Metal Nanoparticle Containing Nanocomposites of Drug Substances and Their Potential Biomedical Applications
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials
3. Methods
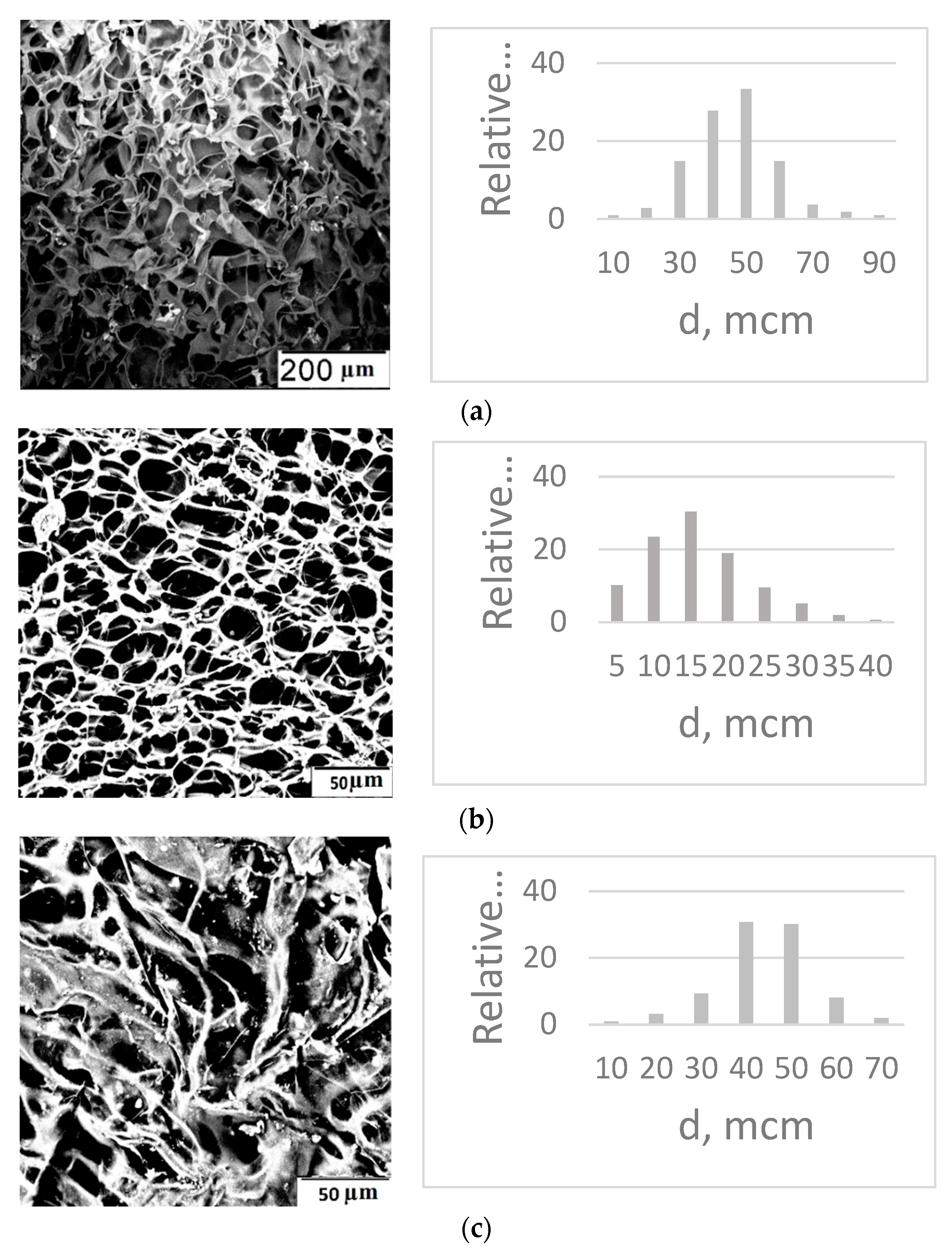
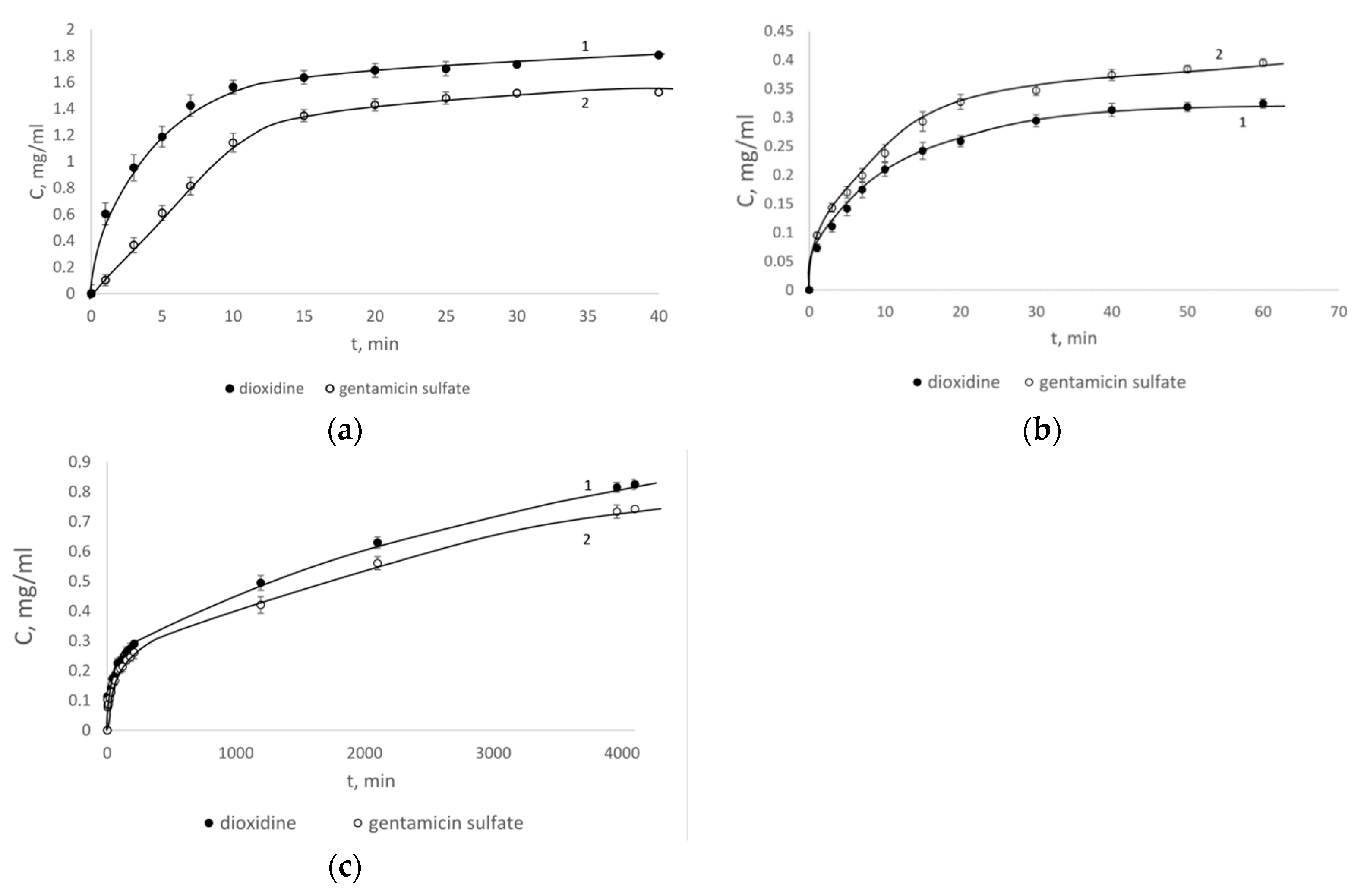
4. Results
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
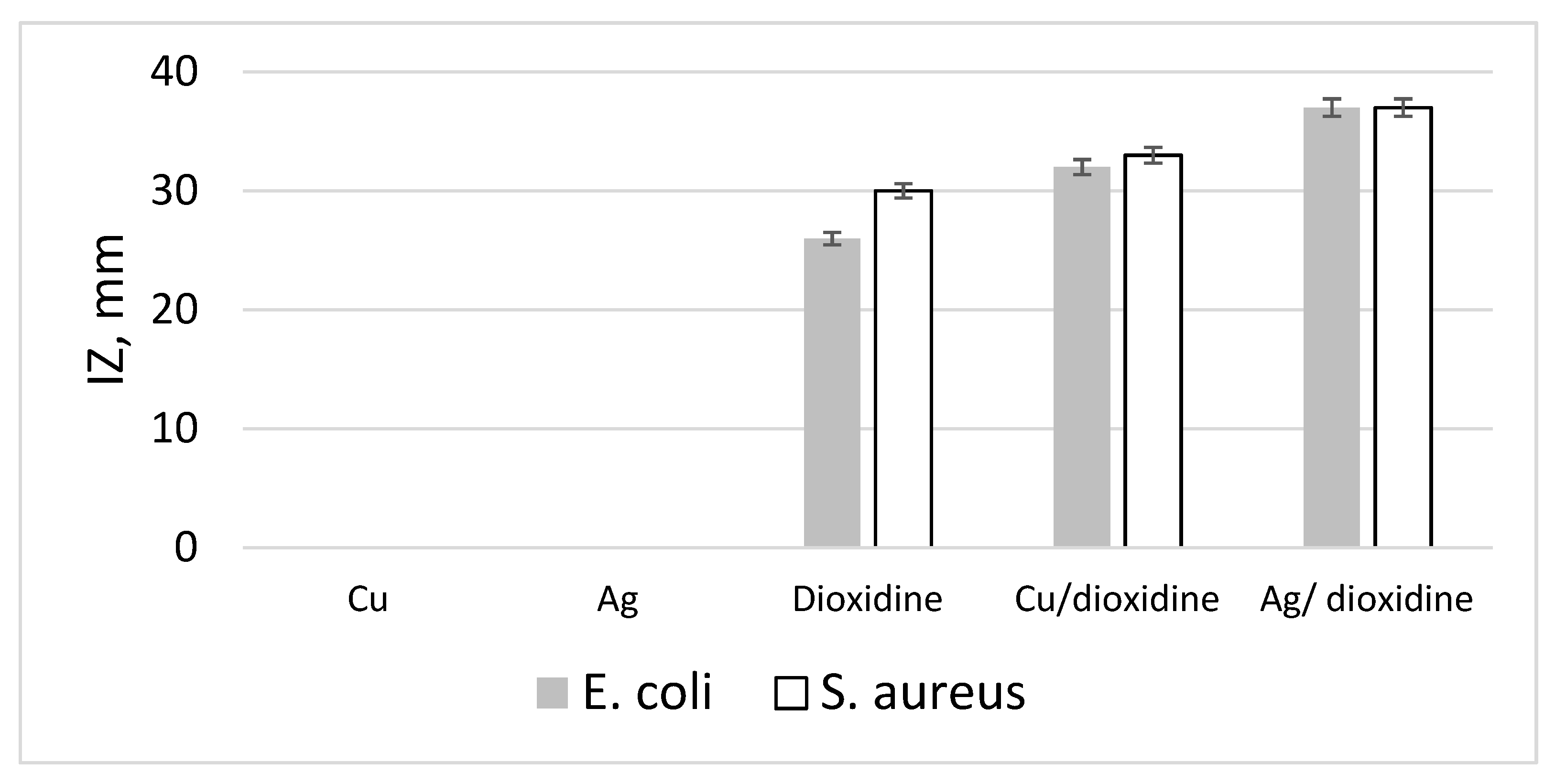
| Bacterial Strain | IZ, Ag, mm | IZ, Cu, mm | IZ, Dioxidine, mm | IZ, Ag/dioxidine, mm | IZ, Cu/Dioxidine, mm |
|---|---|---|---|---|---|
| E. coli 2 | 0 | 0 | 26.2 ± 1.2 | 36.7 ± 0.6 | 32.1 ± 1.2 |
| S. aureus 44 | 0 | 0 | 30.3 ± 0.6 | 37.1 ± 0.6 | 33.3 ± 1.2 |
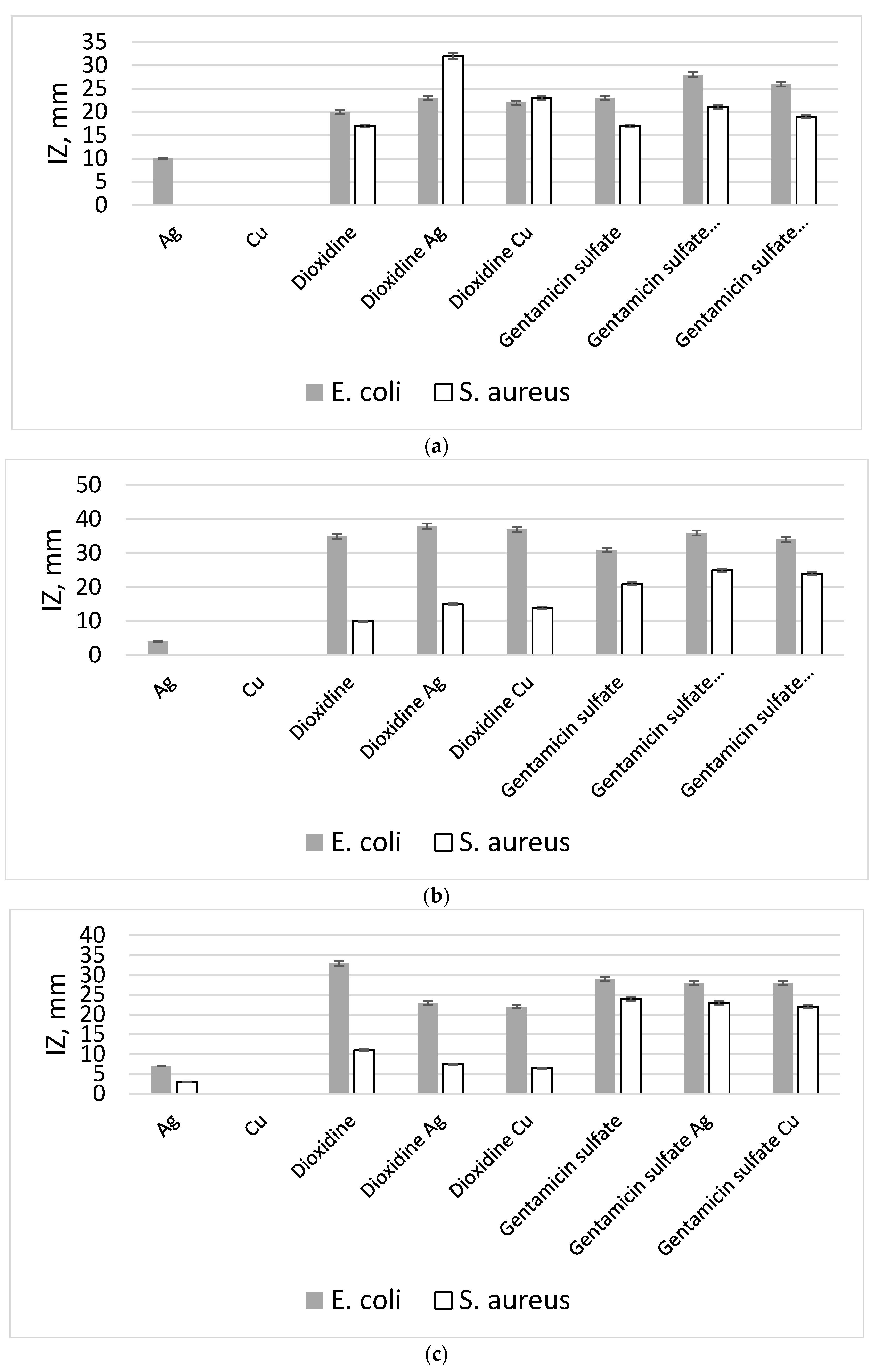
| Biopolymer Matrix | Components Included in the Hybrid Nanoform | E. col 52, IZ, mm | S. aureus 144, IZ, mm |
|---|---|---|---|
| Ca-Alginate | Ag | 10.0 ± 0.6 | 0 |
| Cu | 0 | 0 | |
| Dioxidine | 20.0 ± 0.6 | 17 ± 0.6 | |
| Dioxidine Ag | 23.0 ± 0.8 | 32 ± 0.8 | |
| Dioxidine Cu | 22.1 ± 1.2 | 23 ± 0.6 | |
| Gentamicin sulfate | 23.0 ± 0.6 | 18 ± 0.6 | |
| Gentamicin sulfate Ag | 28 ± 0.6 | 21 ± 0.6 | |
| Gentamicin sulfate Cu | 26 ± 0.6 | 19 ± 0.8 | |
| Gelatin | Ag | 4 ± 1.2 | 0 |
| Cu | 0 | 0 | |
| Dioxidine | 35 ± 0.6 | 10 ± 0.6 | |
| Dioxidine Ag | 38 ± 1.2 | 15 ± 0.6 | |
