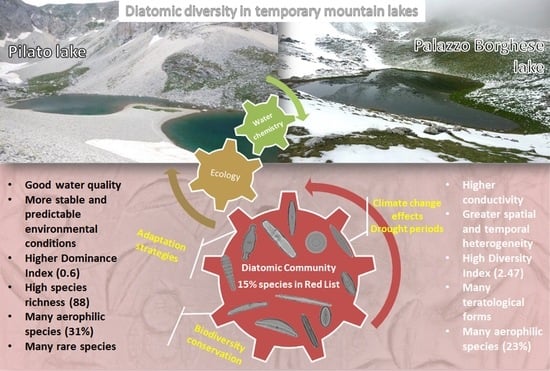
The Diatomic Diversity of Two Mediterranean High-Elevation Lakes in the Sibillini Mountains National Park (Central Italy)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
2.3. Laboratory Preparation and Techniques
3. Results
3.1. Environmental Characterization
3.2. Diatom Communities
3.3. Ecological Preferences
4. Discussion
Future Perspectives and Management Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morin, S.; Gómez, N.; Tornés, E.; Licursi, M.; Rosebery, J. Benthic diatom monitoring and assessment of freshwater environments: Standard methods and future challenges. In Aquatic Biofilms: Ecology, Water Quality and Wastewater Treatment; Romaní, A.M., Guasch, H., Balaguer, M.D., Eds.; Caister Academic Press: Norfolk, UK, 2016; pp. 111–124. [Google Scholar] [CrossRef] [Green Version]
- Falkowski, P.G.; Fenchel, T.; Delong, E.F. The microbial engines that drive Earth’s biogeochemical cycles. Microb. Ecol. 2008, 320, 1034–1039. [Google Scholar] [CrossRef] [Green Version]
- Soininen, J.; Jamoneau, A.; Rosebery, J.; Passy, S.I. Global patterns and drivers of species and trait composition in diatoms. Glob. Ecol. Biogeogr. 2016, 25, 940–950. [Google Scholar] [CrossRef]
- Poulíčková, A.; Manoylov, K. Ecology of freshwater diatoms. Current trends and applications. In Diatoms: Fundamentals and Applications; Seckbach, J., Gordon, R., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 289–310. [Google Scholar] [CrossRef]
- Blanco, S.; Olenici, A.; Ortega, F.; Jiménez-Gómez, F.; Guerrero, F. Identifying environmental drivers of benthic diatom diversity: The case of Mediterranean mountain ponds. PeerJ 2020, 8, e8825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixit, S.S.; Smol, J.P.; Kingston, J.C.; Charles, D.F. Diatoms: Powerful indicators of environmental change. Environ. Sci. Technol. 1992, 26, 23–33. [Google Scholar] [CrossRef]
- Feret, L.; Bouchez, A.; Rimet, F. Benthic diatom communities in high altitude lakes: A large scale study in the French Alps. Ann. Limnol. Int. J. Limnol. 2017, 53, 411–423. [Google Scholar] [CrossRef] [Green Version]
- Todeschini, S.; Papiri, S.; Sconfietti, R. Impact assessment of urban wet-weather sewer discharges on the Vernavola river (Northern Italy). Civ. Eng. Environ. Syst. 2017, 28, 209–229. [Google Scholar] [CrossRef]
- Rzodkiewicz, M.; Gąbka, M.; Szpikowska, G.; Woszczyk, M. Diatom assemblages as indicators of salinity gradients: A case study from a coastal lake. Oceanol. Hydrobiol. Stud. 2017, 46, 325–339. [Google Scholar] [CrossRef]
- Lane, C.R.; Brown, M.T. Diatoms as indicators of isolated herbaceous wetland condition in Florida, USA. Ecol. Indic. 2007, 7, 521–540. [Google Scholar] [CrossRef]
- Pellegrino, L.; Dela Pierre, F.; Natalicchio, M.; Carnevale, G. The Messinian diatomite deposition in the Mediterranean region and its relationships to the global silica cycle. Earth Sci. Rev. 2018, 178, 154–176. [Google Scholar] [CrossRef]
- Pellegrino, L.; Abeb, K.; Gennaria, R.; Lozara, F.; Dela Pierre, F.; Natalicchio, M.; Mikami, Y.; Jordanc, R.W.; Carnevale, G. Integrated micropaleontological study of the Messinian diatomaceous deposits of the Monferrato Arc (Piedmont basin, NW Italy): New insights into the paleoceanographic evolution of the northernmost Mediterranean region. Mar. Micropaleontol. 2020, 160, 101910. [Google Scholar] [CrossRef]
