Influence of Forest Management and Sylvicultural Treatments on Abundance of Snags and Tree Cavities in Mountain Mixed Beech Forests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
2.3. Data Analysis
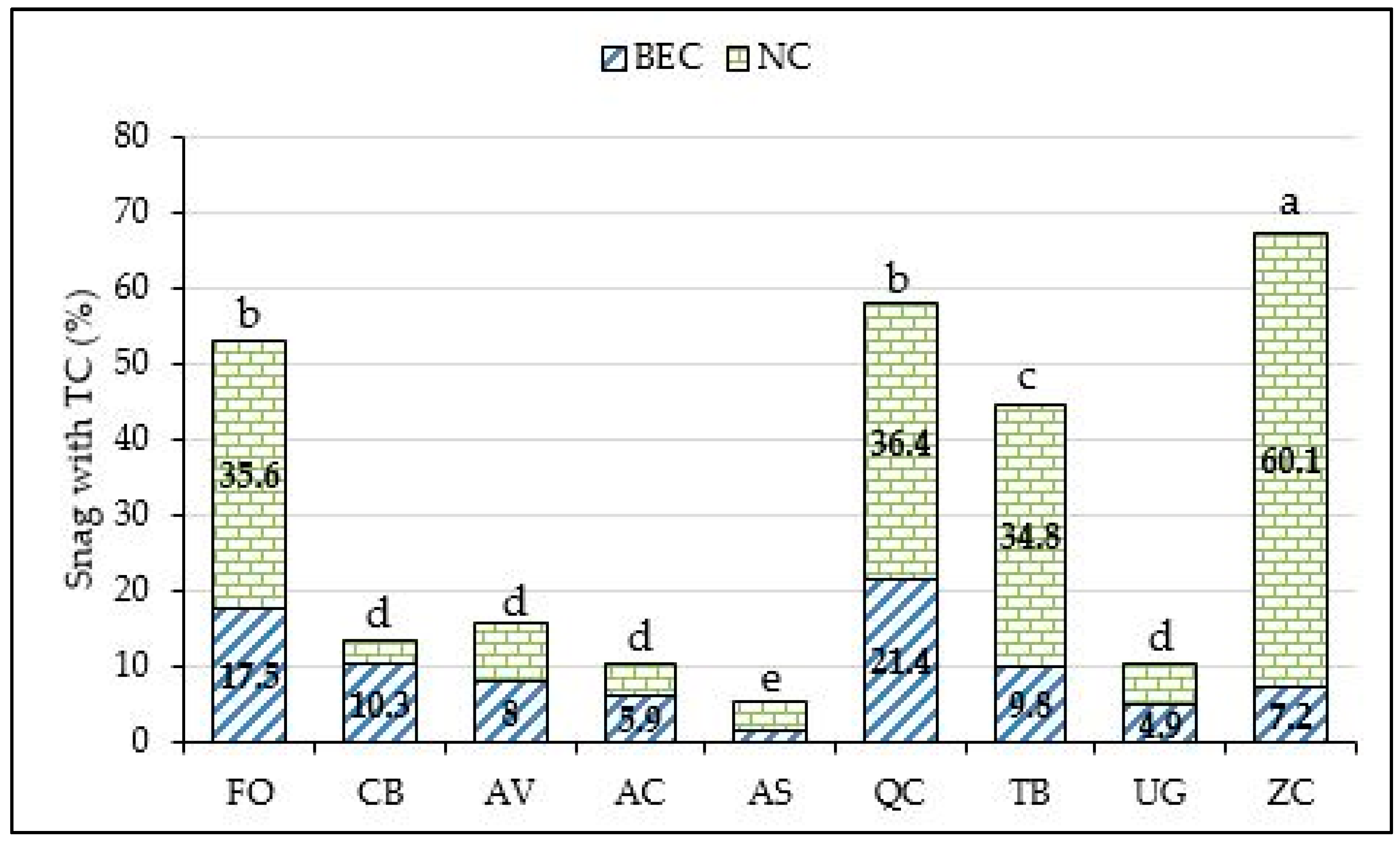
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Oettel, J.; Lapin, K. Linking forest management and biodiversity indicators to strengthen sustainable forest management in Europe. Ecol. Indic. 2021, 122, 107275. [Google Scholar] [CrossRef]
- Ganey, J.L. Snag density and composition of snag populations on two National Forests in northern Arizona. For. Ecol. Manag. 1999, 117, 169–178. [Google Scholar] [CrossRef]
- Tavankar, F.; Nikooy, M.; Picchio, R.; Venanzi, R.; Lo Monaco, A. Long-term effects of single-tree selection cutting management on coarse woody debris in natural mixed beech stands in the Caspian forest (Iran). iForest-Biogeosci. For. 2017, 10, 652. [Google Scholar] [CrossRef] [Green Version]
- Lo Monaco, A.; Luziatelli, G.; Latterini, F.; Tavankar, F.; Picchio, R. Structure and Dynamics of Deadwood in Pine and Oak Stands and their Role in CO 2 Sequestration in Lowland Forests of Central Italy. Forests 2020, 11, 253. [Google Scholar] [CrossRef] [Green Version]
- Lutz, J.A.; Struckman, S.; Furniss, T.J.; Birch, J.D.; Yocom, L.L.; McAvoy, D.J. Large-diameter trees, snags, and deadwood in southern Utah, USA. Ecol. Process. 2021, 10, 1–12. [Google Scholar]
- Öder, V.; Petritan, A.M.; Schellenberg, J.; Bergmeier, E.; Walentowski, H. Patterns and drivers of deadwood quantity and variation in mid-latitude deciduous forests. For. Ecol. Manag. 2021, 487. [Google Scholar] [CrossRef]
- Horton, S.P.; Mannan, R.W. Effects of prescribed fire on snags and cavity-nesting birds in southeastern Arizona pine forests. Wildl. Soc. Bull. 1988, 16, 37–44. [Google Scholar]
- Leverkus, A.B.; Buma, B.; Wagenbrenner, J.; Burton, P.J.; Lingua, E.; Marzano, R.; Thorn, S. Tamm review: Does salvage logging mitigate subsequent forest disturbances? For. Ecol. Manag. 2021, 481, 118721. [Google Scholar] [CrossRef]
- Waskiewicz, J.D.; Fulé, P.Z.; Beier, P. Comparing classification systems for ponderosa pine snags in northern Arizona. West. J. Appl. For. 2007, 22, 233–240. [Google Scholar] [CrossRef] [Green Version]
