Method Validation: Extraction of Microplastics from Organic Fertilisers
Abstract
1. Introduction
2. Materials and Methods
2.1. Origin of the Organic Waste Products (OWP)
2.2. Sampling Methods
2.3. Microplastics Preparation and Extraction Protocol
2.3.1. Spiked Microplastics
2.3.2. Sample Pre-Treatment
Sample Storage and Drying
Dry and Wet Sieving
Chemical Digestion
2.3.3. MP Extraction
Density Separation and MP Recovery
Microplastics Extraction Rate
2.4. Microplastics Identification and Characterisation
2.5. Contamination Prevention
2.6. A Posteriori Analysis
2.6.1. Materials
2.6.2. Recovery Rate
2.7. Confidence Interval
3. Results
3.1. Spiked Microplastics: Extraction Rate and Organic Matter Recovery
3.2. Evaluation of the Degradation of the Spiked Micoplastics
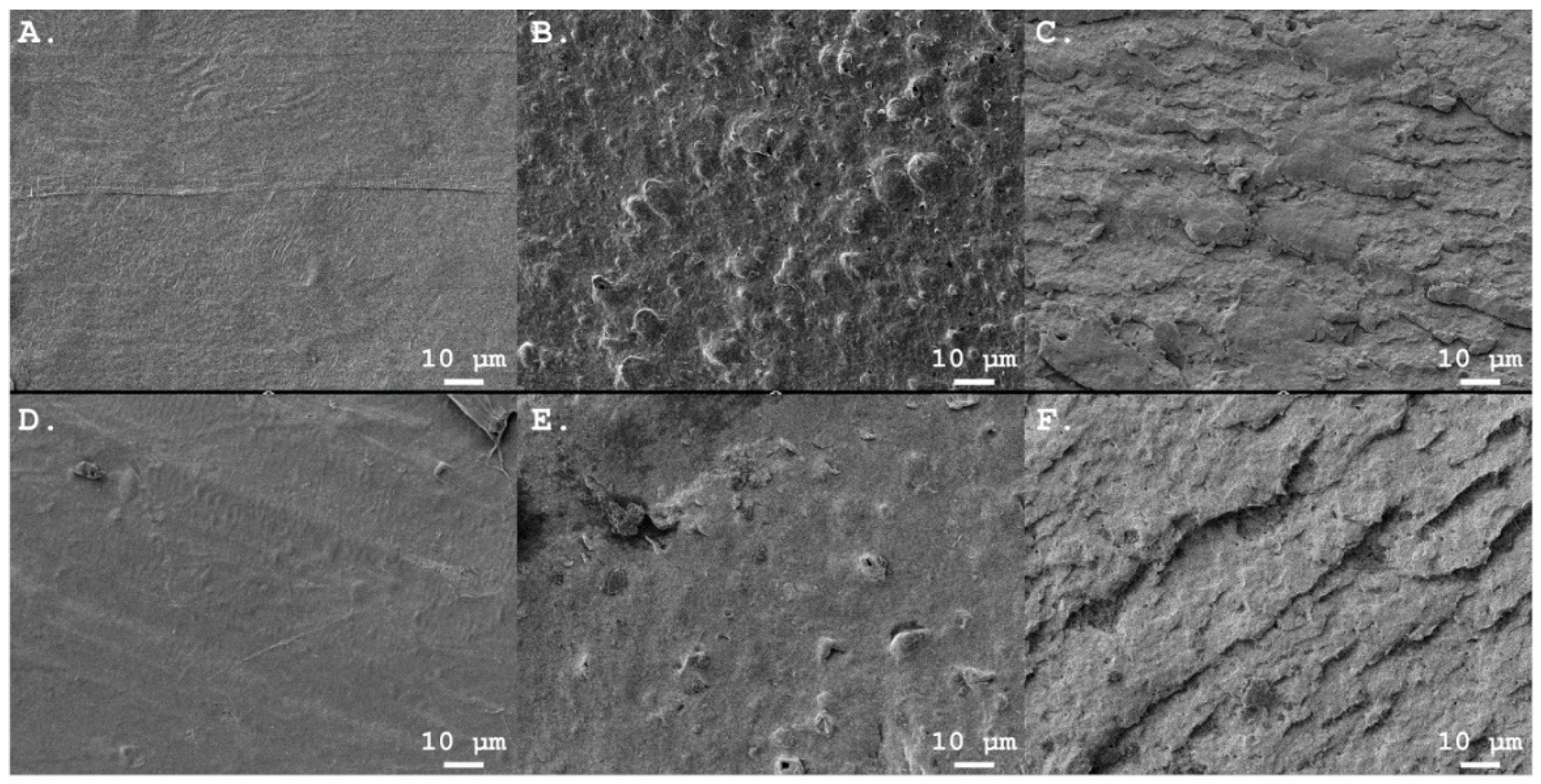
3.2.1. Microplastic Shape and Surface
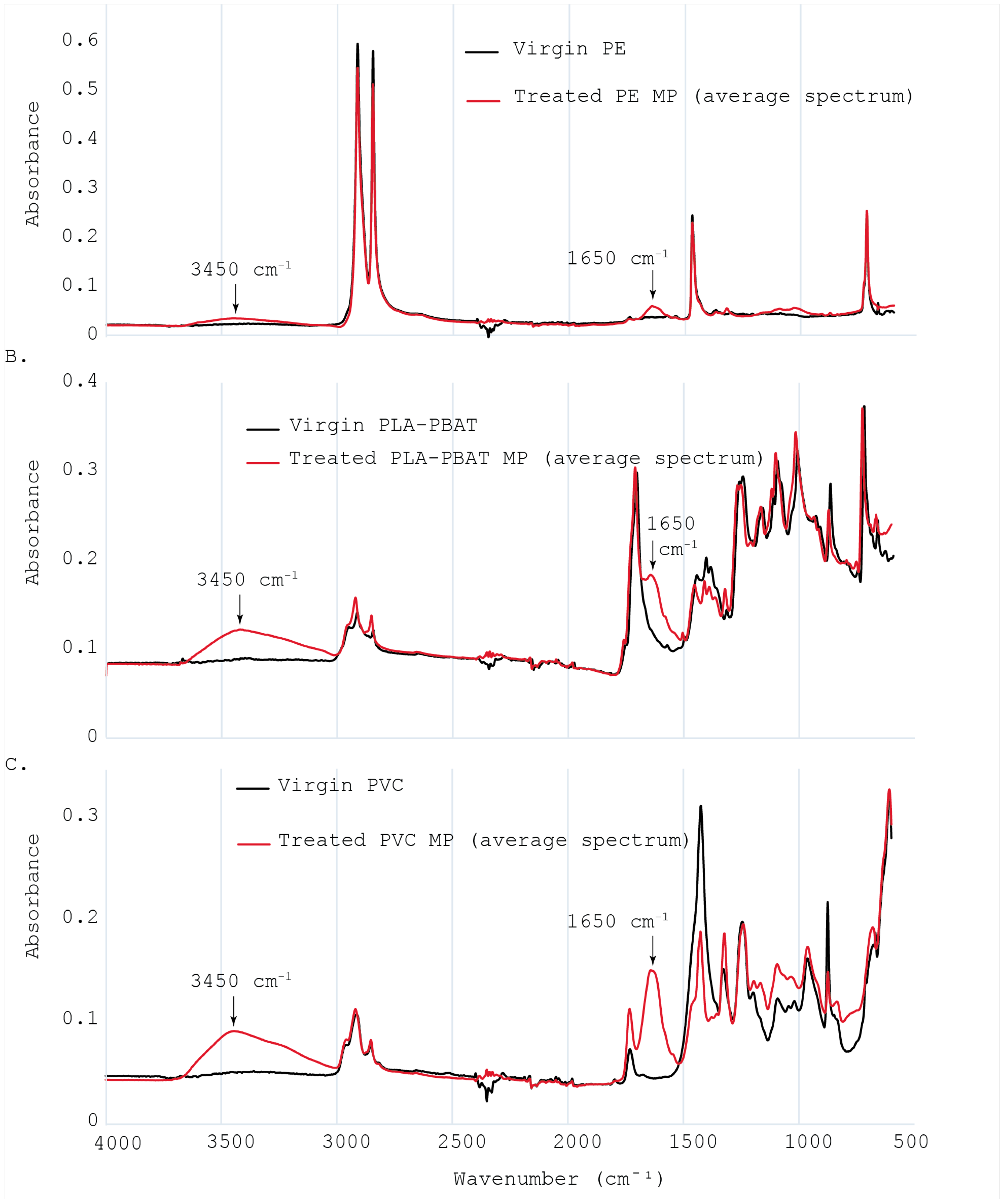
3.2.2. Microplastics Chemical Analysis
3.3. A Posteriori Analysis of Results
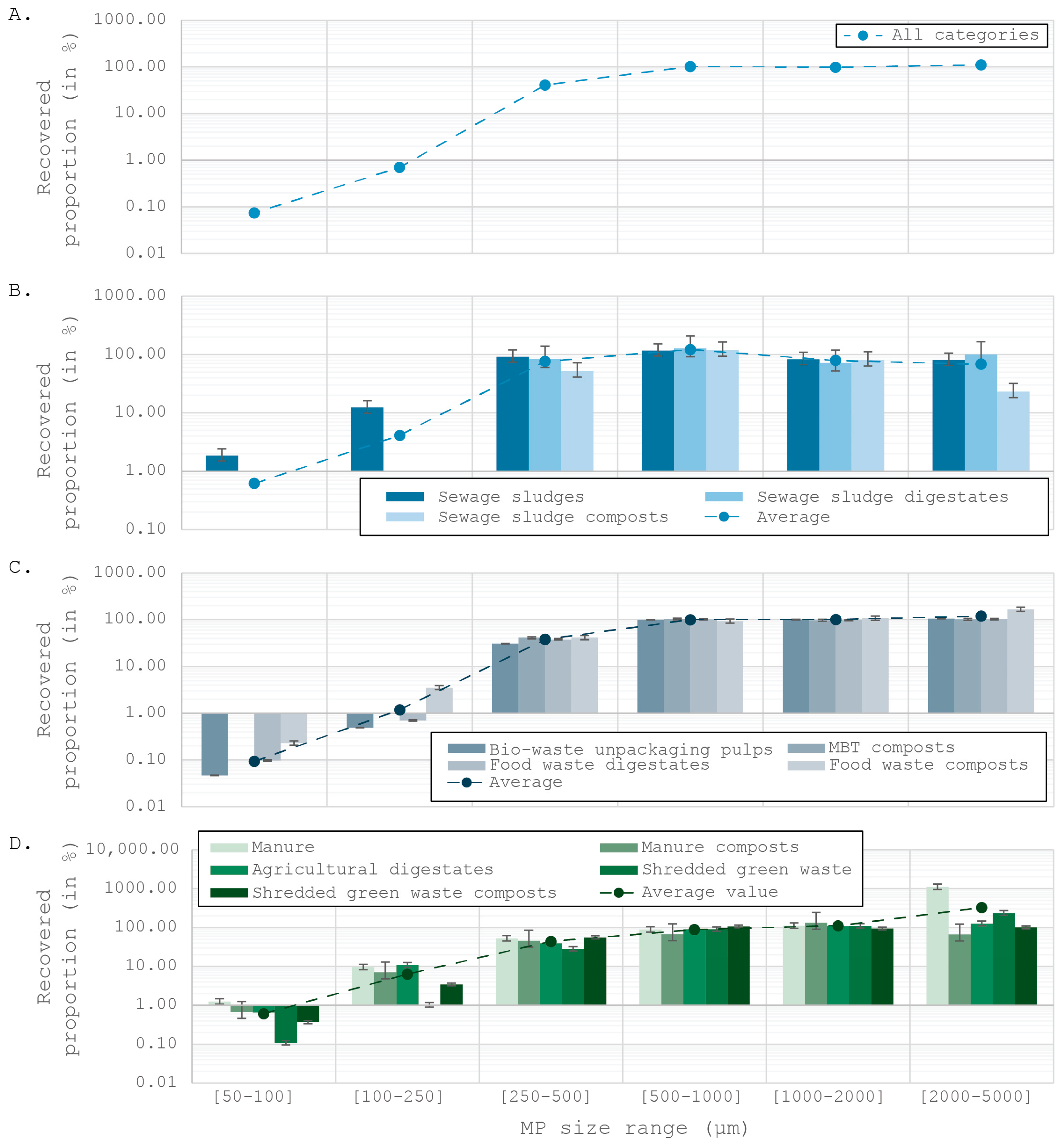
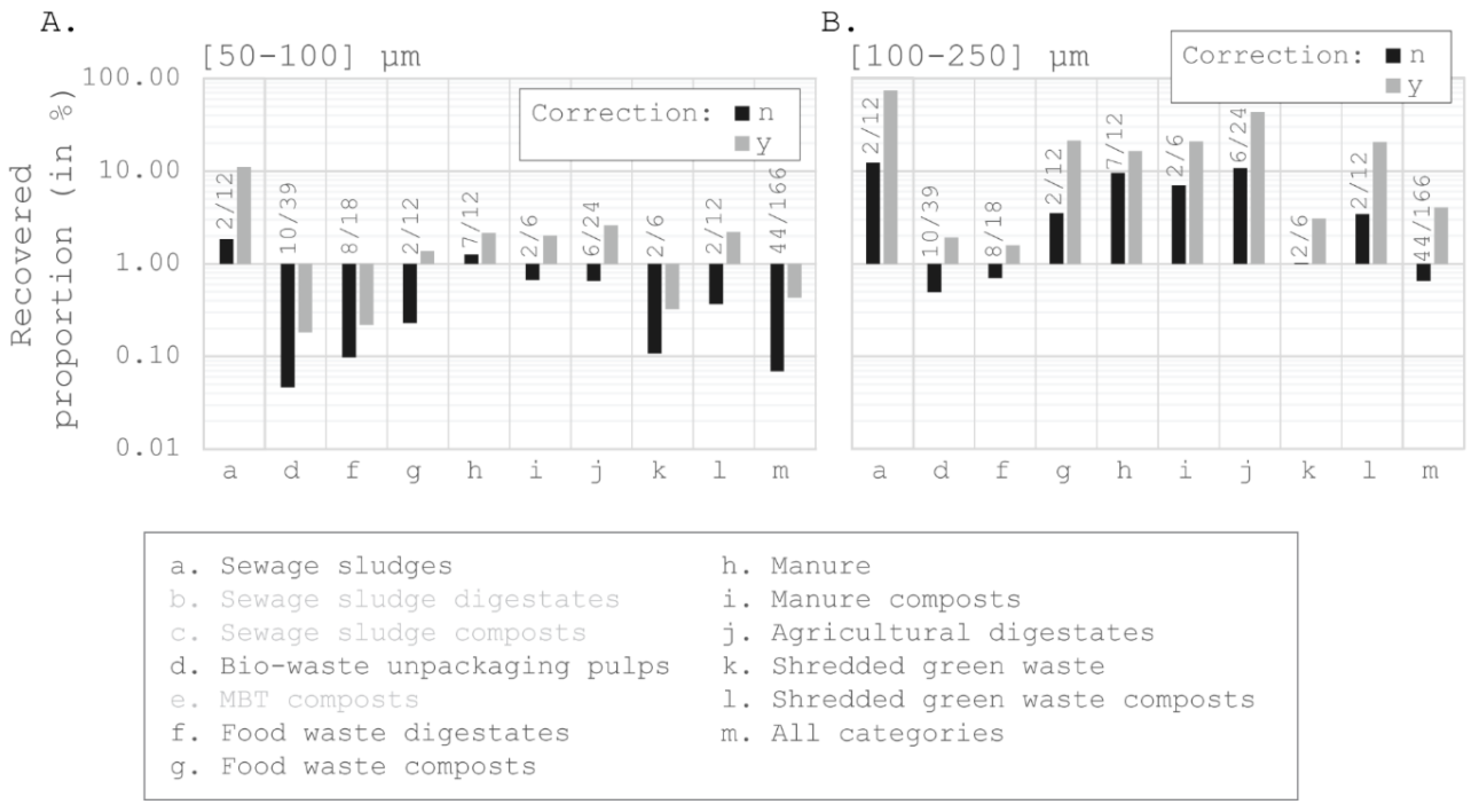
Estimating the Microplastic Recovery
4. Discussion
4.1. The Spiking Method: An a Priori Approach for Assessing Microplastic Extraction Rates
4.1.1. Microplastic Recovery
4.1.2. Residues from the Matrix
4.1.3. Consequences of These Results for the Project
4.2. An Original Approach: A Posteriori Estimation of Extraction Rates
4.2.1. An Underestimated Number of Microplastics