- Ognjanova-Rumenova, N.; Wojtal, A.Z.; Sienkiewicz, E.; Botev, I.; Trichkova, T. Biodiversity of high mountain lakes in Europe with special regards to Rila Mountains (Bulgaria) and Tatra Mountains (Poland). In Diatoms: Fundamentals and Applications; Seckbach, J., Gordon, R., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 335–354. [Google Scholar] [CrossRef]
- Catalan, J.; Camarero, L.; Felip, M.; Pla, S.; Ventura, M.; Buchaca, T.; Bartumeus, F.; de Mendoza, G.; Miró, A.; Casamayor, E.O.; et al. High mountain lakes: Extreme habitats and witnesses of environmental changes. Limnetica 2006, 25, 551–584. [Google Scholar]
- Carosi, A.; Barelli, M.G.; Ambrosi, A.; Rossetti, A.; Padula, R.; Bifulco, C.; Morandi, F.; Lorenzoni, M. Conservation status of Chirocephalus marchesonii Ruffo & Vesentini, 1957 in the Pilato Lake (Sibillini Mountains National Park, Central Italy). Fundam. Appl. Limnol. 2021, 194, 171–185. [Google Scholar] [CrossRef]
- Marchesoni, V.; Moretti, G. Appunti idrobiologici sul Lago di Pilato nei Monti Sibillini. Boll. Della Soc. Eustachiana 1954, 3, 131–144. [Google Scholar]
- Cottarelli, V.; Mura, G. Una nuova specie di Anostraco (Crustacea, Branchiopoda) dell’Italia Peninsulare: Chirocephalus sibyllae. Boll. Zool. 1975, 42, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Petitta, M.; Mastrorillo, L.; Preziosi, E.; Banzato, F.; Barberio, M.D.; Billi, A.; Doglioni, C. Water-table and discharge changes associated with the 2016–2017 seismic sequence in central Italy: Hydrogeological data and a conceptual model for fractured carbonate aquifers. Hydrogeol. J. 2018, 26, 1009–1026. [Google Scholar] [CrossRef] [Green Version]
- Martarelli, L.; Gafà, R.M.; Guarino, P.M.; Monti, G.M.; Puzzilli, L.M.; Silvi, A. The Pilato Lake (Sibillini Mts., Central Italy): First results of a study on the supposed variations of its hydrogeological conditions induced by the seismic sequence 2016–2017. Ital. J. Groundw. 2019, 8, 23–28. [Google Scholar] [CrossRef]
- Belk, D. Evolution of egg size strategies in fairy shrimps. Southwest. Nat. 1977, 22, 99–105. [Google Scholar] [CrossRef]
- Mura, G.; Calzecchi-Onesti, B. Osservazioni sul ciclo biologico in natura di Chirocephalus sibyllae. Riv. Idrobiol. 1983, 22, 215–227. [Google Scholar]
- Toro, M.; Granados, I. Restoration of a small high mountain lake after recent tourist impact: The importance of limnological monitoring and palaeolimnology. Water Air Soil Pollut. 2002, 2, 295–310. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, B.; Zhang, J.; Wang, L.; Pan, Y.; Wang, Q.; Jian, D.; Deng, G. Lake macroinvertebrate assemblages and relationship with natural environment and tourism stress in Jiuzhaigou Natural Reserve, China. Ecol. Indic. 2016, 62, 182–190. [Google Scholar] [CrossRef]
- Agenzia Per la Protezione Dell’Ambiente e Per i Servizi Tecnici, Consiglio Nazionale delle Ricerche, & Istituto di Ricerca Sulle Acque (APAT). Analytical Methods for Water. Manuals and Guidelines; IGER SRL: Rome, Italy, 2003. Available online: http://www.isprambiente.gov.it/it/pubblicazioni/manuali-e-linee-guida/metodi-analitici-per-le-acque (accessed on 11 August 2021).
- Clesceri, L.S.; Greenberg, A.E.; Eaton, A.D.; American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF). Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- ISPRA. Manuali e Linee Guida 111/2014 Metodi Biologici per le Acque Superficiali Interne; ISPRA: Rome, Italy, 2014. Available online: https://www.isprambiente.gov.it/files/pubblicazioni/manuali-lineeguida/MLG__111_2014_Metodi_Biologici_acque.pdf (accessed on 11 August 2021).