- Knoke, T.; Kindu, M.; Schneider, T.; Gobakken, T. Inventory of Forest Attributes to Support the Integration of Non-provisioning Ecosystem Services and Biodiversity into Forest Planning—From Collecting Data to Providing Information. Curr. For. Reports 2021, 7, 38–58. [Google Scholar]
- Rueegger, N. Variation in summer and winter microclimate in multi-chambered bat boxes in eastern Australia: Potential eco-physiological implications for bats. Environments 2019, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Savilaakso, S.; Johansson, A.; Häkkilä, M.; Uusitalo, A.; Sandgren, T.; Mönkkönen, M.; Puttonen, P. What are the effects of even-aged and uneven-aged forest management on boreal forest biodiversity in Fennoscandia and European Russia? A systematic review. Environ. Evid. 2021, 10, 1–38. [Google Scholar] [CrossRef]
- Jaroszewicz, B.; Cholewińska, O.; Chećko, E.; Wrzosek, M. Predictors of diversity of deadwood-dwelling macrofungi in a European natural forest. For. Ecol. Manag. 2021, 490, 119123. [Google Scholar] [CrossRef]
- Ganey, J.L.; Vojta, S.C. Characteristics of snags containing excavated cavities in northern Arizona mixed-conifer and ponderosa pine forests. For. Ecol. Manag. 2004, 199, 323–332. [Google Scholar] [CrossRef]
- Nagaike, T. Snag abundance and species composition in a managed forest landscape in central Japan composed of Larix kaempferi plantations and secondary broadleaf forests. Silva Fenn. 2009, 43, 755–766. [Google Scholar] [CrossRef] [Green Version]
- Bujoczek, L.; Bujoczek, M.; Zięba, S. How much, why and where? Deadwood in forest ecosystems: The case of Poland. Ecol. Indic. 2021, 121. [Google Scholar] [CrossRef]
- Alberdi, I.; Moreno-Fernández, D.; Cañellas, I.; Adame, P.; Hernández, L. Deadwood stocks in south-western European forests: Ecological patterns and large scale assessments. Sci. Total Environ. 2020, 747, 141237. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Li, X.; Liu, Z.; Ming, A.; Lan, H.; Ye, S. The Abundance and Structure of Deadwood: A Comparison of Mixed and Thinned Chinese Fir Plantations. Front. Plant Sci. