Phytoremediation: The Sustainable Strategy for Improving Indoor and Outdoor Air Quality
Abstract
:1. Introduction
2. Air Pollutants
2.1. Particulate Matter (PM)
2.2. Volatile Organic Compounds (VOCs)
2.3. Inorganic Air Pollutants (IAC)
2.4. Others
3. Air Pollution and Human Diseases
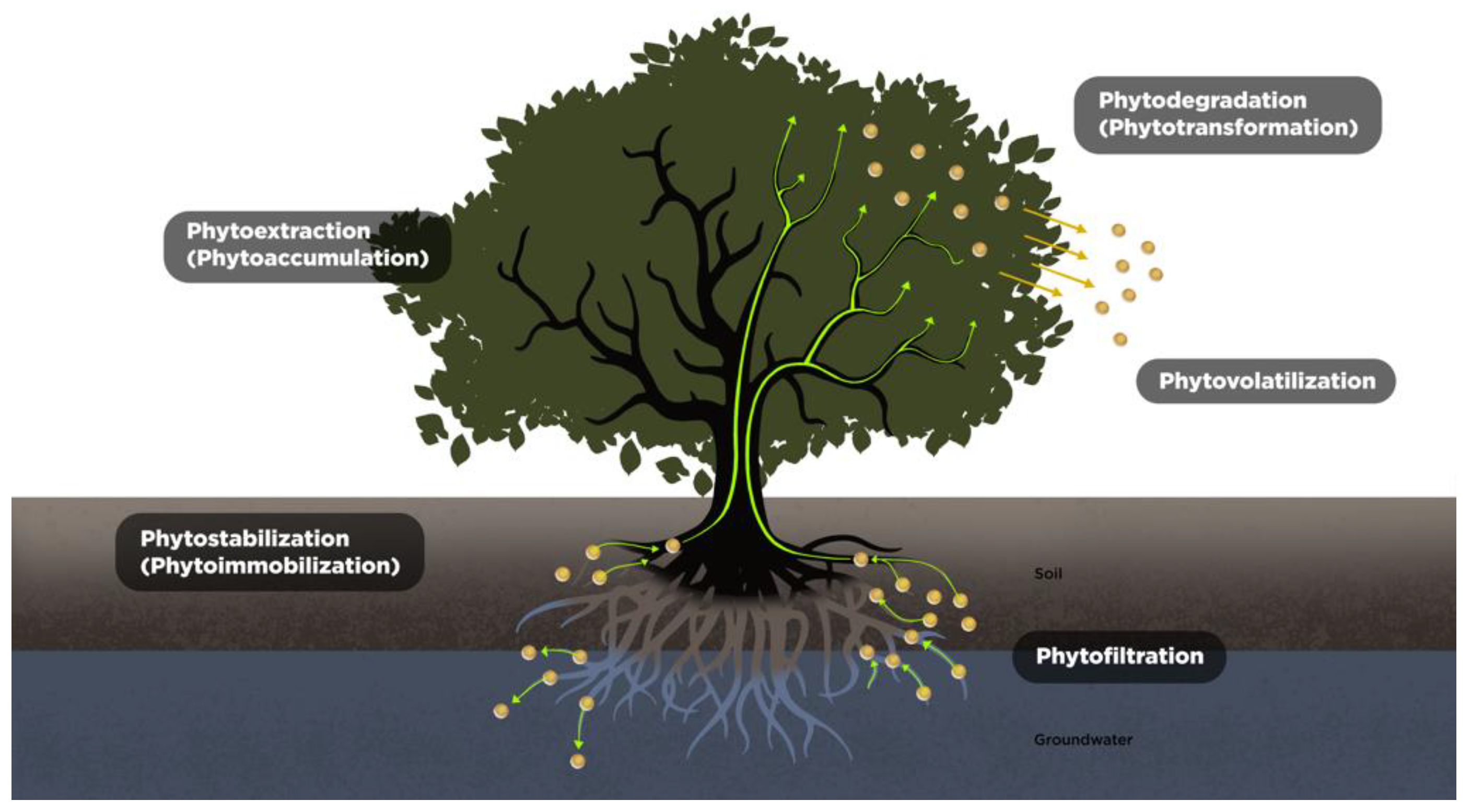
4. Mechanisms of Phytoremediation
4.1. Phytoextraction (Phytoaccumulation)
4.2. Phytostabilization (Phytoimmobilization)
4.3. Phytovolatilization
4.4. Phytodegradation (Phytotransformation)
4.5. Phytofiltration
5. Phytoremediation Mechanisms of Main Air Pollutants
5.1. Removal of Gaseous Pollutants
5.2. Removal of Aerosol Pollutants (Prticulate Matter)
6. Applications of the Phytoremediation for Improving Air Quality
6.1. Indoor Air Quality
6.2. Outdoor Air Quality
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gawronski, S.W.; Gawronska, H.; Lomnicki, S.; Sæbo, A.; Vangronsveld, J. Plants in Air Phytoremediation. Adv. Bot. Res. 2017, 83, 319–346. [Google Scholar] [CrossRef]
- World Health Organization. Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Farrow, A.; Miller, K.A.; Myllyvirta, L.; Newport, E.; Son, M. Toxic Air: The Price of Fossil Fuels; Greenpeace Southeast Asia: Bangkok, Thailand, 2020. [Google Scholar]
- Truu, J.; Truu, M.; Espenberg, M.; Nõlvak, H.; Juhanson, J. Phytoremediation and Plant-Assisted Bioremediation in Soil and Treatment Wetlands: A Review. Open Biotechnol. J. 2015, 9, 85–92. [Google Scholar] [CrossRef]
- Boyajian, G.E.; Carreira, L.H. Phytoremediation: A Clean Transitionfrom Laboratory to Marketplace? Nat. Biotechnol. 1997, 15, 127–128. [Google Scholar] [CrossRef] [PubMed]
- Pilon-Smits, E. Phytoremediation. Annu. Rev. Plant Biol. 2005, 56, 15–39. [Google Scholar] [CrossRef]
- Singh, O.V.; Labana, S.; Pandey, G.; Budhiraja, R.; Jain, R.K. Phytoremediation: An Overview of Metallic Ion Decontamination from Soil. Appl. Microbiol. Biotechnol. 2003, 61, 405–412. [Google Scholar] [CrossRef]
- Yang, W.; Omaye, S.T. Air Pollutants, Oxidative Stress and Human Health. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2009, 674, 45–54. [Google Scholar] [CrossRef]
- Agarwal, P.; Sarkar, M.; Chakraborty, B.; Banerjee, T. Phytoremediation of Air Pollutants: Prospects and Challenges. In Phytomanagement of Polluted Sites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 221–241. ISBN 9780128139134. [Google Scholar]
- Davidson, C.I.; Phalen, R.F.; Solomon, P.A. Airborne Particulate Matter and Human Health: A Review. Aerosol Sci. Technol. 2005, 39, 737–749. [Google Scholar] [CrossRef]
- World Health Organization; Regional Office for Europe. Health Effects of Particulate Matter; World Health Organization: Geneva, Switzerland, 2013; ISBN 9789289000017. [Google Scholar]
- Lin, Y.; Zou, J.; Yang, W.; Li, C.Q. A Review of Recent Advances in Research on PM2.5 in China. Int. J. Environ. Res. Public Health 2018, 15, 438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araujo, J.A.; Nel, A.E. Particulate Matter and Atherosclerosis: Role of Particle Size, Composition and Oxidative Stress. Part. Fibre Toxicol. 2009, 6, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Fiordelisi, A.; Piscitelli, P.; Trimarco, B.; Coscioni, E.; Iaccarino, G.; Sorriento, D. The Mechanisms of Air Pollution and Particulate Matter in Cardiovascular Diseases. Heart Fail. Rev. 2017, 22, 337–347. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Code of Federal Regulations, 40: Chapter 1, Subchapter C, Part 51, Subpart F, 51100. Available online: https://www.epa.gov/indoor-air-quality-iaq/technical-overview-volatile-organic-compounds (accessed on 8 February 2009).
- Ulker, O.C.; Ulker, O.; Hiziroglu, S. Volatile Organic Compounds (VOCs) Emitted from Coated Furniture Units. Coatings 2021, 11, 806. [Google Scholar] [CrossRef]
- Deng, B.; Kim, C.N. An Analytical Model for VOCs Emission from Dry Building Materials. Atmos. Environ. 2004, 38, 1173–1180. [Google Scholar] [CrossRef]
- Weyens, N.; Thijs, S.; Popek, R.; Witters, N.; Przybysz, A.; Espenshade, J.; Gawronska, H.; Vangronsveld, J.; Gawronski, S.W. The Role of Plant–Microbe Interactions and Their Exploitation for Phytoremediation of Air Pollutants. Int. J. Mol. Sci. 2015, 16, 25576–25604. [Google Scholar] [CrossRef] [Green Version]
