Effects of Colored Light on Growth and Nutritional Composition of Tilapia, and Biofloc as a Food Source
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Laboratory Facilities and Fish Stocking
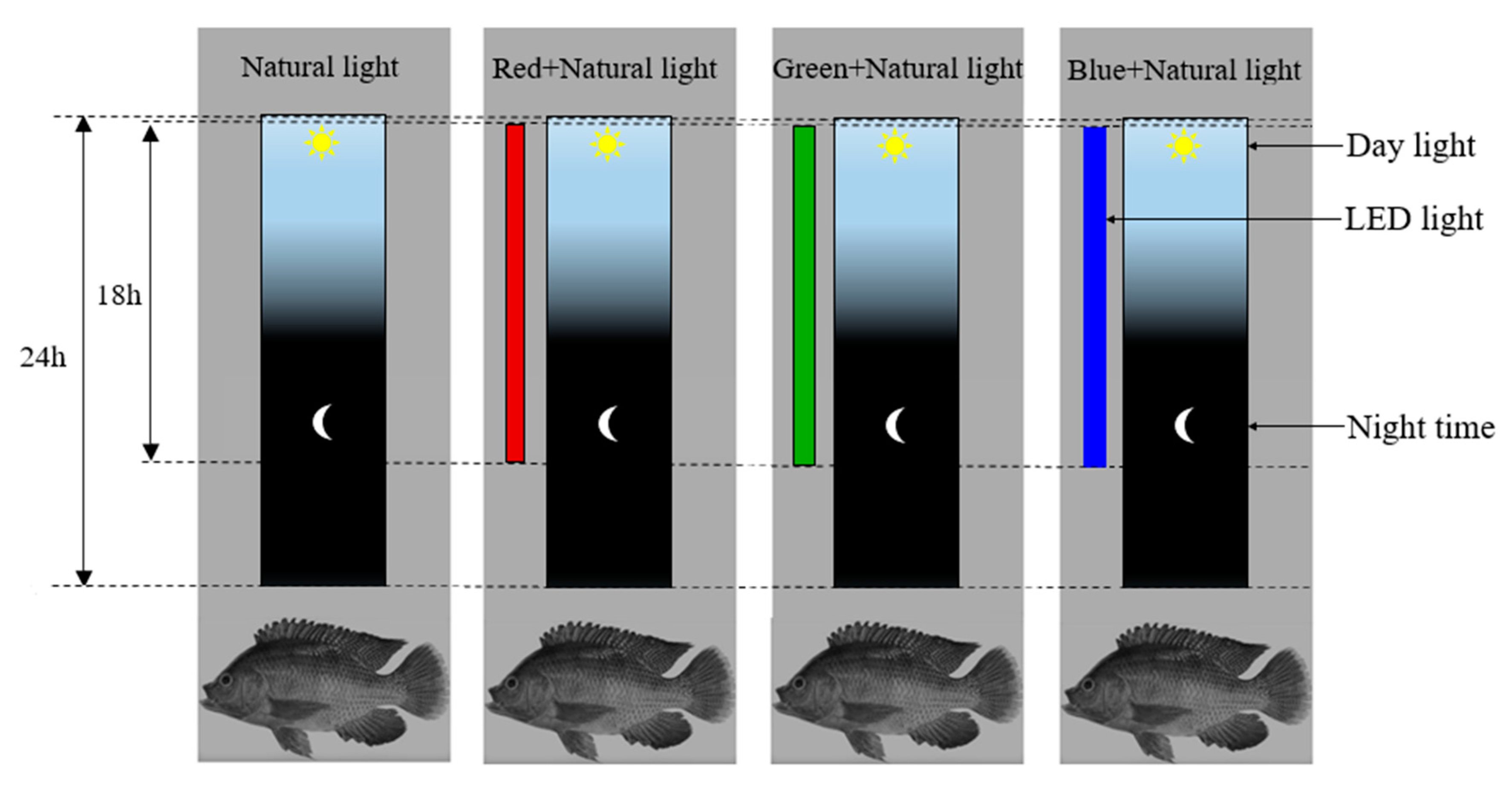
2.3. Experimental Design and Setup
2.4. Characterization of LED Lamps
2.5. Nile Tilapia Fingerlings and the Biofloc System
2.6. Water Quality Parameters
2.7. Growth Rates
2.8. Nutritional Composition of the Fish Body and Bioflocs
2.9. Statistical Analysis
3. Results
3.1. Water Quality Parameters
3.2. Growth Rates
3.3. Nutritional Composition of the Fish Body and Bioflocs
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Belton, B.; Bush, S.R.; Little, D.C. Not just for the wealthy: Rethinking farmed fish consumption in the Global South. Glob. Food Secur. 2017, 16, 85–92. [Google Scholar] [CrossRef]
- FAO. Contributing to food security and nutrition for all. In The State of World Fisheries and Aquaculture, 2016; FAO: Rome, Italy, 2016; p. 172. [Google Scholar]
- Pauly, D.; Zeller, D. Comments on FAOs state of world fisheries and aquaculture (SOFIA 2016). Mar. Policy 2017, 77, 176–181. [Google Scholar] [CrossRef]
- WWAP. The United Nations World Water Development Report 2015: Water for a Sustainable World; UNESCO: Paris, France, 2015. [Google Scholar]
- Arias-Moscoso, J.L.; Espinoza-Barrón, L.G.; Miranda-Baeza, A.; Rivas-Vega, M.E.; Nieves-Soto, M. Effect of commercial probiotics addition in a biofloc shrimp farm during the nursery phase in zero water exchange. Aquac. Rep. 2018, 11, 47–52. [Google Scholar] [CrossRef]
- Avnimelech, Y. Feeding with microbial flocs by tilapia in minimal discharge bio-flocs technology ponds. Aquaculture 2007, 264, 140–147. [Google Scholar] [CrossRef]
- Avnimelech, Y.; Verdegem, M.; Kurup, M.; Keshavanath, P. Sustainable land-based aquaculture: Rational utilization of water, land and feed resources. Mediterr. Aquac. J. 2008, 1, 45–55. [Google Scholar] [CrossRef] [Green Version]
- Azim, M.E.; Little, D.C. The biofloc technology (BFT) in indoor tanks: Water quality, biofloc composition, and growth and welfare of Nile tilapia (Oreochromis niloticus). Aquaculture 2008, 283, 29–35. [Google Scholar] [CrossRef]
- Emerenciano, M.; Ballester, E.L.; Cavalli, R.O.; Wasielesky, W. Biofloc technology application as a food source in a limited water exchange nursery system for pink shrimp Farfantepenaeus brasiliensis (Latreille, 1817). Aquac. Res. 2012, 43, 447–457. [Google Scholar] [CrossRef]