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Mataji, A.; Sagheb-Talebi, K.; Eshaghi-Rad, J. Deadwood assessment in different developmental stages of beech (Fagus orientalis Lipsky) stands in Caspian forest ecosystems. Int. J. Environ. Sci. Technol. 2014, 11, 1215–1222. [Google Scholar] [CrossRef] [Green Version]
- Jahed, R.R.; Kavousi, M.R.; Farashiani, M.E.; Sagheb-Talebi, K.; Babanezhad, M.; Courbaud, B.; Wirtz, R.; Müller, J.; Larrieu, L. A comparison of the formation rates and composition of tree-related microhabitats in beech-dominated primeval carpathian and hyrcanian forests. Forests 2020, 11, 144. [Google Scholar] [CrossRef] [Green Version]
- Walankiewicz, W.; Czeszczewik, D.; Stański, T.; Sahel, M.; Ruczyński, I. Tree cavity resources in spruce-pine managed and protected stands of the Białowieża Forest, Poland. Nat. Areas J. 2014, 34, 423–428. [Google Scholar] [CrossRef]
- Veridiano, R.K.; Schröder, J.M.; Come, R.; Baldos, A.; Günter, S. Towards Forest Landscape Restoration Programs in the Philippines: Evidence from Logged Forests and Mixed-Species Plantations. Environments 2020, 7, 20. [Google Scholar] [CrossRef] [Green Version]
- Corace, R.G.; Seefelt, N.E.; Goebel, P.C.; Shaw, H.L. Snag longevity and decay class development in a recent jack pine clearcut in Michigan. North. J. Appl. For. 2010, 27, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Alidadi, F.; Mohadjer, M.R.M.; Etemad, V.; Sefidi, K. Decay dynamics of oriental beech (Fagus orientalis lipsky) and hornbeam (Carpinus betulus L.) deadwood in mixed beech stands. Iran. J. For. Poplar Res. 2014, 22, Pe624–Pe634. [Google Scholar]
- Remm, J.; Lohmus, A.; Remm, K. Tree cavities in riverine forests: What determines their occurrence and use by hole-nesting passerines? For. Ecol. Manag. 2006, 221, 267–277. [Google Scholar] [CrossRef]
- Assadi, S.B.; Kaboli, M.; Etemad, V.; Khanaposhtani, M.G.; Tohidifar, M. Habitat selection of cavity-nesting birds in the Hyrcanian deciduous forests of northern Iran. Ecol. Res. 2015, 30, 889–897. [Google Scholar] [CrossRef]
