Preparation and the Effect of Surface-Functionalized Calcium Carbonate Nanoparticles on Asphalt Binder
Abstract
:1. Introduction
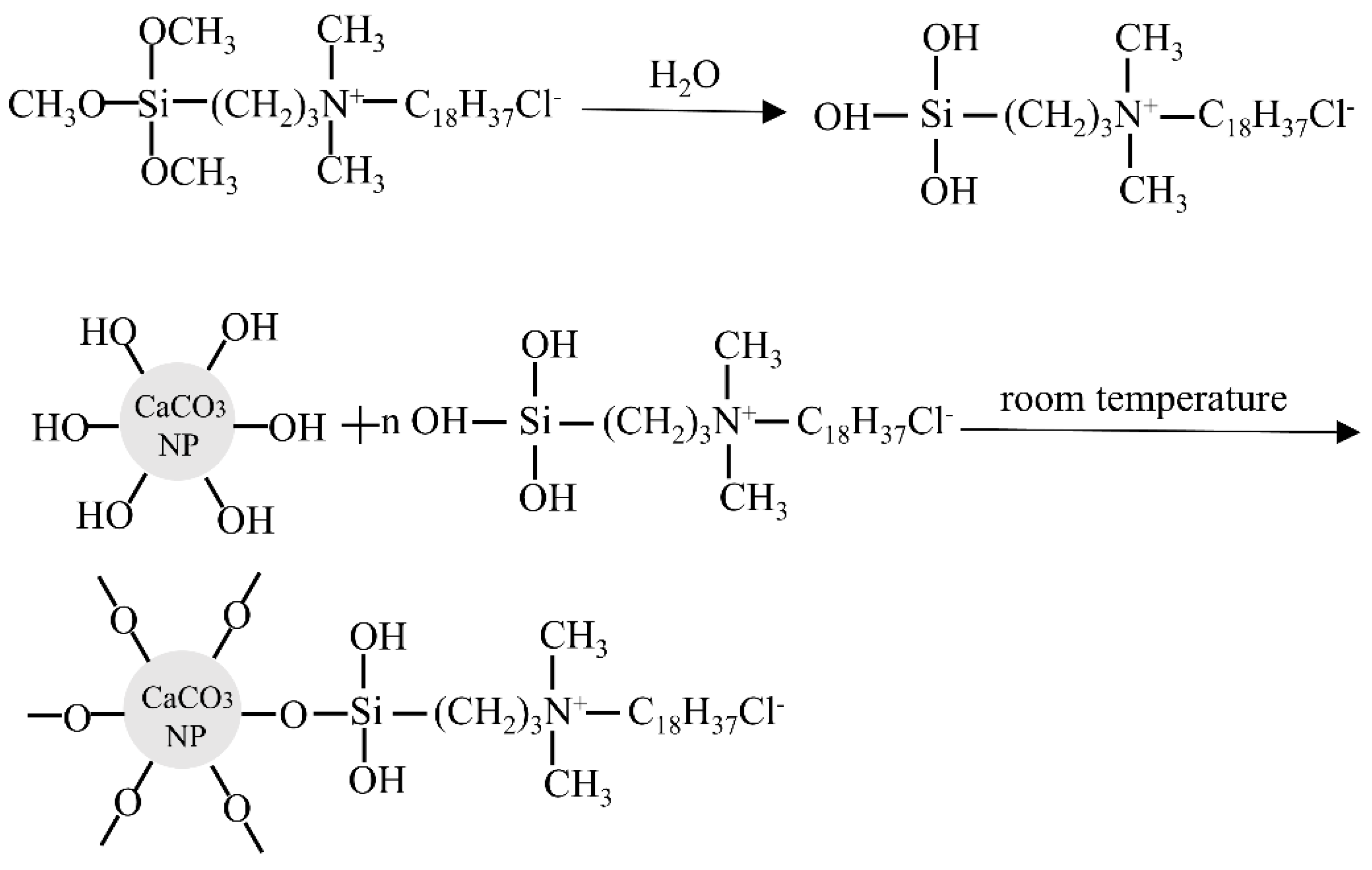
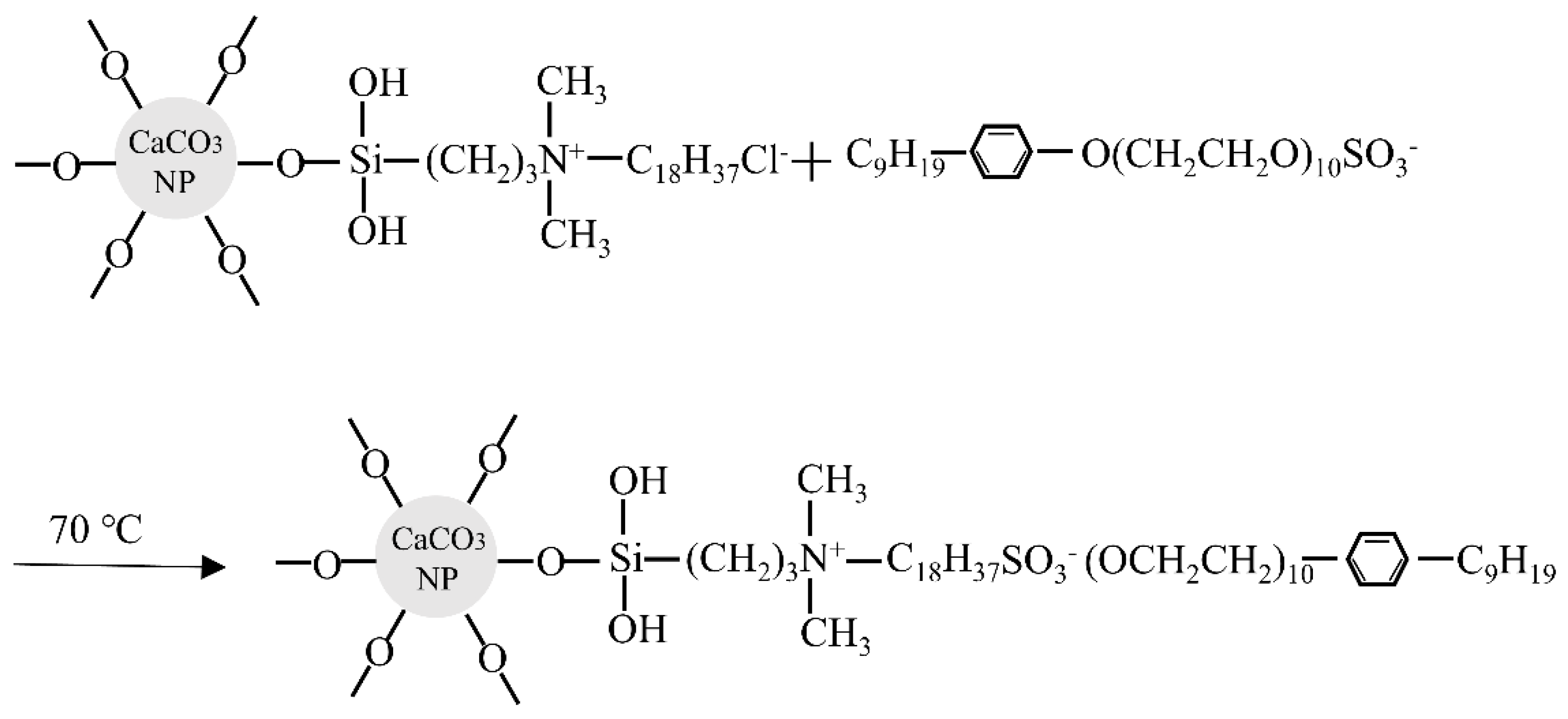
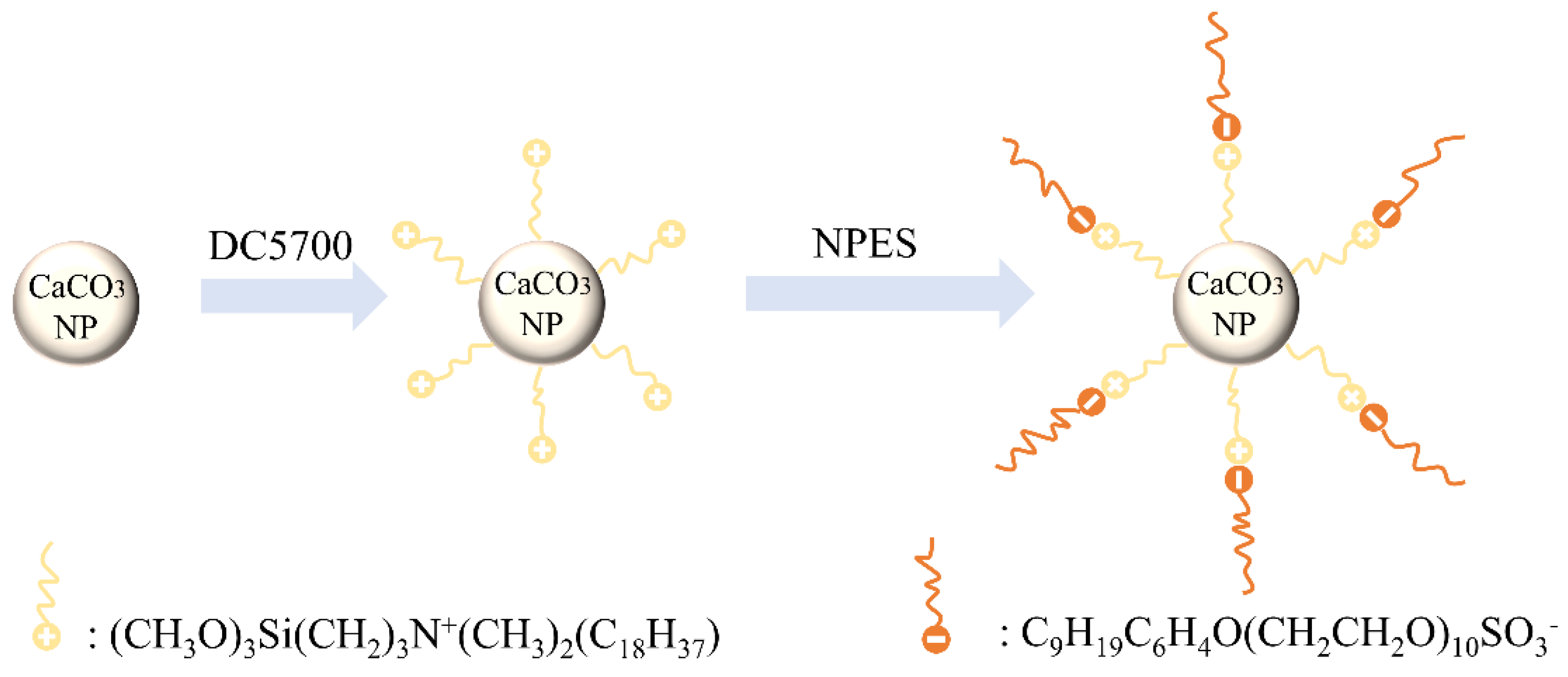