- Green, B.W.; Rawles, S.D.; Webster, C.D.; McEntire, M.E. Effect of Stocking Rate on Growing Juvenile Sunshine Bass, Morone chrysops x M. saxatilis, in an Outdoor Biofloc Production System. J. World Aquac. Soc. 2018, 49, 827–836. [Google Scholar] [CrossRef]
- Avnimelech, Y. Biofloc technology. In A Practical Guide Book; The World Aquaculture Society: Baton Rouge, LA, USA, 2009; p. 182. [Google Scholar]
- Crab, R.; Defoirdt, T.; Bossier, P.; Verstraete, W. Biofloc technology in aquaculture: Beneficial effects and future challenges. Aquaculture 2012, 356, 351–356. [Google Scholar] [CrossRef]
- Boeuf, G.; Le Bail, P.Y. Does light have an influence on fish growth? Aquaculture 1999, 177, 129–152. [Google Scholar] [CrossRef]
- Ridha, M.; Cruz, E. Effect of light intensity and photoperiod on Nile tilapia Oreochromis niloticus L. seed production. Aquac. Res. 2000, 31, 609–617. [Google Scholar] [CrossRef]
- Moyle, P.B.; Cech, J.J. Fishes: An Introduction to Ichthyology; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2004. [Google Scholar]
- Heydarnejad, M.S.; Parto, M.; Pilevarian, A.A. Influence of light colours on growth and stress response of rainbow trout (Oncorhynchus mykiss) under laboratory conditions. J. Anim. Physiol. Anim. Nutr. 2013, 97, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Karakatsouli, N.; Papoutsoglou, E.S.; Sotiropoulos, N.; Mourtikas, D.; Stigen-Martinsen, T.; Papoutsoglou, S.E. Effects of light spectrum, rearing density and light intensity on growth performance of scaled and mirror common carp Cyprinus carpio reared under recirculating system conditions. Aquac. Eng. 2010, 42, 121–127. [Google Scholar] [CrossRef]
- Ruchin, A.B. Environmental colour impact on the life of lower aquatic vertebrates: Development, growth, physiological and biochemical processes. Rev. Aquac. 2018, 1–18. [Google Scholar] [CrossRef]
- Ruchin, A.B. Influence of Colored Light on Growth rate of Juveniles of Fish. Fish Physiol. Biochem. 2004, 30, 175–178. [Google Scholar] [CrossRef]
- Karakatsouli, N.; Papoutsoglou, S.E.; Pizzonia, G.; Tsatsos, G.; Tsopelakos, A.; Chadio, S.; Kalogiannis, D.; Dalla, C.; Polissidis, A.; Papadopoulou-Daifoti, Z. Effects of light spectrum on growth and physiological status of gilthead seabream Sparus aurata and rainbow trout Oncorhynchus mykiss reared under recirculating system conditions. Aquac. Eng. 2007, 36, 302–309. [Google Scholar] [CrossRef]
- Bayarri, M.; Madrid, J.; Sánchez-Vázquez, F. Influence of light intensity, spectrum and orientation on sea bass plasma and ocular melatonin. J. Opineal Res. 2002, 32, 34–40. [Google Scholar] [CrossRef]