- CEMAGREF. Etude de Méthodes Biologiques Quantitatives d’Appreciation de la Qualité des eaux. Rapport. Qualité des Eaux Lyon; Agence financiè de Bassin Rhone-Méditerarée: Pierre-Bénite, France, 1982. [Google Scholar]
- Rott, E.; Hofmann, G.; Pall, K.; Pfister, P.; Pipp, E. Indikationslisten fur Aufwurchsalgen. Teil 1. Saprobielle Indikation; Bundesministerium fur Land und Forstwirtschaft: Wien, Austria, 1999. [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Falasco, E.; Bona, F.; Badino, G.; Hoffmann, L.; Ector, L. Diatom teratological forms and environmental alteration: A review. Hydrobiologia 2009, 623, 1–35. [Google Scholar] [CrossRef]
- Lange-Bertalot, H.; Rupperl, M. Zur Revision Taxonomisch Problematischer, Ökologisch Jedoch Wichtiger Sippen der Gattung Achnanthes BORY. Arch. Hydrobiol. 1980, 60, 1–31. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Süßwasserflora von Mitteleuropa. In Bacillariophyceae. 2/1 Naviculaceae; 2/2 Bacillariaceae, Epithemiaceae, Surirellaceae; 2/3 Centrales, Fragilariaceae, Eunotiaceae; 2/4 Achnanthaceae, Kritische erganzungen zu Achnanthes sl, Navicula s.str., Gomphonema; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Spektrum: Berlin, Germany, 1986–1991. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Süßwasserflora von Mitteleuropea. Bacillariophyceae. Achnanthaceae; G. Fisher Verlag: Stuttgart, Germany, 2004. [Google Scholar]
- Lange-Bertalot, H.; Krammer, K. Diatoms of Europe 1 The genus Pinnularia; 2 Navicula Sensu Stricto, 10 Genera Separated from Navicula Sensu lato, Frustulia; 3 Cymbella; Vol.4 Cymbopleura, Delicata, Navicymbula, Gomphocymbellopsis, Afrocymbella; 5 Amphora sensu lato; Koeltz Scientific Books: Königstein, Germany, 2000–2004. [Google Scholar]
- Houk, V.; Klee, R.; Tanaka, H. Atlas of freshwater centric diatoms with a brief key and descriptions. Part III: Stephanodiscaceae A. Fottea 2010, 10, 1–498. [Google Scholar]
- Bey, M.Y.; Ector, L. Atlas des diatomées des cours d’eau de la région Rhône-Alpes. 1 Centriques, Monoraphidées; 2 Araphidées, Brachyraphidées; 3 Naviculacées, Naviculoidées; 4 Naviculacées, Naviculoidées; 5 Naviculacées Cymbelloidées, Gomphonématoidées; 6 Bacillariacées, Rhopalodiacées, Surirellacées; Direction régionale de l’Environnement, de l’Aménagement et du Logement Rhône-Alpes. 2013. Available online: http://www.rhone-alpes.developpement-durable.gouv.fr (accessed on 11 August 2021).
- Hofmann, G.; Werum, M.; Lange-Bertalot, H. Diatomeen im Süßwasser—Benthos von Mitteleuropa. Bestimmungsflora Kieselalgen für die Ökologische Praxis. Über 700 der Häufigsten Arten und ihre Ökologie; Koeltz Scientific Books: Königstein, Germany, 2013. [Google Scholar]