4.2.2. Implications of This Gap
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dignac, M.-F.; Houot, S.; Francou, C.; Derenne, S. Pyrolytic Study of Compost and Waste Organic Matter. Org. Geochem. 2005, 36, 1054–1071. [Google Scholar] [CrossRef]
- Braun, M.; Mail, M.; Heyse, R.; Amelung, W. Plastic in Compost: Prevalence and Potential Input into Agricultural and Horticultural Soils. Sci. Total Environ. 2021, 760, 143335. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.; Mail, M.; Krupp, A.E.; Amelung, W. Microplastic Contamination of Soil: Are Input Pathways by Compost Overridden by Littering? Sci. Total Environ. 2023, 855, 158889. [Google Scholar] [CrossRef]
- Hargreaves, J.; Adl, M.; Warman, P. A Review of the Use of Composted Municipal Solid Waste in Agriculture. Agric. Ecosyst. Environ. 2008, 123, 1–14. [Google Scholar] [CrossRef]
- Diacono, M.; Montemurro, F. Long-Term Effects of Organic Amendments on Soil Fertility. A Review. Agron. Sustain. Dev. 2010, 30, 401–422. [Google Scholar] [CrossRef]
- Weithmann, N.; Möller, J.N.; Löder, M.G.J.; Piehl, S.; Laforsch, C.; Freitag, R. Organic Fertilizer as a Vehicle for the Entry of Microplastic into the Environment. Sci. Adv. 2018, 4, eaap8060. [Google Scholar] [CrossRef]