| Dioxidine Cu | 37 ± 1.2 | 14 ± 0.6 | |
| Gentamicin sulfate | 32 ± 0.6 | 21 ± 0.6 | |
| Gentamicin sulfate Ag | 34 ± 1.2 | 24 ± 0.6 | |
| Gentamicin sulfate Cu | 36 ± 0.6 | 24 ± 1.2 | |
| Chitosan | Ag | 7 ± 1.0 | 6.6 ± 0.5 |
| Cu | 0 | 0 | |
| Dioxidine | 33.2 ± 1.6 | 11.5 ± 2.5 | |
| Dioxidine Ag | 23.4 ± 1.2 | 7.5 ± 0.6 | |
| Dioxidine Cu | 22.0 ± 1.2 | 6.5 ± 0.5 | |
| Gentamicin sulfate | 29.0 ± 1.0 | 24.0 ± 0.2 | |
| Gentamicin sulfate Ag | 28.3 ± 1.4 | 23.0 ± 1.3 | |
| Gentamicin sulfate Cu | 28.3 ± 0.8 | 22.5 ± 0.5 |
| Bacterial Strain | IZ, Ag, mm | IZ, Cu, mm | IZ, Gentamicin Sulfate, mm | IZ, Ag/Gentamicin Sulfate, mm | IZ, Cu/Gentamicin Sulfate, mm |
|---|---|---|---|---|---|
| E. coli 52 | 0 | 0 | 33.8 ± 0.8 | 36.5 ± 0.6 | 35.0 ± 0.8 |
| S. aureus 144 | 0 | 0 | 18.0 ± 0.6 | 21.1 ± 0.6 | 20.2 ± 0.6 |
References
- Gupta, A.; Saleh, N.M.; Das, R.; Landis, R.F.; Bigdeli, A.; Motamedchaboki, K.; Campos, A.R.; Pomeroy, K.; Mahmoudi, M.; Rotello, V.M. Synergistic antimicrobial therapy using nanoparticles and antibiotics for the treatment of multidrug-resistant bacterial infection. Nano Futures 2017, 1, 015004. [Google Scholar] [CrossRef]