- van Dam, H.; Mertens, A.; Sinkeldam, J. A coded checklist and ecological indicator values of freshwater diatoms from Netherland. Neth. J. Aquat. Ecol. 1994, 28, 117–133. [Google Scholar]
- Lange-Bertalot, H.; Steindorf, A. Rote Liste der limnischen Kieselalgen (Bacillariophyceae) Deutschlands. Schr. Veg. 1996, 28, 633–675. [Google Scholar]
- Lange-Bertalot, H. Diatomeen im Süßwasser—Benthos von Mitteleuropa; Koeltz Scientific Books: Königstein, Germany, 2013; p. 908. [Google Scholar]
- Cianficconi, F.; Moretti, G.P.; Pirisinu, Q.; Tucciarelli, F. Composizione sistematica delle comunità acquatiche del settore meridionale dei Monti Sibillini, con considerazioni zoogeografiche. J. Integr. Biogeogr. 1979, 6, 479–523. [Google Scholar] [CrossRef] [Green Version]
- Falasco, E.; Mobili, L.; Risso, A.M.; Bona, F. Considerazioni sull’applicazione dell’indice diatomico ICMi (Intercalibration Common Metric index) nell’Italia nord-occidentale. Biol. Ambient. 2012, 26, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Winder, M.; Reuter, J.E.; Schladow, S.G. Lake warming favours small-sized planktonic diatom species. Proc. R. Soc. B 2009, 276, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Saros, J.E.; Anderson, N.J. The ecology of the planktonic diatom Cyclotella and its implications for global environmental change studies. Biol. Rev. 2015, 90, 522–541. [Google Scholar] [CrossRef]
- Bramburger, A.J.; Reavie, E.D.; Sgro, G.V.; Estepp, L.R.; Shaw Chraïbi, V.L.; Pillsbury, R.W. Decreases in diatom cell size during the 20th century in the Laurentian Great Lakes: A response to warming waters? J. Plankton Res. 2017, 39, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Litchman, E.; Klausmeier, C.A.; Miller, J.R.; Schofield, O.M.; Falkowski, P.G. Multi-nutrient, multi-group model of present and future oceanic phytoplankton communities. Biogeosciences 2006, 3, 585–606. [Google Scholar] [CrossRef] [Green Version]
- Falasco, E.; Ector, L.; Wetzel, C.E.; Badino, G.; Bona, F. Looking back, looking forward: A review of the new literature on diatom teratological forms (2010–2020). Hydrobiologia 2021, 848, 1675–1753. [Google Scholar] [CrossRef]
- Antonarakou, A.; Kontakiotis, G.; Zarkogiannis, S.; Mortyn, P.G.; Drinia, H.; Koskeridou, E.; Anastasakis, G. Planktonic foraminiferal abnormalities in coastal and open marine eastern Mediterranean environments: A natural stress monitoring approach in recent and early Holocene marine systems. J. Mar. Syst. 2018, 181, 63–78. [Google Scholar] [CrossRef]



| Pilato Lake | Palazzo Borghese Lake | |||||
|---|---|---|---|---|---|---|
| Environmental Variables and Diatomic Indices | Range | Mean ± SD | Range | Mean ± SD | t | p |