- Rillig, M.C. Microplastic in Terrestrial Ecosystems and the Soil? Environ. Sci. Technol. 2012, 46, 6453–6454. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, Z.; Wollmann, C.; Schaefer, M.; Buchmann, C.; David, J.; Tröger, J.; Muñoz, K.; Frör, O.; Schaumann, G.E. Plastic Mulching in Agriculture. Trading Short-Term Agronomic Benefits for Long-Term Soil Degradation? Sci. Total Environ. 2016, 550, 690–705. [Google Scholar] [CrossRef]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in Freshwater and Terrestrial Environments: Evaluating the Current Understanding to Identify the Knowledge Gaps and Future Research Priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef]
- Ng, E.-L.; Huerta Lwanga, E.; Eldridge, S.M.; Johnston, P.; Hu, H.-W.; Geissen, V.; Chen, D. An Overview of Microplastic and Nanoplastic Pollution in Agroecosystems. Sci. Total Environ. 2018, 627, 1377–1388. [Google Scholar] [CrossRef]
- Bläsing, M.; Amelung, W. Plastics in Soil: Analytical Methods and Possible Sources. Sci. Total Environ. 2018, 612, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Watteau, F.; Dignac, M.-F.; Bouchard, A.; Revallier, A.; Houot, S. Microplastic Detection in Soil Amended With Municipal Solid Waste Composts as Revealed by Transmission Electronic Microscopy and Pyrolysis/GC/MS. Front. Sustain. Food Syst. 2018, 2, 81. [Google Scholar] [CrossRef]
- Crossman, J.; Hurley, R.R.; Futter, M.; Nizzetto, L. Transfer and Transport of Microplastics from Biosolids to Agricultural Soils and the Wider Environment. Sci. Total Environ. 2020, 724, 138334. [Google Scholar] [CrossRef] [PubMed]