- Li, P.; Li, J.; Wu, C.; Wu, Q. Synergistic antibacterial effects of β-lactam antibiotic combined with silver nanoparticles. Nanotechnology 2005, 16, 1912–1917. [Google Scholar] [CrossRef]
- Dong, X.; Awak, M.Al.; Tomlinson, N.; Tang, Y.; Sun, Y.-P.; Yang, L. Antibacterial effects of carbon dots in combination with other antimicrobial reagents. PLoS ONE 2017, 12, e0185324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitran, R.A.; Băjenaru, L.; Moisescu, M.G. Controlling drug release from mesoporous silica through an amorphous, nanoconfined 1-tetradecanol layer. Eur. J. Pharm. Biopharm. 2018, 127, 318–325. [Google Scholar] [CrossRef]
- Ding, C.; Li, Z. A review of drug release mechanisms from nanocarrier systems. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 1440–1453. [Google Scholar] [CrossRef]
- Sami, A.J.; Khalid, M.; Jamil, T.; Aftab, S.; Mangat, S.A.; Shakoori, A.R.; Iqbal, S. Formulation of Novel Chitosan Guargum based Hydrogels for Sustained Drug Release of Paracetamol. Int. J. Biol. Macromol. 2018, 108, 324–332. [Google Scholar] [CrossRef]
- Ito, T.; Takami, T.; Uchida, Y.; Murakami, Y. Chitosan gel sheet containing drug carriers with controllable drug-release properties. Colloids Surf. B Biointerfaces 2018, 163, 257–265. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nature Rev. Mater. 2016, 1. [Google Scholar] [CrossRef]