| Water temperature (°C) | 11.3–16.8 | 14.53 ± 2.03 | 7.8–19.8 | 13.8 ± 13.8 | 0.11 | 0.915 |
| Dissolved oxygen (mg L−1) | 6.4–8.8 | 7.95 ± 0.95 | 8–9.8 | 8.9 ± 1.27 | 1.26 | 0.275 |
| Chlorides (mg L−1) | 6–19 | 12.13 ± 3.76 | 14–23 | 18.5 ± 6.4 | 2.15 | 0.097 |
| Sulphates (mg L−1) | 0 | 0 | 0 | 0 | - | - |
| Ammonia (mg L−1 NH4+) | 0.053–0.125 | 0.08 ± 0.02 | 0.06–0.063 | 0.0615 ± 0.002 | 0.99 | 0.375 |
| Nitrates (mg L−1 N–NO3) | 0.5–1.6 | 0.75 ± 0.39 | 1.3–0.6 | 0.45 ± 0.21 | 1.23 | 0.285 |
| Nitrites (mg L−1 N-NO2) | 0.05–0.09 | 0.07 ± 0.01 | 0.05–0.07 | 0.06 ± 0.01 | 0.44 | 0.685 |
| Phosphates (mg L−1 P–PO4) | 0.03–0.10 | 0.059 ± 0.03 | 0.09–0.22 | 0.155 ± 0.092 | 2.17 | 0.096 |
| BOD5 (mg L−1) | 0.6–4.8 | 2.1 ± 1.66 | 0.8–1.8 | 1.3 ± 0.71 | 0.36 | 0.736 |
| COD (mg L−1) | 3.5–11.6 | 8.96 ± 2.91 | 8–13.3 | 10.65 ± 3.75 | 0.62 | 0.568 |
| Conductivity (μS cm−1) | 132–180 | 151.38 ± 16.84 | 197–203 | 200 ± 4.24 | 3.73 | 0.020 |
| pH (units) | 7.4–8.5 | 7.94 ± 0.58 | 8.28–8.6 | 8.44 ± 0.23 | 1.04 | 0.358 |
| IPS | 19.2–19.6 | 19.4 ± 0.14 | 12.7–17.2 | 14.47 ± 2.40 | 4.80 | 0.001 |
| TI | 1.17–1.34 | 1.24 ± 0.07 | 2.26–2.84 | 2.55–0.29 | 10.08 | 0.026 |
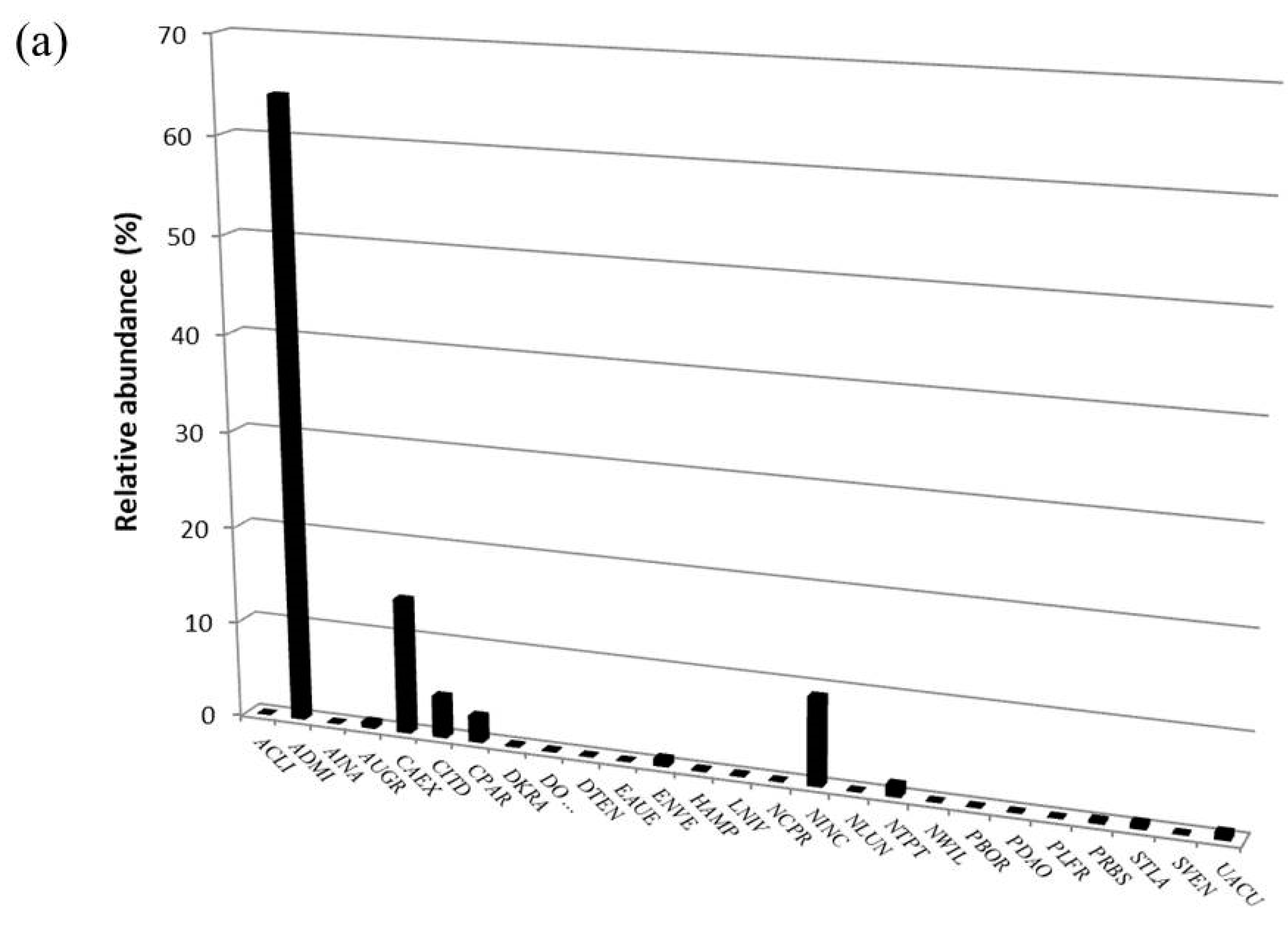
| Taxon | OMNIDIA Code | Pilato Lake | Palazzo Borghese Lake | IPS Sensitivity | TI Sensitivity | Threatened Status |
|---|---|---|---|---|---|---|
| Achnanthes coarctata (Brebisson) Grunow and Grun. 1880 | ACOA | X | X | 4.5 | 0.9 | ? |
| Achnanthidium lineare W. Smith 1999 | ACLI | X | 5.0 | 1.8 | 3 | |
| Achnanthidium minutissimum (Kützing) Czarnecki 1994 | ADMI | X | X | 5.0 | 1.2 | ? |
| Amphora inariensis Krammer 1980 | AINA | X | X | 5.0 | 2.1 | 3 |
| Amphora meridionalis Levkov 2009 | AMDN | X | 2.6 | Z | ||
| Amphora ovalis (Kützing) Kützing var. ovalis 1844 | AOVA | X | 3.0 | 3.3 | ? | |
| Aulacoseira granulata (Ehr.) Simonsen 1979 | AUGR | X | X | 2.9 | ? | |
| Aulacoseira granulata var. angustissima (Ehr.) Simonsen var. angustissima (O.M.) Simonsen 1979 | AUGA | X | X | 2.8 | ? | |
| Caloneis silicula (Ehr.) Cleve 1894 | CSIL | X | 4.5 | 2.5 | * | |
| Cocconeis euglypta Ehrenberg emend Romero and Jahn 1854 | CEUG | X | 3.6 | 2.3 | ? | |
| Cocconeis euglyptoides (Geitler) Lange-Bertalot 2004 | CEUO | X | 3.5 | Z | ||
| Cocconeis pediculus Ehrenberg 1838 | CPED | X | 4.0 | 2.6 | ? | |
| Craticula ambigua (Ehrenberg) Mann 1990 | CAMB | X | 3.0 | * | ||
| Craticula molestiformis (Hustedt) Lange-Bertalot 2000 | CMLF | X | X | 2.0 | 2.9 | ? |
| Cyclotella intermedia (Manguin) Houk Klee and Tanaka 2010 | CITD | X | X | 5.0 | Z | |
| Cyclotella meneghiniana Kützing 1844 | CMEN | X | 2.0 | 2.8 | ? | |
| Cymbella excisa Kützing 1844 | CAEX | X | 4.0 | Z | ||
| Cymbella excisa var. procera Krammer 2002 | CEPR | X | 4.0 | Z | ||