- Hoang, T.; Barchiesis, M.; Lee, S.Y.; Keenan, C.P.; Marsden, G.E. Influences of light intensity and photoperiod on moulting and growth of Penaeus merguiensis cultured under laboratory conditions. Aquaculture 2003, 216, 343–354. [Google Scholar] [CrossRef]
- Neal, R.S.; Coyle, S.D.; Tidwell, J.H.; Boudreau, B.M. Evaluation of stocking density and light level on the growth and survival of the Pacific white shrimp, Litopenaeus vannamei, reared in zero-exchange systems. J. World Aquac. Soc. 2010, 41, 533–544. [Google Scholar] [CrossRef]
- Njiru, M.; Okeyo-Owuor, J.; Muchiri, M.; Cowx, I. Shifts in the food of Nile tilapia, Oreochromis niloticus (L.) in Lake Victoria, Kenya. Afr. J. Ecol. 2004, 42, 163–170. [Google Scholar] [CrossRef]
- Moreno, I.; Contreras, U. Color distribution from multicolor LED arrays. Opt. Express 2007, 15, 3607–3618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, I.; Sun, C.C. Modeling the radiation pattern of LEDs. Opt. Express 2008, 16, 1808–1819. [Google Scholar] [CrossRef] [PubMed]
- De Schryver, P.; Crab, R.; Defoirdt, T.; Boon, N.; Verstraete, W. The basics of bio-flocs technology: The added value for aquaculture. Aquaculture 2008, 277, 125–137. [Google Scholar] [CrossRef]
- Crab, R.; Kochva, M.; Verstraete, W.; Avnimelech, Y. Bio-flocs technology application in over-wintering of tilapia. Aquac. Eng. 2009, 40, 105–112. [Google Scholar] [CrossRef]
- Poli, M.A.; Legarda, E.C.; de Lorenzo, M.A.; Martins, M.A.; do Nascimento Vieira, F. Pacific white shrimp and Nile tilapia integrated in a biofloc system under different fish-stocking densities. Aquaculture 2019, 498, 83–89. [Google Scholar] [CrossRef]
- Boyd, C.E.; Tucker, C.S. Pond Aquaculture Water Quality Management; Springer Science and Business Media: Medford, MA, USA, 2012; ISBN 978-1-4615-5407-3. [Google Scholar]
- Mansour, A.T.; Esteban, M.Á. Effects of carbon sources and plant protein levels in a biofloc system on growth performance, and the immune and antioxidant status of Nile tilapia (Oreochromis niloticus). Fish Shellfish. Immunol. 2017, 64, 202–209. [Google Scholar] [CrossRef]
- Nhi, N.H.Y.; Da, C.T.; Lundh, T.; Lan, T.T.; Kiessling, A. Comparative evaluation of Brewer’s yeast as a replacement for fishmeal in diets for tilapia (Oreochromis niloticus), reared in clear water or biofloc environments. Aquaculture 2018, 495, 654–660. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- NMX Water Analysis-Measurement of Extractables Fats and Oils in Natural Waters, Wastewaters and Treated Wastewaters; Diario Oficial de la Federacion de la Secretaria de Economia del Gobierno de Mexico: Ciudad de Mexico, CDMX, Mexico, 2013.