- Van Schothorst, B.; Beriot, N.; Huerta Lwanga, E.; Geissen, V. Sources of Light Density Microplastic Related to Two Agricultural Practices: The Use of Compost and Plastic Mulch. Environments 2021, 8, 36. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Kang, S.; Wang, Z.; Wu, C. Microplastics in Soil: A Review on Methods, Occurrence, Sources, and Potential Risk. Sci. Total Environ. 2021, 780, 146546. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, Q.; Jia, W.; Yan, C.; Wang, J. Agricultural Plastic Mulching as a Source of Microplastics in the Terrestrial Environment. Environ. Pollut. 2020, 260, 114096. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Löffler, P.; Eichhöfer, S.; David, J.; Muñoz, K.; Schaumann, G.E. Are Agricultural Plastic Covers a Source of Plastic Debris in Soil? A First Screening Study. SOIL 2022, 8, 31–47. [Google Scholar] [CrossRef]
- Khalid, N.; Aqeel, M.; Noman, A.; Fatima Rizvi, Z. Impact of Plastic Mulching as a Major Source of Microplastics in Agroecosystems. J. Hazard. Mater. 2023, 445, 130455. [Google Scholar] [CrossRef]
- Accinelli, C.; Saccà, M.L.; Mencarelli, M.; Vicari, A. Deterioration of Bioplastic Carrier Bags in the Environment and Assessment of a New Recycling Alternative. Chemosphere 2012, 89, 136–143. [Google Scholar] [CrossRef]