- Vicent, M.J.; Duncan, R. Polymer conjugates: Nanosized medicines for treating cancer. Trends Biotechnol. 2006, 24, 39–47. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Nayak, A.K.; Singh, M.; Tabish, M.; Ara, J. Alginate-based bipolymeric-nanobioceramic composite matrices for sustained drug release. Int. J. Biol. Macromol. 2016, 83, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Jalababu, R.; Veni, S.; Reddy, K.V.N.S. Synthesis and characterization of dual responsive sodium alginate-g-acryloyl phenylalanine-poly N-isopropyl acrylamide smart hydrogels for the controlled release of anticancer drug. J. Drug Deliv. Sci. Technol. 2018, 44, 190–204. [Google Scholar] [CrossRef]
- Bini, R.A.; Silva, M.F.; Varanda, L.C.; da Silva, M.A.; Dreiss, C.A. Soft nanocomposites of gelatin and poly (3-hydroxybutyrate) nanoparticles for dual drug release. Colloids Surf. B Biointerfaces 2017, 157, 191–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talebian, A.; Mansourian, A. Release of Vancomycin from electrospun gelatin/chitosan nanofibers. Mater. Today Proc. 2017, 4, 7065–7069. [Google Scholar] [CrossRef]
- Saikova, S.V.; Vorob’ev, S.A.; Nikolaeva, R.B.; Mikhlin, Y.L. Conditions for the formation of copper nanoparticles by reduction of copper (II) ions with hydrazine hydrate solutions. Russ. J. Gen. Chem. 2010, 80, 1122–1127. [Google Scholar] [CrossRef]
- Vernaya, O.I.; Khvatov, D.I.; Nuzhdina, A.V.; Fedorov, V.V.; Shabatin, V.P.; Semenov, A.M.; Shabatina, T.I. Cu/Dioxidine Hybrid Nanocomposites: Cryochemical Synthesis. Mosc. Univ. Chem. Bull. 2016, 71, 224–226. [Google Scholar]
- Vernaya, O.I.; Shabatin, V.P.; Semenov, A.M.; Shabatina, T.I. Cryochemical Synthesis and Antibacterial Activity of a Hybrid Composition Based on Ag Nanoparticles and Dioxidine. Mosc. Univ. Chem. Bull. 2017, 72, 6–9. [Google Scholar] [CrossRef]
- Zvukova, N.D.; Klimova, T.P.; Ivanov, R.V.; Ryabev, A.N.; Tsiskarashvili, A.V.; Lozinsky, V.I. Cryostructuring of polymeric systems. 52. Properties, microstructure and an example of a potential biomedical use of the wide-pore alginate cryostructurastes. Gels 2019, 5, 25. [Google Scholar] [CrossRef] [Green Version]
- Sazhnev, N.A.; Drozdova, M.G.; Rodionov, I.A.; Kil’deeva, N.R.; Balabanova, T.V.; Markvicheva, E.A.; Lozinsky, V.I. Preparation of chitosan cryostructurates with controlled porous morphology and their use as 3D-scaffolds for culturing of animal cells. Appl. Biochem. Microbiol. 