- Azaza, M.; Dhraief, M.; Kraiem, M. Effects of water temperature on growth and sex ratio of juvenile Nile tilapia Oreochromis niloticus (Linnaeus) reared in geothermal waters in southern Tunisia. J. Therm. Biol. 2008, 33, 98–105. [Google Scholar] [CrossRef]
- El-Sayed, A.F.M.; Kawanna, M. Optimum water temperature boosts the growth performance of Nile tilapia (Oreochromis niloticus) fry reared in a recycling system. Aquac. Res. 2008, 39, 670–672. [Google Scholar] [CrossRef]
- Evans, J.J.; Shoemaker, C.A.; Klesius, P.H. Effects of sublethal dissolved oxygen stress on blood glucose and susceptibility to Streptococcus agalactiae in Nile tilapia Oreochromis niloticus. J. Aquat. Anim. Health 2003, 15, 202–208. [Google Scholar] [CrossRef]
- El-Sherif, M.S.; El-Feky, A.M.I. Performance of Nile tilapia (Oreochromis niloticus) fingerlings. I. Effect of pH. Int. J. Agric. Biol. 2009, 11, 297–300. [Google Scholar]
- Zeitoun, M.M.; EL-Azrak, K.E.D.M.; Zaki, M.A.; Nemat-Allah, B.R.; Mehana, E.S.E. Effects of ammonia toxicity on growth performance, cortisol, glucose and hematological response of Nile Tilapia (Oreochromis niloticus). Aceh. J. Anim. Sci. 2016, 1, 21–28. [Google Scholar] [CrossRef] [Green Version]
- El-Shafai, S.A.; El-Gohary, F.A.; Nasr, F.A.; van der Steen, N.P.; Gijzen, H.J. Chronic ammonia toxicity to duckweed-fed tilapia (Oreochromis niloticus). Aquaculture 2004, 232, 117–127. [Google Scholar] [CrossRef]
- Volpato, G.L.; Bovi, T.S.; de Freitas, R.H.; da Silva, D.F.; Delicio, H.C.; Giaquinto, P.C.; Barreto, R.E. Red light stimulates feeding motivation in fish but does not improve growth. PLoS ONE 2013, 8, e59134. [Google Scholar] [CrossRef] [Green Version]
- Karakatsouli, N.; Papoutsoglou, S.E.; Panopoulos, G.; Papoutsoglou, E.S.; Chadio, S.; Kalogiannis, D. Effects of light spectrum on growth and stress response of rainbow trout Oncorhynchus mykiss reared under recirculating system conditions. Aquac. Eng. 2008, 38, 36–42. [Google Scholar] [CrossRef]
- Luchiari, A.; Freire, F. Effects of environmental colour on growth of Nile tilapia, Oreochromis niloticus (Linnaeus, 1758), maintained individually or in groups. J. Appl. Ichthyol. 2009, 25, 162–167. [Google Scholar] [CrossRef]
