Giulia Silan, Marion Adelheid Wolf, Katia Sciuto, and Andrea Augusto Sfriso. 2024. "Macrophytes as Key Element to Determine Ecological Quality Changes in Transitional Water Systems: The Venice Lagoon as Study Case" Environments 11, no. 9: 209. https://doi.org/10.3390/environments11090209
APA StyleSfriso, A., Buosi, A., Tomio, Y., Silan, G., Wolf, M. A., Sciuto, K., & Sfriso, A. A. (2024). Macrophytes as Key Element to Determine Ecological Quality Changes in Transitional Water Systems: The Venice Lagoon as Study Case. Environments, 11(9), 209. https://doi.org/10.3390/environments11090209