- Accinelli, C.; Abbas, H.K.; Bruno, V.; Khambhati, V.H.; Little, N.S.; Bellaloui, N.; Shier, W.T. Field Studies on the Deterioration of Microplastic Films from Ultra-Thin Compostable Bags in Soil. J. Environ. Manag. 2022, 305, 114407. [Google Scholar] [CrossRef]
- Rani, M.; Ducoli, S.; Depero, L.E.; Prica, M.; Tubić, A.; Ademovic, Z.; Morrison, L.; Federici, S. A Complete Guide to Extraction Methods of Microplastics from Complex Environmental Matrices. Molecules 2023, 28, 5710. [Google Scholar] [CrossRef] [PubMed]
- Kedzierski, M.; Le Tilly, V.; César, G.; Sire, O.; Bruzaud, S. Efficient Microplastics Extraction from Sand. A Cost Effective Methodology Based on Sodium Iodide Recycling. Mar. Pollut. Bull. 2017, 115, 120–129. [Google Scholar] [CrossRef]
- Primpke, S.; Christiansen, S.H.; Cowger, W.; De Frond, H.; Deshpande, A.; Fischer, M.; Holland, E.B.; Meyns, M.; O’Donnell, B.A.; Ossmann, B.E.; et al. Critical Assessment of Analytical Methods for the Harmonized and Cost-Efficient Analysis of Microplastics. Appl. Spectrosc. 2020, 74, 1012–1047. [Google Scholar] [CrossRef] [PubMed]
- Primpke, S.; Cross, R.K.; Mintenig, S.M.; Simon, M.; Vianello, A.; Gerdts, G.; Vollertsen, J. Toward the Systematic Identification of Microplastics in the Environment: Evaluation of a New Independent Software Tool (siMPle) for Spectroscopic Analysis. Appl. Spectrosc. 2020, 74, 1127–1138. [Google Scholar] [CrossRef]