- Volpato, G.L.; Barreto, R. Environmental blue light prevents stress in the fish Nile tilapia. Braz. J. Med. Biol. Res. 2001, 34, 1041–1045. [Google Scholar] [CrossRef] [Green Version]
- Aly, H.; Abdel-Rahim, M.; Lotfy, A.; Abdelaty, B. Impact of Different Colors of Artificial Light on Pigmentation and Growth Performance of Hybrid Red Tilapia (Oreochromis mosambicus x O. hornorum) Reared in Saline Well Water. J. Mar. Sci. Res. Dev. 2017, 7, 229. [Google Scholar] [CrossRef]
- Sabri, D.M.; Elnwishy, N.; Nwonwu, F. Effect of environmental color on the behavioral and physiological response of Nile tilapia, Oreochromis niloticus. Glob. J. Front. Res. 2012, 12, 11–20. [Google Scholar]
- Douglas, R.H.; Hawryshyn, C.W. Behavioural studies of fish vision: An analysis of visual capabilities. In The Visual System of Fish; Douglas, R., Djamgoz, M., Eds.; Springer: Dordrecht, The Netherlands, 1990; pp. 373–418. ISBN 978-94-009-0411-8. [Google Scholar]
- Younis, E.S.M.; Al-Quffail, A.S.; Al-Asgah, N.A.; Abdel-Warith, A.W.A.; Al-Hafedh, Y.S. Effect of dietary fish meal replacement by red algae, Gracilaria arcuata, on growth performance and body composition of Nile tilapia Oreochromis niloticus. Saudi J. Biol. Sci. 2018, 25, 198–203. [Google Scholar] [CrossRef]
- He, A.Y.; Ning, L.J.; Chen, L.Q.; Chen, Y.L.; Xing, Q.; Li, J.M.; Qiao, F.; Li, D.L.; Zhang, M.L.; Du, Z.Y. Systemic adaptation of lipid metabolism in response to low- and high-fat diet in Nile tilapia (Oreochromis niloticus). Physiol. Rep. 2015, 3, e12485. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; Ramos-Romero, I.R. Light spectrum for maximum luminous efficacy of radiation and high color quality. In Current Developments in Lens Design and Optical Engineering XIX; International Society for Optics and Photonics: Bellingham, WA, USA, 2018; Volume 10745. [Google Scholar]




| Parameters | LED Lamps | ||
|---|---|---|---|
| R | G | B | |
| λ (nm) | 627.27 | 513.33 | 451.67 |
| Δλ (nm) | 16.92 | 32.80 | 23.75 |
| Irradiance () | 0.87 | 0.83 | 0.80 |
| Feed | ||||||
|---|---|---|---|---|---|---|
| Commercial Diet I (Manufacturer) | Commercial Diet I (Laboratory) | Commercial Diet II (Manufacturer) | Commercial Diet II (Laboratory) | Cane Sugar (Manufacturer) | Cane Sugar (Laboratory) | |
| Moisture (%) | 12.00 | 7.90 | 12.00 | 8.10 | 0.00 | 1.80 |
| Crude protein (%) | 53.00 | 43.00 | 32.00 | 40.90 | 0.00 | 0.30 |
| Lipids (%) | 15.00 | 12.30 | 6.00 | 12.80 | 0.00 | 0.00 |
| Crude fiber (%) | 2.50 | 1.70 | 6.00 | 2.20 | 0.00 | 3.30 |
| Ash (%) | 12.00 | 7.80 | 15.00 | 10.00 | 0.00 | 0.10 |