- Soni, V.; Singh, P.; Shree, V.; Goel, V. Effects of VOCs on Human Health. In Energy, Environment, and Sustainability; Springer Nature: Basingstoke, UK, 2018; pp. 119–142. [Google Scholar]
- Frumkin, H.; Hess, J.; Luber, G.; Malilay, J.; McGeehin, M. Climate Change: The Public Health Response. Am. J. Public Health 2008, 98, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Sadatshojaie, A.; Rahimpour, M.R. CO2 emission and air pollution (volatile organic compounds, etc.)–related problems causing climate change. In Current Trends and Future Developments on (Bio-) Membranes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–30. [Google Scholar]
- Florentina Gheorghe, I.; Ion, B. The Effects of Air Pollutants on Vegetation and the Role of Vegetation in Reducing Atmospheric Pollution. In The Impact of Air Pollution on Health, Economy, Encironment and Agricultural Sources; In Tech: Kwun Tong, Hong Kong, 2011; pp. 241–280. [Google Scholar]
- Srivastava, R.K.; Jozewicz, W.; Singer, C. SO2 Scrubbing Technologies: A Review. Environ. Prog. 2001, 20, 219–228. [Google Scholar] [CrossRef]
- Agudelo-Castaneda, D.M.; Teixeira, E.C. Time-Series Analysis of Surface Ozone and Nitrogen Oxides Concentrations in an Urban Area at Brazil. Atmos. Pollut. Res. 2014, 5, 411–420. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.X.Y.; Hadibarata, T.; Yuniarto, A. Phytoremediation Mechanisms in Air Pollution Control: A Review. Water Air Soil Pollut. 2020, 231, 1–13. [Google Scholar] [CrossRef]
- Ran, J.; Kioumourtzoglou, M.A.; Sun, S.; Han, L.; Zhao, S.; Zhu, W.; Li, J.; Tian, L. Source-Specific Volatile Organic Compounds and Emergency Hospital Admissions for Cardiorespiratory Diseases. Int. J. Environ. Res. Public Health 2020, 17, 6210. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, Y.; Chai, X.; Xu, L.; Zhang, L.; Ning, P.; Huang, J.; Tian, S. Interaction of Inhalable Volatile Organic Compounds and Pulmonary Surfactant: Potential Hazards of VOCs Exposure to Lung. J. Hazard. Mater. 2019, 369, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Zhang, Y.; Jiang, J.; Luan, H.; Yu, C.; Nan, P.; Luo, B.; You, M. Short-Term Effects of Ambient Air Pollution on Hospitalization for Respiratory Disease in Taiyuan, China: A Time-Series Analysis. Int. J. Environ. Res. Public Health 2018, 15, 2160. [Google Scholar] [CrossRef] [Green Version]
- Ren, M.; Li, N.; Wang, Z.; Liu, Y.; Chen, X.; Chu, Y.; Li, X.; Zhu, Z.; Tian, L.; Xiang, H. The Short-Term Effects of Air Pollutants on Respiratory Disease Mortality in Wuhan, China: Comparison of Time-Series and Case-Crossover Analyses. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Bonyadi, Z.; Arfaeinia, H.; Fouladvand, M.; Farjadfard, S.; Omidvar, M.; Ramavandi, B. Impact of Exposure to Ambient Air Pollutants on the Admission Rate of Hospitals for Asthma Disease in Shiraz, Southern Iran. Chemosphere 2021, 262, 128091. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Ju, S.W.; Lin, C.L.; Hsu, W.H.; Lin, C.C.; Ting, I.W.; Kao, C.H. Air Pollutants and Subsequent Risk of Chronic Kidney Disease and End-Stage Renal Disease: A Population-Based Cohort Study. Environ. Pollut. 2020, 261, 114154. [Google Scholar] [CrossRef]
- Gorguner, M.; Akgun, M. Acute Inhalation Injury. Eurasian J. Med. 2010, 42, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Øvrevik, J.; Refsnes, M.; Låg, M.; Holme, J.A.; Schwarze, P.E. Activation of Proinflammatory Responses in Cells of the Airway Mucosa by Particulate Matter: Oxidant- and Non-Oxidant-Mediated Triggering Mechanisms. Biomolecules 2015, 5, 1399–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, R.; Tao, L.; Jian, H.; Chang, Y.; Cheng, Y.; Feng, Y.; Zhang, H. NLRP3 Inflammasome Activation and Lung Fibrosis Caused by Airborne Fine Particulate Matter. Ecotoxicol. Environ. Saf. 2018, 163, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Liu, X.; Li, W.; Zu, Y.; Zhou, F.; Shou, Q.; Ding, Z. PM2.5 Exposure Induces Inflammatory Response in Macrophages via the TLR4/COX-2/NF-ΚB Pathway. Inflammation 2020, 43, 1948–1958. [Google Scholar] [CrossRef]
- Hayes, R.B.; Lim, C.; Zhang, Y.; Cromar, K.; Shao, Y.; Reynolds, H.R.; Silverman, D.T.; Jones, R.R.; Park, Y.; Jerrett, M.; et al. PM2.5 Air Pollution and Cause-Specific Cardiovascular Disease Mortality. Int. J. Epidemiol. 2020, 49, 25–35. [Google Scholar] [CrossRef]