| Cymbella parva (W.Sm.) Kirchner 1878 | CPAR | X | X | 5.0 | Z | |
| Cymbella perparva Krammer 2002 | CPPV | X | 5.0 | Z | ||
| Cymbella vulgata var. plitvicensis Krammer 2002 | CVPL | X | Z | |||
| Cymbopleura yateana (Maillard) Krammer and Lange-Bertalot 1998 | CBYA | X | 4.8 | Z | ||
| Denticula tenuis Kützing 1844 | DTEN | X | 5.0 | 1.4 | * | |
| Diadesmis contenta (Grunow ex V. Heurck) Mann 1990 | DCOT | X | 4.0 | ? | ||
| Diadesmis perpusilla (Grunow) D.G. Mann et al. 1990 | DPER | X | 5.0 | 1.2 | ? | |
| Diatoma moniliformis Kützing 1833 | DMON | X | 4.0 | 2.0 | ? | |
| Diploneis krammerii Lange-Bertalot and Reichardt 2000 | DKRA | X | 4.0 | V | ||
| Diploneis oculata (Brebisson) Cleve 1894 | DOCU | X | 4.0 | 1.0 | * | |
| Encyonema auerswaldi Rabenhorst 1853 | EAUE | X | 4.0 | 2.1 | Z | |
| Encyonema lange-bertalotii Krammer morphotype 1 1997 | ENLB | X | 4.0 | Z | ||
| Encyonema minutum (Hilse in Rabh.) Mann and Mann 1990 | ENMI | X | 4.6 | 2.6 | * | |
| Encyonema ventricosum (Agardh) Grunow et al. 1875 | ENVE | X | 4.0 | * | ||
| Epithemia adnata (Kützing) Brébisson 1838 | EADN | X | 4.0 | 2.2 | ? | |
| Epithemia sorex Kützing 1844 | ESOR | X | 4.0 | 2.7 | ? | |
| Eucocconeis laevis (Oestrup) Lange-Bertalot 1999 | EULA | X | 5.0 | 1.2 | * | |
| Eunotia arcus Ehrenberg 1838 | EARC | X | 5.0 | 1.0 | 2 | |
| Eunotia bilunaris (Ehr.) Mills 1934 | EBIL | X | 5.0 | 1.7 | ? | |
| Eunotia minor (Kützing) Grunow 1881 | EMIN | 4.6 | 1.5 | * | ||
| Fallacia insociabilis (Krasske) Mann 1990 | FINS | X | X | 3.0 | * | |
| Fistulifera saprophila (Lange-Bertalot and Bonik) Lange-Bertalot 1997 | FSAP | X | 2.0 | 2.6 | ? | |
| Fragilaria acidoclinata Lange-Bertalot and Hofmann 1993 | FACD | X | 5.0 | G | ||
| Gomphonema angustatum (Kützing) Rabenhorst 1864 | GANG | X | 3.0 | * | ||
| Gomphonema cuneolus Reichardt 1997 | GCUN | X | 5.0 | Z | ||
| Gomphonema cymbelliclinum Reichardt and Lange-Bertalot, 1999 | GCBC | X | 3.8 | Z | ||
| Gomphonema drutelingense Reichardt 1999 | GDRU | X | 3.8 | Z | ||
| Gomphonema longilineare Reichardt 1999 | GLGL | X | Z | |||
| Gomphonema micropus Kützing 1844 | GMIC | X | X | 3.0 | 2.0 | * |
| Gomphonema minutum (Ag.) Agardh 1831 | GMIN | X | X | 4.0 | 2.0 | ? |
| Gomphonema olivaceum (Hornemann) Brébisson 1838 | GOLI | X | 4.6 | 2.1 | ? | |
| Gomphonema pumilum (Grunow) Reichardt and Lange-Bertalot, 1991 | GPUM | X | 4.5 | 1.6 | * | |
| Gomphonema rosenstokianum Lange-Bertalot and Reichardt 1993 | GROS | X | 5.0 | Z | ||
| Gomphonema sarcophagus Gregory 1856 | GSAR | X | 3.2 | 1.3 | V | |
| Gomphonema tergestinum (Grunow) Fricke 1902 | GTER | X | X | 4.0 | 1.4 | G |
| Gyrosigma attenuatum (Kützing) Rabenhorst 1853 | GYAT | X | 4.0 | 2.6 | ? | |