2. Raw Materials and Preparation
3. Experimental Plan
3.1. Microscopic Structure Tests of CaCO3 NFs
3.2. Performance Evaluation of Asphalt Binders
3.2.1. Compatibility Performance
3.2.2. Conventional Performance
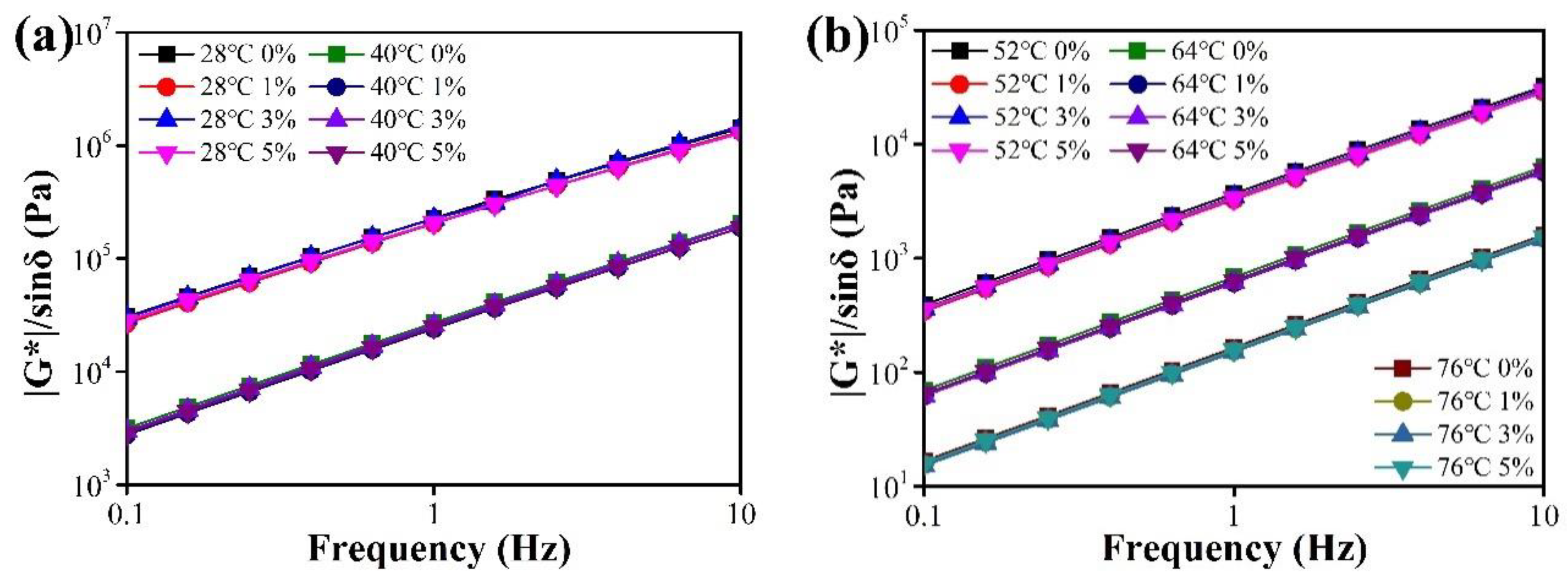
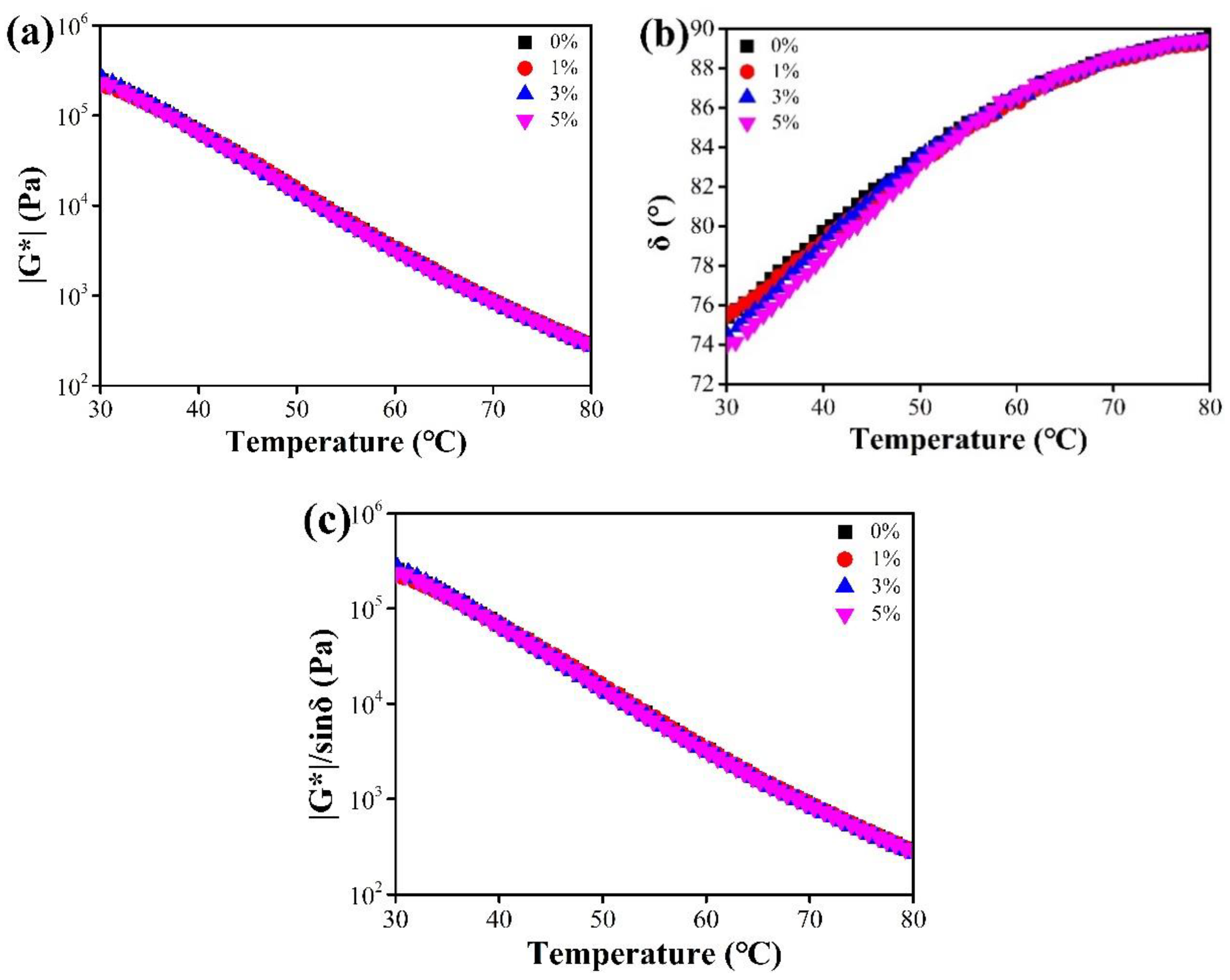