- Miller, K.A.; Siscovick, D.S.; Sheppard, L.; Shepherd, K.; Sullivan, J.H.; Anderson, G.L.; Kaufman, J.D.; Dss, M.; Health Sci-ences, O.L. Long-Term Exposure to Air Pollution and Incidence of Cardiovascular Events in Women. N. Engl. J. Med. 2007, 356, 447–458. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, Y.H.; Kim, M.K.; Paik, H.J.; Kim, D.H. Different Adverse Effects of Air Pollutants on Dry Eye Disease: Ozone, PM2.5, and PM210. Environ. Pollut. 2020, 265, 115039. [Google Scholar] [CrossRef] [PubMed]
- Weaver, A.M.; Wang, Y.; Wellenius, G.A.; Young, B.; Boyle, L.D.; Hickson, D.M.A.; Diamantidis, C.J. Long-Term Exposure to Ambient Air Pollution and Renal Function in African Americans: The Jackson Heart Study. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhu, G.; Zhang, L.; Chen, K. Particulate Matter Pollution and Hospital Outpatient Visits for Endocrine, Digestive, Urological, and Dermatological Diseases in Nanjing, China. Environ. Pollut. 2020, 261, 114205. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.M.; Jung, C.R.; Chen, W.T.; Hwang, B.F. Exposure to Fine Particulate Matter (PM2.5) and Pediatric Rheumatic Diseases. Environ. Int. 2020, 138, 105602. [Google Scholar] [CrossRef] [PubMed]
- Pei, L.; Wang, X.; Guo, B.; Guo, H.; Yu, Y. Do Air Pollutants as Well as Meteorological Factors Impact Corona Virus Disease 2019 (COVID-19)? Evidence from China Based on the Geographical Perspective. Environ. Sci. Pollut. Res. 2021, 1–13. [Google Scholar] [CrossRef]
- Martelletti, L.; Martelletti, P. Air Pollution and the Novel Covid-19 Disease: A Putative Disease Risk Factor. SN Compr. Clin. Med. 2020, 2, 383–387. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Chen, H.; Yik, X.; Chan, L.; Oliver, B.G. Is There an Association between the Level of Ambient Air Pollution and COVID-19? J. Physiol. Lung Cell Mol. Physiol. 2020, 319, 416–421. [Google Scholar] [CrossRef]
- Leni, Z.; Künzi, L.; Geiser, M. Air Pollution Causing Oxidative Stress. Curr. Opin. Toxicol. 2020, 20–21, 1–8. [Google Scholar] [CrossRef]
- Mudway, I.S.; Kelly, F.J.; Holgate, S.T. Oxidative Stress in Air Pollution Research. Free Radic. Biol. Med. 2020, 151, 2–6. [Google Scholar] [CrossRef]
- Suwa, T.; Hogg, J.C.; Quinlan, K.B.; Ohgami, A.; Vincent, R.; van Eeden, S.F. Particulate Air Pollution Induces Progression of Atherosclerosis. J. Am. Coll. Cardiol. 2002, 39, 935–942. [Google Scholar] [CrossRef] [Green Version]
- Salvi, A.; Liu, H.; Salim, S. Involvement of Oxidative Stress and Mitochondrial Mechanisms in Air Pollution-Related Neurobiological Impairments. Neurobiol. Stress 2020, 12, 100205. [Google Scholar] [CrossRef]
- Boovarahan, S.R.; Kurian, G.A. Mitochondrial Dysfunction: A Key Player in the Pathogenesis of Cardiovascular Diseases Linked to Air Pollution. Rev. Environ. Health 2018, 33, 111–122. [Google Scholar] [CrossRef]
- Daiber, A.; Kuntic, M.; Hahad, O.; Delogu, L.G.; Rohrbach, S.; di Lisa, F.; Schulz, R.; Münzel, T. Effects of Air Pollution Particles (Ultrafine and Fine Particulate Matter) on Mitochondrial Function and Oxidative Stress–Implications for Cardiovascular and Neurodegenerative Diseases. Arch. Biochem. Biophys. 2020, 696, 108662. [Google Scholar] [CrossRef]
- Afsar, B.; Elsurer Afsar, R.; Kanbay, A.; Covic, A.; Ortiz, A.; Kanbay, M. Air Pollution and Kidney Disease: Review of Current Evidence. Clin. Kidney J. 2019, 12, 19–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arthur, E.L.; Rice, P.J.; Rice, P.J.; Anderson, T.A.; Baladi, S.M.; Henderson, K.L.D.; Coats, J.R. Phytoremediation—An Overview. Crit. Rev. Plant Sci. 2005, 24, 109–122. [Google Scholar] [CrossRef] [Green Version]
- Kapourchal, S.A.; Kapourchal, S.A.; Pazira, E.; Homaee, M. Assessing Radish (Raphanus sativus L.) Potential for Phytoremediation of Lead-Polluted Soils Resulting from Air Pollution. Plant Soil Environ. 2009, 55, 202–206. [Google Scholar] [CrossRef] [Green Version]
- Etim, E.E. Phytoremediation and Its Mechanisms: A Review. Int. J. Environ. Bioenergy 2012, 2012, 120–136. [Google Scholar]
- Padmavathiamma, P.K.; Li, L.Y. Phytoremediation Technology: Hyper-Accumulation Metals in Plants. Water Air Soil Pollut. 2007, 184, 105–126. [Google Scholar] [CrossRef]
- Morikawa, H.; Erkin, Ö.C. Basic Processes in Phytoremediation and Some Applications to Air Pollution Control. Chemosphere 2003, 52, 1553–1558. [Google Scholar] [CrossRef]