- Dufour-Pelletier, S.A.; Tremblay, J.; Hébert, C.; Lachat, T.; Ibarzabal, J. Testing the effect of snag and cavity supply on deadwood-associated species in a managed boreal forest. Forests 2020, 11, 424. [Google Scholar] [CrossRef] [Green Version]
- Johnsson, K. The Black Woodpecker as a Keystone Species in Forest. Report 24; The Swedish University of Agricultural Sciences, Department of Wildlife Ecology: Uppsala, Sweden, 1993. [Google Scholar]
- Wesołowski, T. Anti-predator adaptations in nesting marsh tits Parus palustris: The role of nest-site security. Ibis 2002, 144, 593–601. [Google Scholar] [CrossRef]
- Schaaf, A.A.; Tallei, E.; Ruggera, R.A.; Vivanco, C.G.; Rivera, L.; Politi, N. An assessment of the availability of cavities for secondary cavity-nesting birds in certified and conventionally-logged neotropical rainforests. IForest 2020, 13, 318–322. [Google Scholar] [CrossRef]
- Doerfler, I.; Cadotte, M.W.; Weisser, W.W.; Müller, J.; Gossner, M.M.; Heibl, C.; Bässler, C.; Thorn, S.; Seibold, S. Restoration-oriented forest management affects community assembly patterns of deadwood-dependent organisms. J. Appl. Ecol. 2020, 57, 2429–2440. [Google Scholar] [CrossRef]
- Vítková, L.; Bače, R.; Kjučukov, P.; Svoboda, M. Deadwood management in Central European forests: Key considerations for practical implementation. For. Ecol. Manag. 2018, 429, 394–405. [Google Scholar] [CrossRef]
- Christensen, M.; Hahn, K.; Mountford, E.P.; Odor, P.; Standovár, T.; Rozenbergar, D.; Diaci, J.; Wijdeven, S.; Meyer, P.; Winter, S. Dead wood in European beech (Fagus sylvatica) forest reserves. For. Ecol. Manag. 2005, 210, 267–282. [Google Scholar] [CrossRef]
- Sefidi, K. The influence of forest management histories on dead wood and habitat trees in the old growth forest in Northern Iran. Int. J. Biol. Biomol. Agric. Food Biotech. Eng 2015, 9, 1014–1018. [Google Scholar]
- Tavankar, F.; Picchio, R.; Monaco, A.L.; Bonyad, A.E. Forest management and snag characteristics in Northern Iran lowland forests. J. For. Sci. 2014, 60, 431–441. [Google Scholar] [CrossRef] [Green Version]
- Rosenvald, R.; Lõhmus, A.; Kraut, A.; Remm, L. Bird communities in hemiboreal old-growth forests: The roles of food supply, stand structure, and site type. For. Ecol. Manag. 2011, 262, 1541–1550. [Google Scholar] [CrossRef]