- Hurley, R.R.; Lusher, A.L.; Olsen, M.; Nizzetto, L. Validation of a Method for Extracting Microplastics from Complex, Organic-Rich, Environmental Matrices. Environ. Sci. Technol. 2018, 52, 7409–7417. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; da Costa, J.P.; Girão, A.V.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Identifying a Quick and Efficient Method of Removing Organic Matter without Damaging Microplastic Samples. Sci. Total Environ. 2019, 686, 131–139. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Ji, Y.; Li, M.; Dong, B.; Qian, G.; Zhou, J.; Dai, X. Effects of Chemical Pretreatments on Microplastic Extraction in Sewage Sludge and Their Physicochemical Characteristics. Water Res. 2020, 171, 115379. [Google Scholar] [CrossRef] [PubMed]
- Primpke, S.; Lorenz, C.; Rascher-Friesenhausen, R.; Gerdts, G. An Automated Approach for Microplastics Analysis Using Focal Plane Array (FPA) FTIR Microscopy and Image Analysis. Anal. Methods 2017, 9, 1499–1511. [Google Scholar] [CrossRef]
- AFNOR. French Standard—NF U44-108—Boues Des Ouvrages de Traitement Des Eaux Usées Urbaines—Boues Liquides—Échantillonnage En Vue de l’estimation de La Teneur Moyenne d’un Lot (Sludge from Urban Wastewater Plant—Liquid Sludge—Sampling Method for Estimating Average Content of a Production Batch). 1982. Available online: https://www.boutique.afnor.org/en-gb/standard/nf-u44108/fluid-sludge-from-treatment-of-urban-sewage-sampling-for-estimating-the-ave/fa017118/13727 (accessed on 2 February 2021).