- Trapp, S.; Köhler, A.; Larsen, L.C.; Zambrano, K.C.; Karlson, U. Phytotoxicity of Fresh and Willow and Poplar Trees Weathered Diesel and Gasoline to Willow and Poplar Trees. J. Soils Sediments 2001, 1, 71–76. [Google Scholar] [CrossRef]
- Ma, Y.; Oliveira, R.S.; Freitas, H.; Zhang, C. Biochemical and Molecular Mechanisms of Plant-Microbe-Metal Interactions: Relevance for Phytoremediation. Front. Plant Sci. 2016, 7, 918. [Google Scholar] [CrossRef] [PubMed]
- Agbontalor, A. Phytoremediation: An Environmentally Sound Technology for Pollution Prevention, Control and Remediation in Developing Countries. Educ. Res. Rev. 2007, 2, 151–156. [Google Scholar]
- Wei, Z.; van Le, Q.; Peng, W.; Yang, Y.; Yang, H.; Gu, H.; Lam, S.S.; Sonne, C. A Review on Phytoremediation of Contaminants in Air, Water and Soil. J. Hazard. Mater. 2021, 403, 123658. [Google Scholar] [CrossRef] [PubMed]
- Verzera, A.; Ziino, M.; Condurso, C.; Romeo, V.; Zappalà, M. Solid-Phase Microextraction and Gas Chromatography-Mass Spectrometry for Rapid Characterisation of Semi-Hard Cheeses. Anal. Bioanal. Chem. 2004, 380, 930–936. [Google Scholar] [CrossRef]
- Omasa, K.; Tobe, K.; Kondo, T. Absorption of Organic and Inorganic Air Pollutants by Plants. In Air Pollution and Plant Biotechnology; Springer: Berlin/Heidelberg, Germany, 2002; pp. 155–178. [Google Scholar]
- Singh, S.N.; Verma, A. Phytoremediation of Air Pollutants: A Review. In Environmental Bioremediation Technology; Springer: Berlin/Heidelberg, Germany, 2007; pp. 293–314. [Google Scholar]
- Wei, X.; Lyu, S.; Yu, Y.; Wang, Z.; Liu, H.; Pan, D.; Chen, J. Phylloremediation of Air Pollutants: Exploiting the Potential of Plant Leaves and Leaf-Associated Microbes. Front. Plant Sci. 2017, 8, 1318. [Google Scholar] [CrossRef]
- Popek, R.; Gawrońska, H.; Wrochna, M.; Gawroński, S.W.; Sæbø, A. Particulate Matter on Foliage of 13 Woody Species: Deposition on Surfaces and Phytostabilisation in Waxes—A 3-Year Study. Int. J. Phytoremediation 2013, 15, 245–256. [Google Scholar] [CrossRef]
- Weyens, N.; van der Lelie, D.; Taghavi, S.; Newman, L.; Vangronsveld, J. Exploiting Plant–Microbe Partnerships to Improve Biomass Production and Remediation. Trends Biotechnol. 2009, 27, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Weyens, N.; van der Lelie, D.; Taghavi, S.; Vangronsveld, J. Phytoremediation: Plant-Endophyte Partnerships Take the Challenge. Curr. Opin. Biotechnol. 2009, 20, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Irga, P.J.; Pettit, T.J.; Torpy, F.R. The Phytoremediation of Indoor Air Pollution: A Review on the Technology Development from the Potted Plant through to Functional Green Wall Biofilters. Rev. Environ. Sci. Biotechnol. 2018, 17, 395–415. [Google Scholar] [CrossRef]
- Teiri, H.; Pourzamani, H.; Hajizadeh, Y. Phytoremediation of VOCs from Indoor Air by Ornamental Potted Plants: A Pilot Study Using a Palm Species under the Controlled Environment. Chemosphere 2018, 197, 375–381. [Google Scholar] [CrossRef]
- Hossein Mosaddegh, M.; Jafarian, A.; Ghasemi, A.; Mosaddegh, A. Phytoremediation of Benzene, Toluene, Ethylbenzene and Xylene Contaminated Air by D. Deremensis and O. Microdasys Plants. J. Environ. Health Sci. Eng. 2014, 12, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Gawrońska, H.; Bakera, B. Phytoremediation of Particulate Matter from Indoor Air by Chlorophytum comosum L. Plants. Air Qual. Atmos. Health 2015, 8, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Su, Y.; Zhao, S. An Efficient Plant–Microbe Phytoremediation Method to Remove Formaldehyde from Air. Environ. Chem. Lett. 2020, 18, 197–206. [Google Scholar] [CrossRef]
- Shahriari Moghadam, M.; Kool, F.; Nasrabadi, M. Phytoremediation of Air Organic Pollution (Phenol) Using Hydroponic System. J. Air Pollut. Health 2017, 2, 189–198. [Google Scholar]
- Torpy, F.R.; Irga, P.J.; Burchett, M.D. Profiling Indoor Plants for the Amelioration of High CO2 Concentrations. Urban For. Urban Green. 2014, 13, 227–233. [Google Scholar] [CrossRef]
- Treesubsuntorn, C.; Thiravetyan, P. Botanical Biofilter for Indoor Toluene Removal and Reduction of Carbon Dioxide Emission under Low Light Intensity by Using Mixed C3 and CAM Plants. J. Clean. Prod. 2018, 194, 94–100. [Google Scholar] [CrossRef]