| Gyrosigma sciotense (Sullivan and Wormley) Cleve 1894 | GSCI | X | 4.0 | 2.7 | * | |
| Hantzschia abundans Lange-Bertalot 1993 | HABU | X | X | 1.5 | 3.6 | ? |
| Hantzschia amphioxys (Ehr.) Grunow and Grunow 1880 | HAMP | X | X | 1.5 | 3.6 | ? |
| Hantzschia calcifuga Reichardt and Lange-Bertalot 2004 | HCAL | X | D | |||
| Luticola binodis (Hustedt) M.B. Edlund 2001 | LBIN | X | D | |||
| Luticola mutica (Kützing) Mann et al. 1990 | LMUT | X | X | 2.0 | 2.9 | ? |
| Luticola nivalis (Ehrenberg) Mann Crawford and Mann 1990 | LNIV | X | 5.0 | 2.9 | D | |
| Meridion circulare (Greville) Agardh 1831 | MCIR | X | X | 4.2 | 2.5 | ? |
| Muelleria gibbula (Cleve) Spaulding and Stoermer 1997 | MUGI | X | 4.9 | G | ||
| Navicula capitatoradiata Germain 1981 | NCPR | X | 3.0 | 3.3 | ? | |
| Navicula cryptotenella Lange-Bertalot 1985 | NCTE | X | 4.0 | 2.3 | ? | |
| Navicula densilineolata (Lange-Bertalot) Lange-Bertalot 1993 | NDSL | X | 5.0 | 3 | ||
| Navicula gregaria Donkin 1861 | NGRE | X | 3.4 | 3.5 | ? | |
| Navicula lanceolata (Agardh) Ehrenberg 1838 | NLAN | X | 3.8 | 3.5 | ? | |
| Navicula lundi Reichardt 1988 | NLUN | X | X | 4.8 | D | |
| Navicula microdigitoradiata Lange-Bertalot 1993 | NMDG | X | 3.0 | R | ||
| Navicula reichardtiana Lange-Bertalot 1989 | NRCH | X | 3.6 | 2.3 | ? | |
| Navicula tripunctata (Müller) Bory 1822 | NTPT | X | 4.4 | 3.1 | ? | |
| Navicula trivialis Lange-Bertalot 1980 | NTRV | X | 2.0 | 3.3 | ? | |
| Navicula wildii Lange-Bertalot 1993 | NWIL | X | X | 0.3 | 3 | |
| Neidium bisulcatum (Lagerstedt) Cleve 1894 | NBIS | X | X | 5.0 | 0.6 | 3 |
| Neidium longiceps (Gregory) Ross 1947 | NLGI | X | 4.0 | 0.6 | G | |
| Nitzschia acidoclinata Lange-Bertalot 1976 | NACD | X | 5.0 | 2.3 | * | |
| Nitzschia angustata (Smith) Grunow 1880 | NIAN | X | * | |||
| Nitzschia clausii Hantzsch 1860 | NCLA | X | 2.8 | 3.9 | ? | |
| Nitzschia dissipata (Kützing) Grunow 1862 | NDIS | X | 4.0 | 2.4 | ? | |
| Nitzschia inconspicua Grunow 1862 | NINC | X | 2.8 | 3.1 | ? | |
| Nitzschia linearis (Agardh) Smith 1853 | NLIN | X | 3.0 | 3.4 | ? | |
| Nitzschia pusilla (Kützing) Grunow 1862 | NIPU | X | 2.0 | 2.7 | ? | |
| Nitzschia subtilis Grunow and Grunow 1880 | NISU | X | 3.0 | 3.9 | * | |
| Nitzschia sociabilis Hustedt 1957 | NSOC | X | 3.0 | 2.8 | * | |
| Nitzschia supralitorea Lange-Bertalot 1979 | NZSU | X | 1.5 | 2.9 | ? | |
| Pinnularia borealis Ehrenberg 1843 | PBOR | X | X | 5.0 | 1.9 | ? |
| Pinnularia grunowii Krammer 2000 | PGRU | X | X | Z | ||
| Pinnularia microstauron var. angusta (Ehr.) Krammer 2000 | PMIA | X | X | 4.0 | Z | |
| Pinnularia obscura Hustedt 1927 | POMT | X | 3 | Z | ||
| Pinnularia rupestris Hantzsch 1861 | PRUP | X | 4.2 | G | ||