- AFNOR. French Standard—NF EN 12579—Amendements Organiques et Supports de Culture—Échantillonnage (Organic Amendment and Growing Media- Sampling Method). 2013. Available online: https://www.boutique.afnor.org/en-gb/standard/nf-en-12579/soil-improvers-and-growing-media-sampling/fa164455/42577 (accessed on 2 February 2021).
- Plastics Europe. Plastics—The Facts 2021. Plastics Europe. 2021. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2021/ (accessed on 2 February 2021).
- Pyda, M.; Wunderlich, B. Reversing and Nonreversing Heat Capacity of Poly(Lactic Acid) in the Glass Transition Region by TMDSC. Macromolecules 2005, 38, 10472–10479. [Google Scholar] [CrossRef]
- Maw, M.M.; Boontanon, N.; Fujii, S.; Boontanon, S.K. Rapid and Efficient Removal of Organic Matter from Sewage Sludge for Extraction of Microplastics. Sci. Total Environ. 2022, 853, 158642. [Google Scholar] [CrossRef]
- Cerli, C.; Celi, L.; Kalbitz, K.; Guggenberger, G.; Kaiser, K. Separation of Light and Heavy Organic Matter Fractions in Soil—Testing for Proper Density Cut-off and Dispersion Level. Geoderma 2012, 170, 403–416. [Google Scholar] [CrossRef]
- Enders, K.; Lenz, R.; Ivar do Sul, J.A.; Tagg, A.S.; Labrenz, M. When Every Particle Matters: A QuEChERS Approach to Extract Microplastics from Environmental Samples. MethodsX 2020, 7, 100784. [Google Scholar] [CrossRef]
- Li, Q.; Wu, J.; Zhao, X.; Gu, X.; Ji, R. Separation and Identification of Microplastics from Soil and Sewage Sludge. Environ. Pollut. 2019, 254, 113076. [Google Scholar] [CrossRef]
- Thomas, D.; Schütze, B.; Heinze, W.M.; Steinmetz, Z. Sample Preparation Techniques for the Analysis of Microplastics in Soil—A Review. Sustainability 2020, 12, 9074. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2023. Available online: https://www.scirp.org/reference/referencespapers?referenceid=3582659 (accessed on 23 May 2023).
- Patil, I. statsExpressions: R Package for Tidy Dataframes and Expressions with Statistical Details. J. Open Source Softw. 2021, 6, 3236. [Google Scholar] [CrossRef]
- Beltrán, M.; Marcilla, A. Fourier transform infrared spectroscopy applied to the study of pvc decomposition. Eur. Polym. J. 1997, 33, 1135–1142. [Google Scholar] [CrossRef]
- Pfohl, P.; Roth, C.; Meyer, L.; Heinemeyer, U.; Gruendling, T.; Lang, C.; Nestle, N.; Hofmann, T.; Wohlleben, W.; Jessl, S. Microplastic Extraction Protocols Can Impact the Polymer Structure. Microplast. Nanoplast. 2021, 1, 8. [Google Scholar] [CrossRef]
- Hu, H.; Qiang, L.; Xu, J.; Li, G.; Cheng, J.; Zhong, X.; Zhang, R. Enhanced Microplastic Retrieval Efficiency from Cultivated Soil Samples through Optimized Pre-Treatment in Density-Based Extraction. Soil Tillage Res. 2024, 242, 106134. [Google Scholar] [CrossRef]
- Schrank, I.; Möller, J.N.; Imhof, H.K.; Hauenstein, O.; Zielke, F.; Agarwal, S.; Löder, M.G.J.; Greiner, A.; Laforsch, C. Microplastic Sample Purification Methods—Assessing Detrimental Effects of Purification Procedures on Specific Plastic Types. Sci. Total Environ. 2022, 833, 154824. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, W.; Gao, Q.; Huang, F.; Kang, Y.; Pan, Y.; Luo, G.; Zhou, X.; Fan, L.; Wang, A.; et al. Microplastics Extraction from Wastewater Treatment Plants: Two-Step Digestion Pre-Treatment and Application. Water Res. 2023, 230, 119569. [Google Scholar] [CrossRef]