- Siswanto, D.; Permana, B.H.; Treesubsuntorn, C.; Thiravetyan, P. Sansevieria Trifasciata and Chlorophytum Comosum Botanical Biofilter for Cigarette Smoke Phytoremediation in a Pilot-Scale Experiment—Evaluation of Multi-Pollutant Removal Efficiency and CO2 Emission. Air Qual. Atmos. Health 2020, 13, 109–117. [Google Scholar] [CrossRef]
- Rashmi, F.W.; Agarwal, A.; Hrdlicka, J.; Varjani, S. CO2 Separation, Purification and Conversion to Chemicals and Fuels; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Pettit, T.; Irga, P.J.; Abdo, P.; Torpy, F.R. Do the Plants in Functional Green Walls Contribute to Their Ability to Filter Particulate Matter? Build. Environ. 2017, 125, 299–307. [Google Scholar] [CrossRef]
- Ibrahim, I.Z.; Chong, W.T.; Yusoff, S.; Wang, C.T.; Xiang, X.; Muzammil, W.K. Evaluation of Common Indoor Air Pollutant Reduction by a Botanical Indoor Air Biofilter System. Indoor Built Environ. 2021, 30, 7–21. [Google Scholar] [CrossRef]
- Kim, T.H.; An, B.R.; Clementi, M. Phytoremediation as Adaptive Design Strategy to Improve Indoor Air Quality. Experimental Results Relating to the Application of a Vertical Hydroponic Biofilter; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Abdo, P.; Huynh, B.P.; Irga, P.J.; Torpy, F.R. Evaluation of Air Flow through an Active Green Wall Biofilter. Urban For. Urban Green. 2019, 41, 75–84. [Google Scholar] [CrossRef]
- Kazemi, F.; Rabbani, M.; Jozay, M. Investigating the Plant and Air-Quality Performances of an Internal Green Wall System under Hydroponic Conditions. J. Environ. Manag. 2020, 275, 111230. [Google Scholar] [CrossRef]
- Pettit, T.; Irga, P.J.; Torpy, F.R. Functional Green Wall Development for Increasing Air Pollutant Phytoremediation: Substrate Development with Coconut Coir and Activated Carbon. J. Hazard. Mater. 2018, 360, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Torpy, F.; Clements, N.; Pollinger, M.; Dengel, A.; Mulvihill, I.; He, C.; Irga, P. Testing the Single-Pass VOC Removal Efficiency of an Active Green Wall Using Methyl Ethyl Ketone (MEK). Air Qual. Atmos. Health 2018, 11, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Russell, J.A.; Hu, Y.; Chau, L.; Pauliushchyk, M.; Anastopoulos, I.; Anandan, S.; Waring, M.S. Indoor-Biofilter Growth and Exposure to Airborne Chemicals Drive Similar Changes in Plant Root Bacterial Communities. Appl. Environ. Microbiol. 2014, 80, 4805–4813. [Google Scholar] [CrossRef] [Green Version]
- Mikkonen, A.; Li, T.; Vesala, M.; Saarenheimo, J.; Ahonen, V.; Kärenlampi, S.; Blande, J.D.; Tiirola, M.; Tervahauta, A. Biofiltration of Airborne VOCs with Green Wall Systems—Microbial and Chemical Dynamics. Indoor Air 2018, 28, 697–707. [Google Scholar] [CrossRef] [Green Version]
- Amini Parsa, V.; Salehi, E.; Yavari, A.R.; van Bodegom, P.M. Analyzing Temporal Changes in Urban Forest Structure and the Effect on Air Quality Improvement. Sustain. Cities Soc. 2019, 48, 101548. [Google Scholar] [CrossRef]
- Kończak, B.; Cempa, M.; Deska, M. Assessment of the Ability of Roadside Vegetation to Remove Particulate Matter from the Urban Air. Environ. Pollut. 2021, 268, 115465. [Google Scholar] [CrossRef]
- Paull, N.J.; Krix, D.; Irga, P.J.; Torpy, F.R. Airborne Particulate Matter Accumulation on Common Green Wall Plants. Int. J. Phytoremediat. 2020, 22, 594–606. [Google Scholar] [CrossRef]
- Rachmadiarti, F.; Purnomo, T.; Azizah, D.N.; Fascavitri, A. Syzigium Oleina and Wedelia Trilobata for Phytoremediation of Lead Pollution in the Atmosphere. Nat. Environ. Pollut. Technol. 2019, 18, 157–162. [Google Scholar]
- Chen, L.; Liu, C.; Zhang, L.; Zou, R.; Zhang, Z. Variation in Tree Species Ability to Capture and Retain Airborne Fine Particulate Matter (PM2.5). Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Bertold, M.; Joachim, M.; Hoa, N.X.; Cuong, N.T.; van Sinh, N.; Roeland, S. Particulate Matter Accumulation Capacity of Plants in Hanoi, Vietnam. Environ. Pollut. 2019, 253, 1079–1088. [Google Scholar] [CrossRef]
- Weerakkody, U.; Dover, J.W.; Mitchell, P.; Reiling, K. Particulate Matter Pollution Capture by Leaves of Seventeen Living Wall Species with Special Reference to Rail-Traffic at a Metropolitan Station. Urban For. Urban Green. 2017, 27, 173–186. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Chen, Q. Potential of Thirteen Urban Greening Plants to Capture Particulate Matter on Leaf Surfaces across Three Levels of Ambient Atmospheric Pollution. Int. J. Environ. Res. Public Health 2019, 16, 402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, A.S.; Bai, R.S. Phytoremediation for Urban Landscaping and Air Pollution Control—A Case Study in Trivandrum City, Kerala, India. Environ. Sci. Pollut. Res. 2021, 28, 9979–9990. [Google Scholar] [CrossRef] [PubMed]