2018, 54, 459–467. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Kulakova, V.K.; Ivanov, R.V.; Petrenko, A.Y.; Rogulska, O.Y.; Petrenko, Y.A. Cryostructuring of polymer systems. 47. Preparation of wide porous gelatin-based cryostructurates in sterilizing organic media and assessment of the suitability of thus formed matrices as spongy scaffolds for 3D cell culturing. E-Polymers 2018, 18, 175–186. [Google Scholar] [CrossRef]
- Shabatina, T.I.; Vernaya, O.I.; Shabatin, V.P.; Evseeva, I.V.; Melnikov, M.Y.; Fitch, A.N.; Chernyshev, V.V. Cryochemically Obtained Nanoforms of Antimicrobial Drug Substance Dioxidine and Their Physico-chemical and Structural Properties. Crystals 2018, 8, 298. [Google Scholar] [CrossRef] [Green Version]
- Vernaya, O.I.; Shabatin, V.P.; Shabatina, T.I.; Khvatov, D.I.; Semenov, A.M.; Yudina, T.P.; Danilov, V.S. Cryochemical modification, activity, and toxicity of dioxidine. Russ. J. Phys. Chem. A 2017, 91, 229–232. [Google Scholar] [CrossRef]
- Vernaya, O.I.; Shabatin, V.P.; Semenov, A.M.; Shabatina, T.I. Obtaining ultradispersed dioxidine powder modified. Mosc. Univ. Chem. Bull. 2016, 71, 291–298. [Google Scholar] [CrossRef]