- Oettel, J.; Lapin, K.; Kindermann, G.; Steiner, H.; Schweinzer, K.M.; Frank, G.; Essl, F. Patterns and drivers of deadwood volume and composition in different forest types of the Austrian natural forest reserves. For. Ecol. Manag. 2020, 463, 118016. [Google Scholar] [CrossRef]
- Wesołowski, T.; Czeszczewik, D.; Rowiński, P. Effects of forest management on Three-toed Woodpecker Picoides tridactylus distribution in the Białowieża Forest (NE Poland): Conservation implications. Acta Ornithol. 2005, 40, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Czeszczewik, D.; Walankiewicz, W. Logging affects the white-backed woodpecker Dendrocopos leucotos distribution in the Białowieża Forest. Ann. Zool. Fennici 2006, 43, 221–227. [Google Scholar]
- Roberge, J.-M.; Angelstam, P.; Villard, M.-A. Specialised woodpeckers and naturalness in hemiboreal forests–deriving quantitative targets for conservation planning. Biol. Conserv. 2008, 141, 997–1012. [Google Scholar] [CrossRef]
- Sandström, U. Effects of Forest Management on Density of Tree Holes and Holenesting Birds. Report 23; Department of Wildlife Ecology, Swedish University of Agricultural Sciences: Uppsala, Sweden, 1992. [Google Scholar]
- Carlson, A. Cavity breeding birds and clearcuts. Ornis Fenn. 1994, 71, 120. [Google Scholar]
- Kenefic, L.S.; Nyland, R.D. Habitat Diversity in Uneven-Aged Northern Hardwood Stands: A Case Study; US Department of Agriculture, Forest Service, Northeastern Research Station: Washington, DC, USA, 2000. [Google Scholar]
- Moriarty, J.J.; McComb, W.C. The Long-Term Effect of Timber Stand Improvement on Snag and Cavity Densities in the Central Appalachians. In Proceedings of the Snag Habitat Management: Proceedings of the Symposium USDA Forest Service General Technical Report RM-99, Flagstaff, Arizona, 7–9 June 1983; pp. 40–44. [Google Scholar]
- Rendell, W.B.; Robertson, R.J. Nest-site characteristics, reproductive success and cavity availability for Tree Swallows breeding in natural cavities. Condor 1989, 91, 875–885. [Google Scholar] [CrossRef]