- Pettit, T.; Irga, P.J.; Torpy, F.R. The in Situ Pilot-Scale Phytoremediation of Airborne VOCs and Particulate Matter with an Active Green Wall. Air Qual. Atmos. Health 2019, 12, 33–44. [Google Scholar] [CrossRef]

| Air Pollutants | Human Diseases | Observations/Health Impacts | References |
|---|---|---|---|
| VOCs | Chronic obstructive pulmonary diseases | The emergency hospital visits for chronic obstructive pulmonary diseases were positively linked to VOCs derived from household products, architectural paints, and gasoline emission showing excess risk (ER%) of 2.1%, 95% confidence interval (CI%): 0.9% to 3.4%; 1.5%, 95% CI: 0.2% to 2.9%; and 1.5%, 95% CI: 0.2% to 2.8%, respectively. | [26] |
| BTEX | Lung diseases | Exposure to BTEX may increase the risk of pulmonary diseases owing to the alteration of the gas-liquid interface properties of pulmonary surfactants. | [27] |
| SO2, O3, NO2, PM10, and PM2.5 | Respiratory diseases | The exposure to SO2 and NO2 were significantly linked to respiratory disease-related hospitalization. Females and the younger group were more vulnerable to air pollution than males and the older group. | [28] |
| SO2 and NO2 | Respiratory diseases | An increment of 10 μg/m3 in the SO2 concentration led to respiratory disease-related mortality of 1.9% and 2.9% in the time-series and the case-crossover analyses in single pollutant models, respectively. | [29] |
| SO2, O3, and NO2 | Asthmatic diseases | The yearly average SO2 concentration in the studied location was 8.62 times higher than the WHO guideline. Accordingly, the pollutants were closely linked to the high hospitalization rate by acute respiratory diseases and asthmatic symptoms. | [30] |
| SO2, NOx, and PM2.5 | Chronic kidney disease and end-stage renal disease | Compared to exposure to the first quartile of SO2, NO2, and PM2.5, exposure to the fourth quartile exhibited an increased risk of developing CKD and ESRD at 1.46-fold and 1.32-fold, 1.39-fold and 1.70-fold, and 1.74-fold and 1.69-fold, respectively. | [31] |
| PM2.5 | Lung fibrosis | PM2.5 could be internalized into cells and activate the NLRP3 inflammasome through multiple endocytosis processes involving phagocytosis and pinocytosis, leading to lung fibrosis. | [34] |
| PM2.5 | Respiratory diseases related inflammation | Respiratory disease-related inflammation might be induced by PM2.5 exposure via activation of TLR4/NF-kB/COX signaling | [35] |
| PM2.5 | Cardiovascular diseases | Based on an average concentration of 13.5 μg/m3 of PM2.5, an increase of 10 μg/m3 led to a 24% increase in cardiovascular diseases incidence and 76% increase in death by cardiovascular diseases. | [37] |
| PM10, PM2.5, and O3 | Dry eye diseases | Increased O3 and PM2.5 results in aggravated ocular discomfort. Increased PM10 irritated tear film stability in the DED group. | [38] |
| PM2.5, and O3 | Renal dysfunction | After 1-year and 3-year exposure to PM2.5 and O3, 6.5% and 12.7% of participants showed a reduced eGFR level and elevated UACR level, respectively. These results indicated impaired renal function. | [39] |
| PM2.5 | Endocrine, digestive, urological, and dermatological diseases | A 10 μg/m3 increase in PM2.5 exhibited a significant connection with a 0.65%, 0.59%, 0.43%, and 0.36% increase in hospital visits for DERM, ENDO, DIGE, and UROL, respectively. | [40] |
| PM2.5 | Pediatric rheumatic diseases | Exposure to PM2.5 during 11–40 weeks of pregnancy and 1–14 weeks after birth showed a significant association with the incidence of PRDs. | [41] |
| PM10 and PM2.5 | COVID-19 | Air quality index and PM2.5 and PM1.0 concentration exhibited a significant association with the risk of COVID-19. | [42] |
| PM10 and PM2.5 | COVID-19 | Italian northern regions showing an excess of PM10 and PM2.5 levels from legislative standards (50 μg/m3) have been seriously affected by COVID-19. | [43] |
| Location | Pollutants | Observations/Suggested Measures | References |
|---|---|---|---|