- Savino, I.; Campanale, C.; Trotti, P.; Massarelli, C.; Corriero, G.; Uricchio, V.F. Effects and Impacts of Different Oxidative Digestion Treatments on Virgin and Aged Microplastic Particles. Polymers 2022, 14, 1958. [Google Scholar] [CrossRef] [PubMed]
- Vermeiren, P.; Muñoz, C.; Ikejima, K. Microplastic Identification and Quantification from Organic Rich Sediments: A Validated Laboratory Protocol. Environ. Pollut. 2020, 262, 114298. [Google Scholar] [CrossRef] [PubMed]
- Al-Azzawi, M.S.M.; Funck, M.; Kunaschk, M.; Der Esch, E.V.; Jacob, O.; Freier, K.P.; Schmidt, T.C.; Elsner, M.; Ivleva, N.P.; Tuerk, J.; et al. Microplastic Sampling from Wastewater Treatment Plant Effluents: Best-Practices and Synergies between Thermoanalytical and Spectroscopic Analysis. Water Res. 2022, 219, 118549. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, F.; Fischer, E.K. Various Digestion Protocols Within Microplastic Sample Processing—Evaluating the Resistance of Different Synthetic Polymers and the Efficiency of Biogenic Organic Matter Destruction. Front. Environ. Sci. 2020, 8, 263. [Google Scholar] [CrossRef]
- Tan, M.; Sun, Y.; Gui, J.; Wang, J.; Chen, X.; Wu, D. The Efficiency of Different Digestion and Separation Methods for Extracting Microplastics in Typical Organic Solid Waste. Int. J. Environ. Res. 2022, 16, 16. [Google Scholar] [CrossRef]


| 50–100 µm | 100–250 µm | 250–500 µm | 500–1000 µm | 1000–2000 µm | 2000–5000 µm | |
|---|---|---|---|---|---|---|
| CF | 1.5 | 3.8 | 18.2 | 52.8 | 153.5 | 387.5 |
| 1/CF | 0.66 | 0.26 | 0.06 | 0.02 | 0.007 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciréderf Boulant, D.; Simon, M.; Magueresse, A.; Mortas, N.; Thévenin, N.; Yeuch, V.; Durand, G.; Caurant, A.; Goulitquer, S.; Even, A.; et al. Method Validation: Extraction of Microplastics from Organic Fertilisers. Environments 2025, 12, 143. https://doi.org/10.3390/environments12050143
Ciréderf Boulant D, Simon M, Magueresse A, Mortas N, Thévenin N, Yeuch V, Durand G, Caurant A, Goulitquer S, Even A, et al. Method Validation: Extraction of Microplastics from Organic Fertilisers. Environments. 2025; 12(5):143. https://doi.org/10.3390/environments12050143
Chicago/Turabian StyleCiréderf Boulant, Delphine, Mathilde Simon, Anthony Magueresse, Nicolas Mortas, Nicolas Thévenin, Valérie Yeuch, Gaël Durand, Adrien Caurant, Sophie Goulitquer, Aurélie Even, and et al. 2025. "Method Validation: Extraction of Microplastics from Organic Fertilisers" Environments 12, no. 5: 143. https://doi.org/10.3390/environments12050143
APA StyleCiréderf Boulant, D., Simon, M., Magueresse, A., Mortas, N., Thévenin, N., Yeuch, V., Durand, G., Caurant, A., Goulitquer, S., Even, A., Maisonnat, S., Yesbergenova-Cuny, Z., Deportes, I., Bruzaud, S., & Kedzierski, M. (2025). Method Validation: Extraction of Microplastics from Organic Fertilisers. Environments, 12(5), 143. https://doi.org/10.3390/environments12050143