- Chillo, V.; Goldenberg, M.; Pérez-Méndez, N.; Garibaldi, L.A. Diversity, functionality, and resilience under increasing harvesting intensities in woodlands of northern Patagonia. For. Ecol. Manag. 2020, 474, 118349. [Google Scholar] [CrossRef]
- Kooch, Y.; Moghimian, N.; Wirth, S.; Haghverdi, K. Effects of shelterwood and single-tree cutting systems on topsoil quality and functions in northern Iranian forests. For. Ecol. Manag. 2020, 468, 118188. [Google Scholar] [CrossRef]
- Czeszczewik, D.; Zub, K.; Stanski, T.; Sahel, M.; Kapusta, A.; Walankiewicz, W. Effects of forest management on bird assemblages in the Bialowieza Forest, Poland. iForest-Biogeosci. For. 2015, 8, 377. [Google Scholar] [CrossRef] [Green Version]
- Aszalós, R.; Szigeti, V.; Harmos, K.; Csernák, S.; Frank, T.; Ónodi, G. Foraging activity of woodpeckers on various forms of artificially created deadwood. Acta Ornithol. 2020, 55, 63–76. [Google Scholar] [CrossRef]
- Wesołowski, T. “Lifespan” of woodpecker-made holes in a primeval temperate forest: A thirty year study. For. Ecol. Manag. 2011, 262, 1846–1852. [Google Scholar] [CrossRef]

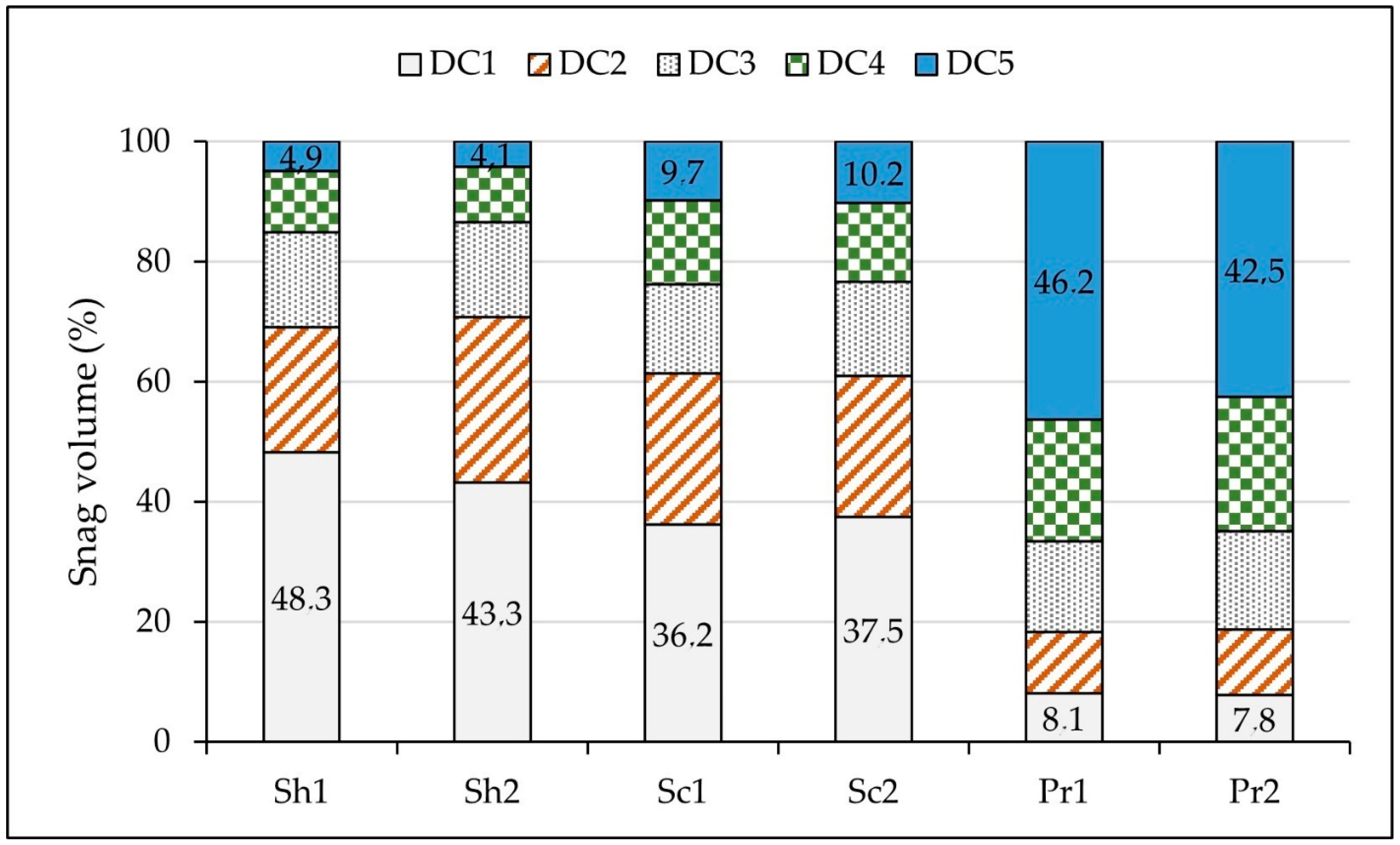


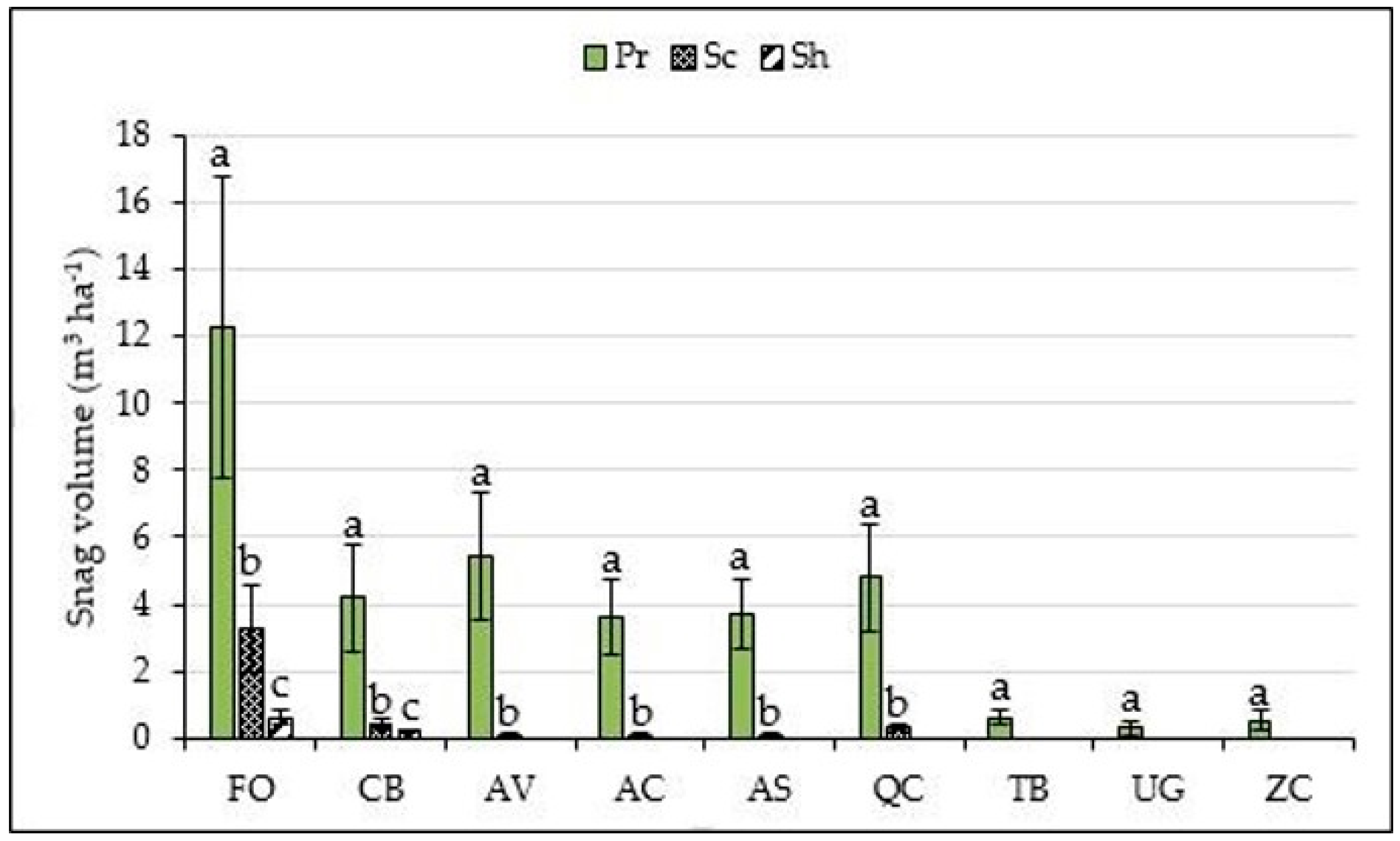
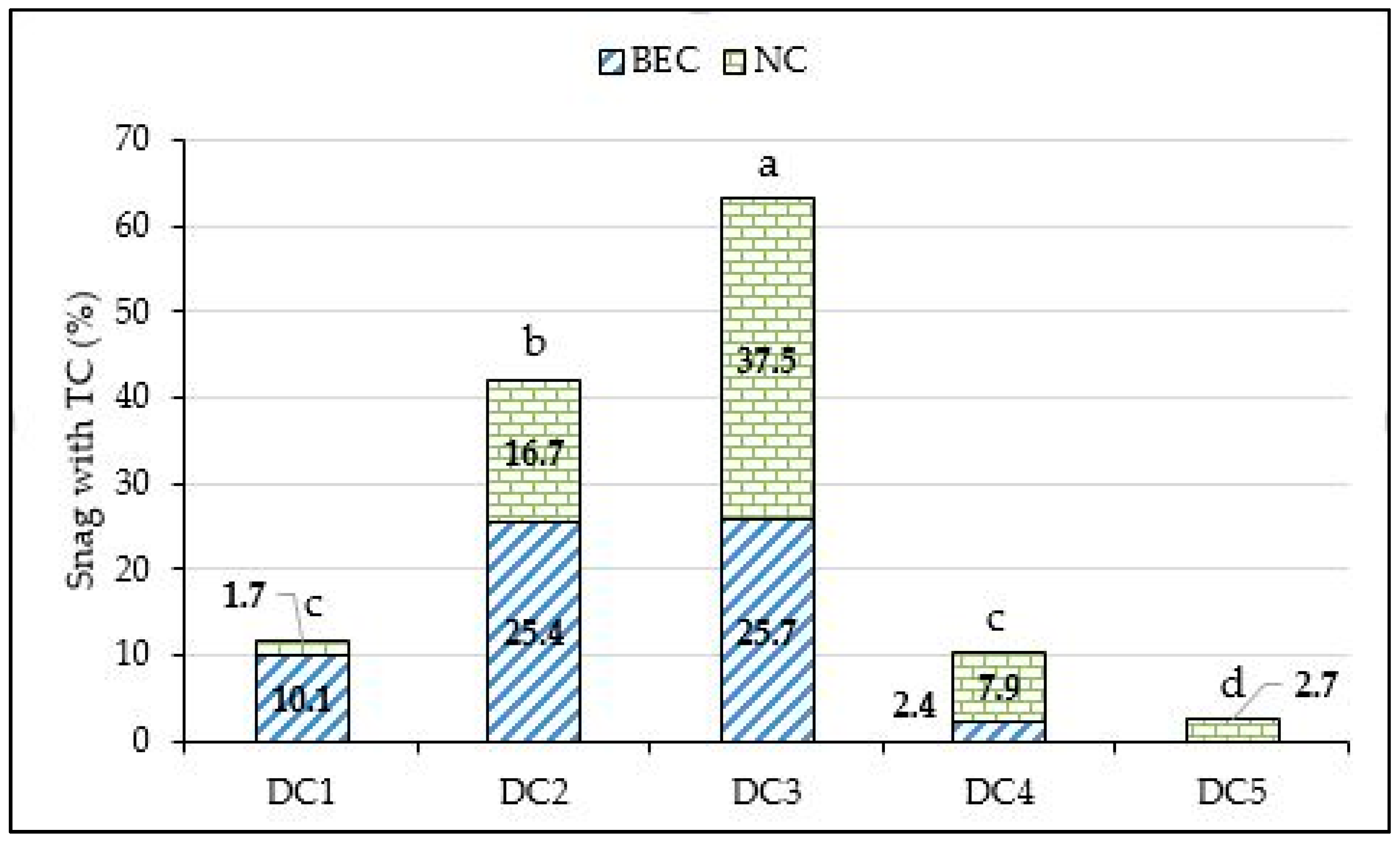
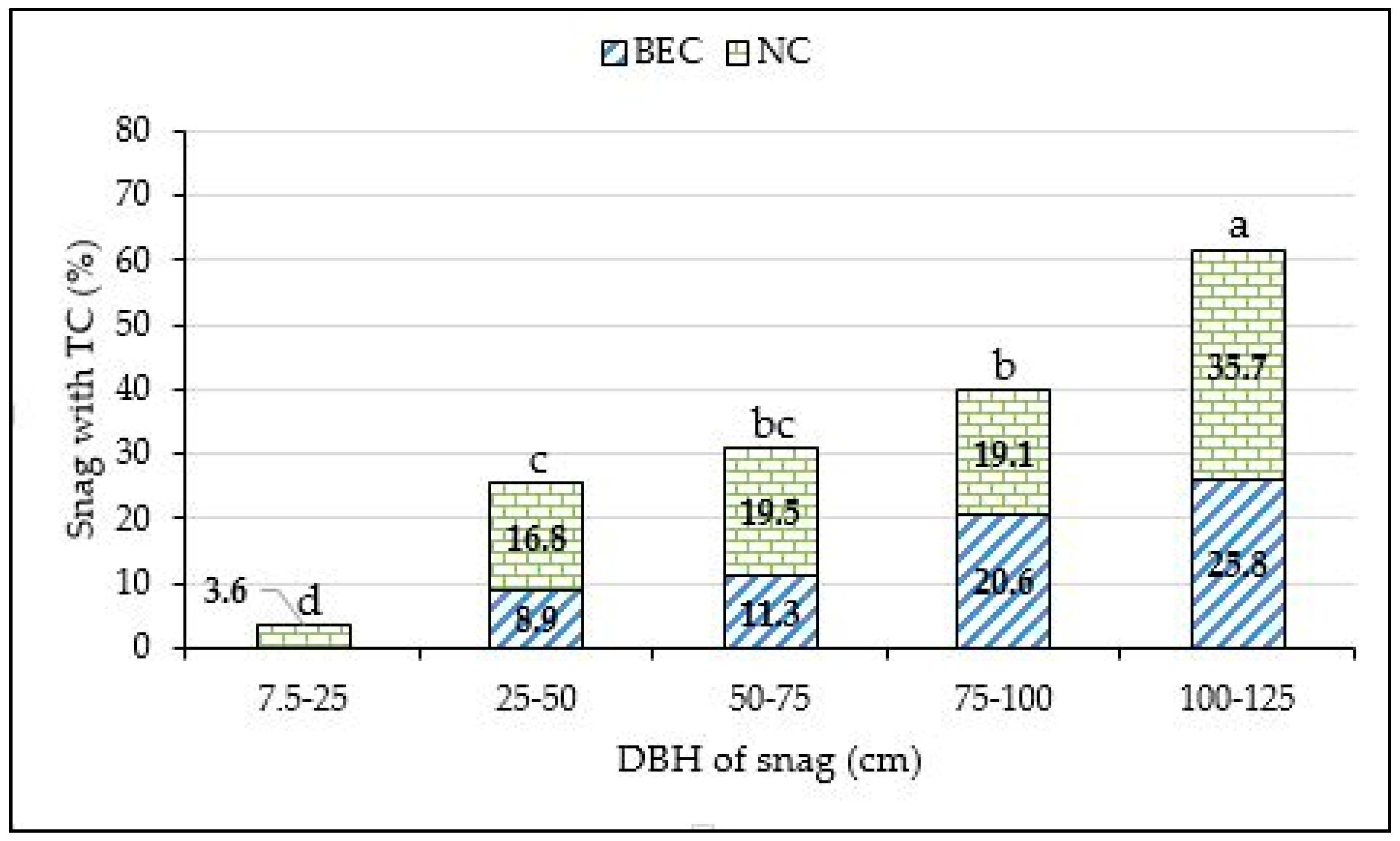
| Management Method | Area of Compartment (ha) | Average Elevation (a.s.l. m) | No. of Sample Plot | Forest Type (% of Number); DBH ≥ 7.5 cm |
|---|---|---|---|---|
| Sh1 | 45 | 1550 | 42 | FO (56.1), CB (23.0), AV (7.3), AC (4.6), AS (3.0), QC (2.1), TB (2.0), UG (1.0), ZC (0.9) |
| Sh2 | 42 | 1450 | 40 | FO (53.4), CB (26.2), AV (7.3), AC (5.2), AS (3.5), QC (2.1), TB (0.9), UG (0.9), ZC (0.5) |
| Sc1 | 45 | 1450 | 43 | FO (48.3), CB (17.6), AV (11.2), AC (9.3), AS (6.2), QC (2.4), TB (2.3), UG (1.6), ZC (1.1) |
| Sc2 | 49 | 1350 | 46 | FO (49.5), CB (14.0), AV (6.0), AC (10.3), AS (13.2), QC (0.9), TB (1.8), UG (2.8), ZC (1.5) |
| Pr1 | 52 | 1400 | 49 | FO (37.6), CB (19.7), AV (11.6), AC (9.9), AS (8.6), QC (6.9), TB (2.8), UG (2.1), ZC (0.8) |
| Pr2 | 51 | 1450 | 49 | FO (45.7), CB (15.6), AV (8.9), AC (7.3), AS (6.5), QC (5.2), TB (4.6), UG (3.4), ZC (2.8) |