| Chamaedorea elegans | Formaldehyde | Potted C. elegans removed 65–100% of formaldehyde in the chamber. The removal capacity depends on the inlet concentration, and the light condition was more efficient than the dark condition. | [69] |
| Opuntia microdasy | BTEX | O. microdays removed BTEX in the chambers with the removal rates 1.35, 1.18, 0.54, and 1.64 mg/m2 d1, respectively. For complete removing 2.5 ppm of BTEX in a 30 m3 room, ten pots of O. microdasys were suggested. | [70] |
| Chlorophytum comosum L. | PM | C. comosum accumulated indoor PM10, PM2.5, and PM0.2 in their waxes. The accumulation occurred more in wax than on the surface to facilitate the attachment tightly to leaves and phytostabilize effectively. The accumulation amount depends on the kind of activity taking place in the room. | [71] |
| Aloe vera (Haw.) Ber, Tradescantia zebrina Bosse, and Vigna radiata (Linn.) Wilczek (V. radiata) | Formaldehyde | Adding microbes to hydro-cultured plants system improved the formaldehyde removal efficiency by 6.7–90.5%. While the remediation process in plant-only systems occurred through redox and enzymatic reactions, that in the plant-microbe systems occurred mainly via microbial degradation mechanisms. | [72] |
| Ophiopogon japonicus | Phenol and PM | A hydroponic system composed of Ophiopogon japonicus and phenol-degrading bacteria, Staphylococus epidermis and Pseudomonas spp., was combined with an air compressor that sucks air and injected it into the bioreactors to circulate in the plant pots. This system showed a high phenol-degrading capacity of about 1000 g/L daily. Additionally, this system absorbed PM and produced oxygen, improving air quality. | [73] |
| Chlorophytum comosum and Sansevieria trifasciata | CO2 | The biofilter containing a combination of C. comosum and S. trifasciata removed 3.9–4.7 mg/m3 toluene within 2–3 h, showing low CO2 emission under both light and dark conditions. | [75] |
| Sansevieria trifasciata and Chlorophytum comosum | PM2.5, VOCs, and CO2 | A biofilter composed of a CAM and C3 plants combination minimized the total CO2 emission accompanying high PM2.5 and COCs removal efficiency, compared to a biofilter composed of individual plant species. | [76] |
| Ficus lyrate, Chlorophytum orchidastrum, Nephrolepis cordifolia duffii, Nephropelis exaltata bostoniensis, Nematanthus glabra, Schefflera amate, Schefflera arboricola | PMs | Plants having fibrous roots revealed higher removal efficiency than those having tap roots, and fern species presented the highest single-pass removal efficiencies (PM03–0.5 = 45.78% and PM5–10 = 92.46%). | [78] |
| Epipremnum aureum | PM10, PM2.5, and VOCs | A botanical biofilter comprising horizontally grown plants in growth media, an evaporative medium, and a mechanical ventilation system showed PM2.5, PM10, and VOCs removal efficiencies of 54.5%, 65.42%, and 46%, respectively. | [79] |
| Schefflera arboricola | VOCs | An air handling unit connected to a biowall removed 3826.4 ppbv of isobutylene, recording an average of 20% single-pass efficiency. | [80] |
| Schefflera arboricola and Chlorophytum comosum ‘variegatum’ | PM10, PM2.5, and VOCs | A fan located at a central opening on the green wall’s back space can drive air through the medium-plant-roots mix, then onward to the plant’s canopy. This enabled more air to pass through the green wall substrate with greater remedy efficacy. Additionally, the wet plant wall modules led to much more air through the modules. | [81] |
| Aptenia cordifolia, Carpobrotus edulis, Peperomia magnoliiaefolia, and Kalanchoe blossfeldiana | 1 n.g. | The organic-rich growing medium of vermicompost along with perlite and cocopeat was suggested as an optimal medium for designing a sustainable internal green wall, especially when this is combined with Aptenia cordifolia. | [82] |
| Nephrolepis exaltata bostoniensis | PM, benzene, and VOCs | Adding granular activated carbon (GAC) into the coconut husk-based substrates, improved the VOCs and benzene deposition rate, while PM removal rate was reduced. | [83] |
| Asplenium antiquum, Philodendron scandens, Philodendron scandens ‘Brazil’, and Syngonium podophyllum. | Methyl ethyl ketone | The plant wall with a soil-less growing medium containing activated carbon was combined with a forced-air system, which draws the polluted air through the biowall and reduced the VOCs significantly, recording a 57% single-pass removal efficiency. | [84] |