| Nitrogen-free extract (%) | 5.50 | 27.30 | 29.00 | 26.00 | 100.00 | 94.50 |
| Parameters | Treatments | |||
|---|---|---|---|---|
| R | G | B | Natural | |
| Temperature (°C) | 28.03 ± 1.03 | 28.38 ± 0.96 | 28.94 ± 1.22 | 28.65 ± 0.93 |
| Dissolved Oxygen (mg/L) | 6.65 ± 0.58 | 6.67 ± 0.43 | 6.71 ± 0.47 | 6.72 ± 0.47 |
| pH | 7.91 ± 0.17 | 7.91 ± 0.16 | 7.92 ± 0.16 | 8.01 ± 0.20 |
| -N (mg/L) | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.08 ± 0.01 | 0.10 ± 0.01 |
| TAN (mg/L) | 0.79 ± 0.18 | 0.76 ± 0.21 | 0.86 ± 0.14 | 0.93 ± 0.22 |
| Parameters | Treatments | |||
|---|---|---|---|---|
| R | G | B | Natural | |
| Initial biomass (g) | 30 | 30 | 30 | 30 |
| Initial weight (g/fish) | 0.2 | 0.2 | 0.3 | 0.3 |
| Initial number of fish | 133 | 132 | 111 | 113 |
| Final Biomass (g) | 2193 | 1330 | 1461 | 1362 |
| Total biomass gained (g) | 2163 | 1300 | 1431 | 1332 |
| Final number of fish | 127 | 79 | 94 | 84 |
| Final individual weight (g/fish) | 17.3 ± 3.3 a | 16.8 ± 3.1 a | 15.5 ± 2.8 b | 16.2 ± 3.0 ab |
| Final individual body length (cm/fish) | 8.2 ± 1.0 a | 8.0 ± 0.9 a | 7.7 ± 0.9 b | 7.9 ± 0.8 ab |
| Survival (%) | 95.5 | 59.9 | 84.7 | 74.3 |
| SGR (%/day) | 6.0 | 5.9 | 5.6 | 5.7 |
| DWG (g/day) | 0.2 | 0.2 | 0.2 | 0.2 |
| FCR | 0.8 | 1.3 | 1.2 | 1.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez-Betancur, D.; Moreno, I.; Guerrero-Mendez, C.; Gómez-Meléndez, D.; Macias P., M.d.J.; Olvera-Olvera, C. Effects of Colored Light on Growth and Nutritional Composition of Tilapia, and Biofloc as a Food Source. Appl. Sci. 2020, 10, 362. https://doi.org/10.3390/app10010362
Lopez-Betancur D, Moreno I, Guerrero-Mendez C, Gómez-Meléndez D, Macias P. MdJ, Olvera-Olvera C. Effects of Colored Light on Growth and Nutritional Composition of Tilapia, and Biofloc as a Food Source. Applied Sciences. 2020; 10(1):362. https://doi.org/10.3390/app10010362
Chicago/Turabian StyleLopez-Betancur, Daniela, Ivan Moreno, Carlos Guerrero-Mendez, Domingo Gómez-Meléndez, Manuel de J. Macias P., and Carlos Olvera-Olvera. 2020. "Effects of Colored Light on Growth and Nutritional Composition of Tilapia, and Biofloc as a Food Source" Applied Sciences 10, no. 1: 362. https://doi.org/10.3390/app10010362
APA StyleLopez-Betancur, D., Moreno, I., Guerrero-Mendez, C., Gómez-Meléndez, D., Macias P., M. d. J., & Olvera-Olvera, C. (2020). Effects of Colored Light on Growth and Nutritional Composition of Tilapia, and Biofloc as a Food Source. Applied Sciences, 10(1), 362. https://doi.org/10.3390/app10010362