| Stand Characteristics | Tree Condition | Sh1 | Sh2 | Sc1 | Sc2 | Pr1 | Pr2 | F-Value | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Density (stem ha−1) | Live | 205.7 ± 22.1 b | 210.1 ± 25.1 b | 183.5 ± 21.3 c | 189.0 ± 22.0 c | 273.4 ± 28.3 a | 270.5 ± 27.6 a | 235.9 | 0.000 |
| Snag | 6.1 ± 0.9 c | 5.3 ± 0.8 c | 11.0 ± 2.2 b | 10.3 ± 2.1 b | 35.8 ± 3.3 a | 36.6 ± 3.6 a | 152.7 | 0.000 | |
| t- test | t = 1305.5 ** | t = 1245 ** | t = 850.2 ** | t = 837.4 ** | t = 450.8 ** | t = 372.2 ** | - | - | |
| DBH (cm) | Live | 21.5 ± 2.0 c | 22.0 ± 2.1 c | 26.6 ± 2.4 b | 27.3 ± 2.0 b | 32.8 ± 4.1 a | 31.7 ± 3.7 a | 139.1 | 0.000 |
| Snag | 15.5 ± 2.2 c | 15.7 ± 2.2 c | 24.7 ± 3.4 b | 23.2 ± 3.5 b | 45.4 ± 4.1 a | 47.5 ± 4.5 a | 126.4 | 0.000 | |
| t- test | t = 17.4 ** | t = 19.6 ** | t = 3.8 N.S. | t = 4.9 N.S. | t = 32.2 ** | t = 44.6 ** | - | - | |
| Height (m) | Live | 19.8 ± 1.9 a | 19.5 ± 1.9 a | 18.7 ± 2.0 b | 17.8 ± 2.1 c | 19.3 ± 2.5 a | 17.6 ± 2.3 b | 11.2 | 0.071 |
| Snag | 8.0 ± 2.1 b | 8.4 ± 2.0 b | 8.2 ± 1.5 b | 7.8 ± 1.4 b | 14.2 ± 2.6 a | 14.0 ± 2.7 a | 140.3 | 0.000 | |
| t- test | t =32.5 ** | t = 31.7 ** | t = 29.6 ** | t = 28.4 ** | t = 13.4 ** | t = 10.2 ** | - | - | |
| Basal area (m2 ha−1) | Live | 7.7 ± 0.6 c | 7.8 ± 0.8 c | 11.8 ± 1.5 b | 12.2 ± 1.5 c | 19.6 ± 2.0 a | 19.4 ± 1.9 a | 101.6 | 0.000 |
| Snag | 0.12 ± 0.02 c | 0.10 ± 0.01 c | 0.54 ± 0.05 b | 0.45 ± 0.05 b | 4.17 ± 0.8 a | 4.76 ± 2.1 a | 95.7 | 0.000 | |
| t- test | t = 438.3 ** | t = 496.1 ** | t = 344.4 ** | t = 350.9 ** | t = 107.6 ** | t = 98.2 ** | - | - | |
| Standing volume (m3 ha−1) | Live | 91.5 ± 10.1 c | 92.7 ± 10.1 c | 133.7 ± 15.0 b | 131.5 ± 13.8 b | 210.5 ± 15.1 a | 205.8 ± 14.5 a | 186.5 | 0.000 |
| Snag | 0.72 ± 0.07 c | 0.70 ± 0.06 c | 4.48 ± 0.86 b | 4.27 ± 0.72 b | 28.94 ± 3.75 a | 39.21 ± 4.40 a | 99.6 | 0.000 | |
| t- test | t = 1450.8 ** | t = 1394.0 ** | t = 170.3 ** | t = 195.6 ** | t = 119.7 ** | t = 100.4 ** | - | - |
| BCN Characteristics | Sh1 | Sh2 | Sc1 | Sc2 | Pr1 | Pr2 | F-Value | p-Value |
|---|---|---|---|---|---|---|---|---|
| Snags with TC (%) | 19.3 ± 2.7 c | 20.4 ± 2.5 c | 33.0 ± 3.1 b | 27.1 ± 2.8 b | 59.7 ± 3.5 a | 63.5 ± 3.9 a | 184.4 | 0.000 |