| Ficus elastica and Schefflera arboricola “Gold Capella” | VOCs | Compared to clean-air exposed and soil-grown plants, VOC-exposed and biowall-grown plants exhibited an enriched level of Hyphomicrobium, a degrader of halogenated and aromatic compounds, surrounding the roots area. | [85] |
| Epipremnum pinnatum cv. Aureum and Davallia fejeensis Hook | VOCs | VOCs formed the microbial communities, enriching the VOCs utilizing bacteria species in the irrigation water, where most of the VOC degradation in the biowall occurs. | [86] |
| Location | Pollutants | Observations/Suggested Measures | References |
|---|---|---|---|
| Tabriz, Iran | O3, SO2, NO2, CO, PM2.5 | In 2015, shrubs and trees removed 238.4 t of air contaminants, and an increase of the elimination up to 814.46 t over the next 20 years is expected if appropriate, feasible urban forest management is performed. | [87] |
| North Katowice, Poland | PM | Among vines, shrubs, and coniferous trees, Parthenocissus quiquefolia and Betula pendula ‘Youngii’ accumulated the highest amounts of PM in their wax. The accumulated PM contained carbon, oxygen, silicon, iron, and heavy metals. | [88] |
| Fifteen different urbanized areas in Sydney, Australia | PM | The leaf traits were not the specific factor to determine the deposition capacities of plants. Among investigated plants, N. glabra, C. comosum variegatum, P. Xanadu, and S. wallisii entrapped the most amount of PM. | [89] |
| Surabaya town, Indonesia | Lead (Pb) | Wedelia trilobata and Syzigium olein are grown on the main roads and exposed to heavy metals. Wedelia trilobata, having wider leaves, absorbed more heavy metals than Syzigium oleina showing a smaller leaf surface area. | [90] |
| Beijing Forestry University, Beijing, China | PM2.5 | Compared to broadleaved plant species, needle-leaved coniferous species accumulated higher amounts of PM2.5. The PM2.5 removal capacity of broadleaved species was correlated to the number of grooves and trichomes. | [91] |
| Hanoi, Vietnam | PM | Leaves with a lower area, hydrophilic traits, and a high abaxial stomatal density entrapped more PM; accordingly, Muntingia calabura showed the highest PM removal capacity among 49 screened plant species. | [92] |
| Birmingham New Street railway, United Kingdom | PM1, PM2.5, and PM10 | Hebe albicans Cockayne, Hebe x youngii Metcalf, Buxus sempervirens L., and Thymus vulgaris L., which have small leaves, revealed the highest PM removal capacity. Leaves with adaxial surfaces showed higher PM densities compared to those with abaxial surfaces. | [93] |
| Kunming City, Southwest China | PM | Platanus acerifolia and Magnolia grandiflora showed the highest PM removal among deciduous and evergreen trees, respectively. PM entrap capacity depends not only on the leaf characteristics, but also on the pollution grade; Loropetalum chinense, Osmanthus fragrans, and Cinnamomum japonicum exhibited significant accumulation of PM in traffic and university campus areas, whereas showing moderate removal efficacy in an industrial area. | [94] |
| Trivandrum City, Kerala, India | Air Pollution Tolerance Indices (APTI) | Based on APTI, plants showing the highest APTI, Agave americana, Anacardium occidentale, Cassia fistula, Cassia roxburghii, Mangifera indica, and Saraca asoca, were suggested for near areas presenting heavy vehicular air pollution, and plants showing the next highest APTI for greenbelts. | [95] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Jun, Z.; Zahra, Z. Phytoremediation: The Sustainable Strategy for Improving Indoor and Outdoor Air Quality. Environments 2021, 8, 118. https://doi.org/10.3390/environments8110118
Lee H, Jun Z, Zahra Z. Phytoremediation: The Sustainable Strategy for Improving Indoor and Outdoor Air Quality. Environments. 2021; 8(11):118. https://doi.org/10.3390/environments8110118
Chicago/Turabian StyleLee, Heayyean, Ziwoo Jun, and Zahra Zahra. 2021. "Phytoremediation: The Sustainable Strategy for Improving Indoor and Outdoor Air Quality" Environments 8, no. 11: 118. https://doi.org/10.3390/environments8110118
APA StyleLee, H., Jun, Z., & Zahra, Z. (2021). Phytoremediation: The Sustainable Strategy for Improving Indoor and Outdoor Air Quality. Environments, 8(11), 118. https://doi.org/10.3390/environments8110118