- Rosenkrantz, B.E.; Greco, J.R.; Hoogerheide, J.G.; Oden, E.M. Gentamicin sulfate. Anal. Profiles Drug Subst. 1981, 9, 295–340. [Google Scholar]
- Deubner, R.; Schollmayer, C.; Wienen, F.; Holzgrabe, U. Assignment of the major and minor components of gentamicin for evaluation of batches. Magn. Reson. Chem. 2003, 41, 589–598. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shabatina, T.I.; Vernaya, O.I.; Shabatin, V.P.; Melnikov, M.Y.; Semenov, A.M.; Lozinsky, V.I. Metal Nanoparticle Containing Nanocomposites of Drug Substances and Their Potential Biomedical Applications. Appl. Sci. 2020, 10, 170. https://doi.org/10.3390/app10010170
Shabatina TI, Vernaya OI, Shabatin VP, Melnikov MY, Semenov AM, Lozinsky VI. Metal Nanoparticle Containing Nanocomposites of Drug Substances and Their Potential Biomedical Applications. Applied Sciences. 2020; 10(1):170. https://doi.org/10.3390/app10010170
Chicago/Turabian StyleShabatina, Tatyana I., Olga I. Vernaya, Vladimir P. Shabatin, Michail Y. Melnikov, Alexandr M. Semenov, and Vladimir I. Lozinsky. 2020. "Metal Nanoparticle Containing Nanocomposites of Drug Substances and Their Potential Biomedical Applications" Applied Sciences 10, no. 1: 170. https://doi.org/10.3390/app10010170