| Frequency of TC (N ha−1) | 1.2 ± 0.6 c | 1.2 ± 0.7 c | 5.5 ± 0.8 b | 4.7 ± 0.9 b | 70.5 ± 3.9 a | 81.3 ± 4.1 a | 140.3 | 0.000 |
| Frequency of BEC (N ha−1) | 0.3 ± 0.1 c | 0.2 ± 0.1 c | 2.2 ± 0.4 b | 2.3 ± 0.3 b | 25.1 ± 3.6 a | 28.9 ± 3.5 a | 106.8 | 0.000 |
| Frequency of NC (N ha−1) | 0.9 ± 0.3 c | 1.0 ± 0.3 c | 3.3 ± 0.4 b | 2.4 ± 0.3 b | 45.4 ± 5.8 a | 52.4 ± 6.3 a | 117.0 | 0.000 |
| Intensity of TC (N snag−1) | 1.0 ± 0.05 c | 1.1 ± 0.05 c | 1.5 ± 0.11 b | 1.7 ± 0.12 b | 3.3 ± 0.62 a | 3.5 ± 0.70 a | 95.7 | 0.000 |
| Height of TC from ground (m) | 7.1 ± 0.7 a | 7.7 ± 1.0 a | 9.0 ± 1.1 a | 9.0 ± 1.2 a | 8.7 ± 1.0 a | 8.8 ± 1.1 a | 9.68 | 0.204 |
| Height of BEC from ground (m) | 7.7 ± 0.6 a | 7.6 ± 0.7 a | 9.2 ± 0.8 a | 9.3 ± 1.0 a | 8.9 ± 1.5 a | 8.5 ± 1.5 a | 3.24 | 0.102 |
| Height of NC from ground (m) | 6.9 ± 0.5 a | 7.0 ± 0.7 a | 8.9 ± 1.1 a | 8.8 ± 1.0 a | 8.5 ± 1.4 a | 9.0 ± 1.6 a | 1.80 | 0.110 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavankar, F.; Latterini, F.; Nikooy, M.; Venanzi, R.; Naghdi, R.; Picchio, R. Influence of Forest Management and Sylvicultural Treatments on Abundance of Snags and Tree Cavities in Mountain Mixed Beech Forests. Environments 2021, 8, 55. https://doi.org/10.3390/environments8060055
Tavankar F, Latterini F, Nikooy M, Venanzi R, Naghdi R, Picchio R. Influence of Forest Management and Sylvicultural Treatments on Abundance of Snags and Tree Cavities in Mountain Mixed Beech Forests. Environments. 2021; 8(6):55. https://doi.org/10.3390/environments8060055
Chicago/Turabian StyleTavankar, Farzam, Francesco Latterini, Mehrdad Nikooy, Rachele Venanzi, Ramin Naghdi, and Rodolfo Picchio. 2021. "Influence of Forest Management and Sylvicultural Treatments on Abundance of Snags and Tree Cavities in Mountain Mixed Beech Forests" Environments 8, no. 6: 55. https://doi.org/10.3390/environments8060055
APA StyleTavankar, F., Latterini, F., Nikooy, M., Venanzi, R., Naghdi, R., & Picchio, R. (2021). Influence of Forest Management and Sylvicultural Treatments on Abundance of Snags and Tree Cavities in Mountain Mixed Beech Forests. Environments, 8(6), 55. https://doi.org/10.3390/environments8060055