| Pinnularia schoenfelderi Krammer 1992 | PSHO | X | X | 4.5 | G | |
| Placoneis placentula (Ehr.) Heinzerling 1908 | PPLC | X | X | 4.0 | 1.6 | V |
| Planothidium frequentissimum (Lange-Bertalot) Lange-Bertalot 1999 | PLFR | X | 3.4 | 2.8 | ? | |
| Planothidium joursacense (Héribaud) Lange-Bertalot 1999 | PJOU | X | 3.0 | 3 | ||
| Planothidium lanceolatum (Brebisson) Lange-Bertalot 1999 | PTLA | X | 4.6 | 3.3 | ? | |
| Psammothidium daonense (Lange-Bertalot) Lange-Bertalot 1999 | PDAO | X | 4.5 | 1.1 | G | |
| Pseudostaurosira robusta (Fusey) Williams and Round 1987 | PRBS | X | 4.8 | 1.0 | Z | |
| Reimeria sinuata (Gregory) Kociolek and Stoermer 1987 | RSIN | X | 4.8 | 2.0 | ? | |
| Rhoicosphenia abbreviata (Agardh) Lange-Bertalot 1980 | RABB | X | 4.0 | 2.9 | ? | |
| Sellaphora atomoides (Grunow) Wetzel and Van de Vijver 2015 | SEAT | X | Z | |||
| Sellaphora pseudopupula (Krasske) Lange-Bertalot 1996 | SPPU | X | X | 2.0 | Z | |
| Sellaphora saugerresi Wetzel et al. 2015 | SSGE | X | ? | |||
| Stauroneis gracilis Ehrenberg 1843 | SGRC | X | 5.0 | V | ||
| Stauroneis reichardtii Lange-Bertalot et al. 2003 | SRCH | X | Z | |||
| Staurosira construens Ehrenberg 1843 | SCON | X | 4.0 | 1.4 | ? | |
| Staurosira venter (Ehr.) Cleve and Moeller 1881 | SSVE | X | X | 4.0 | ? | |
| Staurosirella lapponica (Grunow) Williams and Round 1987 | STLA | X | 5.0 | Z | ||
| Tryblionella apiculata Gregory 1857 | TAPI | X | 2.4 | 2.8 | ? | |
| Ulnaria acus (Kützing) Aboal 2003 | UACU | X | X | 5.0 | 1.2 | * |
| Ulnaria ulna (Nitzsch) Compère 2001 | UULN | X | 5.0 | 1.2 | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padula, R.; Carosi, A.; Rossetti, A.; Lorenzoni, M. The Diatomic Diversity of Two Mediterranean High-Elevation Lakes in the Sibillini Mountains National Park (Central Italy). Environments 2021, 8, 79. https://doi.org/10.3390/environments8080079
Padula R, Carosi A, Rossetti A, Lorenzoni M. The Diatomic Diversity of Two Mediterranean High-Elevation Lakes in the Sibillini Mountains National Park (Central Italy). Environments. 2021; 8(8):79. https://doi.org/10.3390/environments8080079
Chicago/Turabian StylePadula, Rosalba, Antonella Carosi, Alessandro Rossetti, and Massimo Lorenzoni. 2021. "The Diatomic Diversity of Two Mediterranean High-Elevation Lakes in the Sibillini Mountains National Park (Central Italy)" Environments 8, no. 8: 79. https://doi.org/10.3390/environments8080079
APA StylePadula, R., Carosi, A., Rossetti, A., & Lorenzoni, M. (2021). The Diatomic Diversity of Two Mediterranean High-Elevation Lakes in the Sibillini Mountains National Park (Central Italy). Environments, 8(8), 79. https://doi.org/10.3390/environments8080079