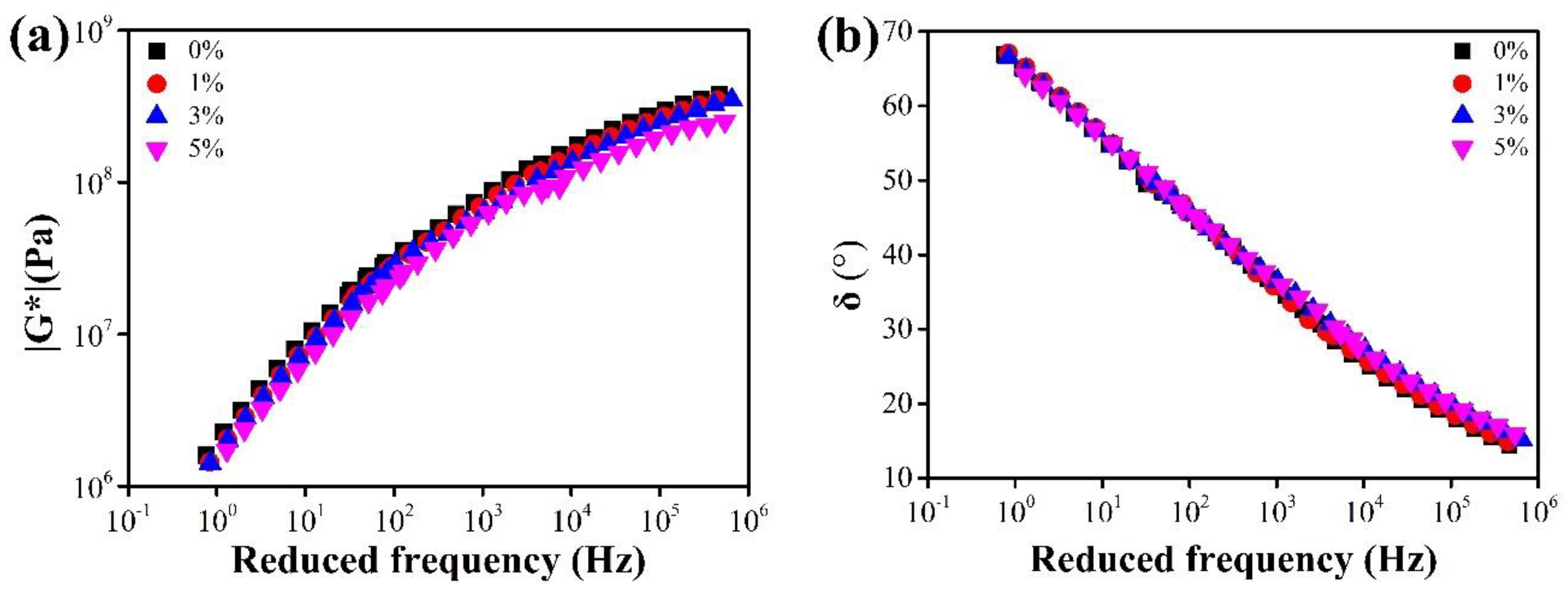
3.2.3. Rheological Properties
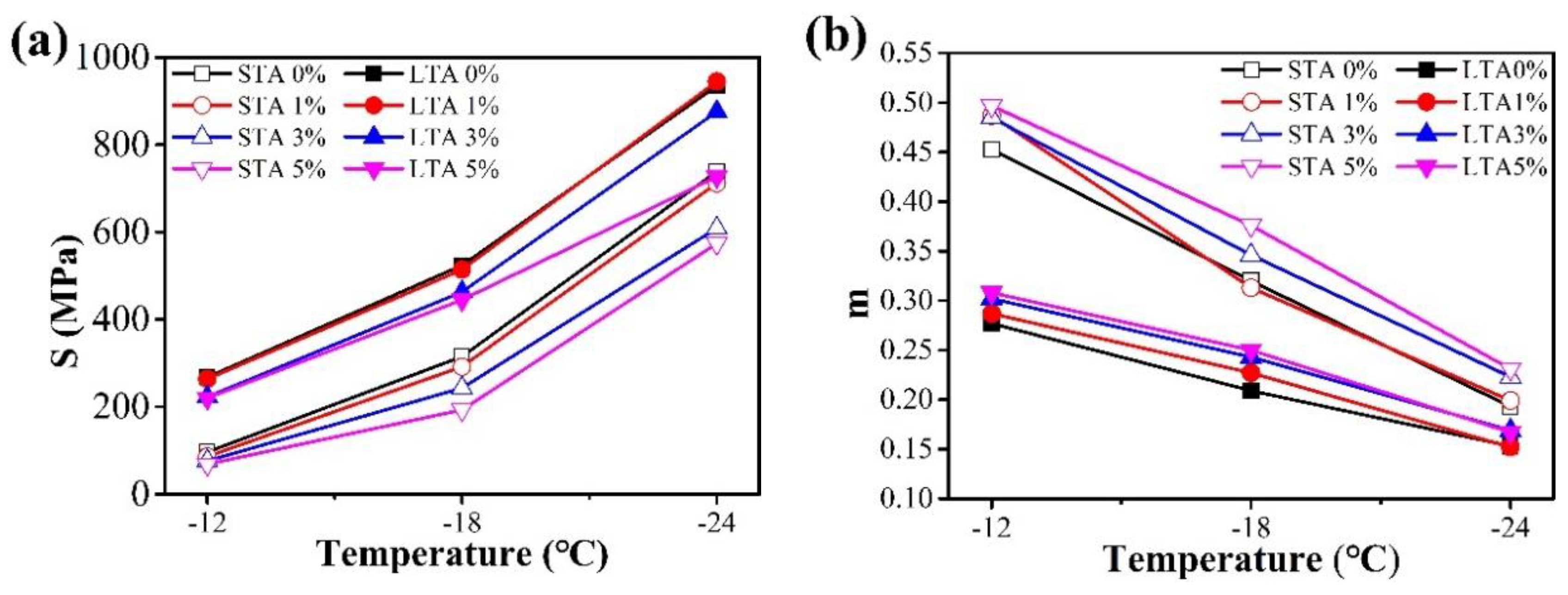
3.2.4. Low-Temperature Creep Properties
4. Results and Discussion
4.1. Microscopic Characterization of CaCO3 NFs
4.1.1. FTIR Analysis
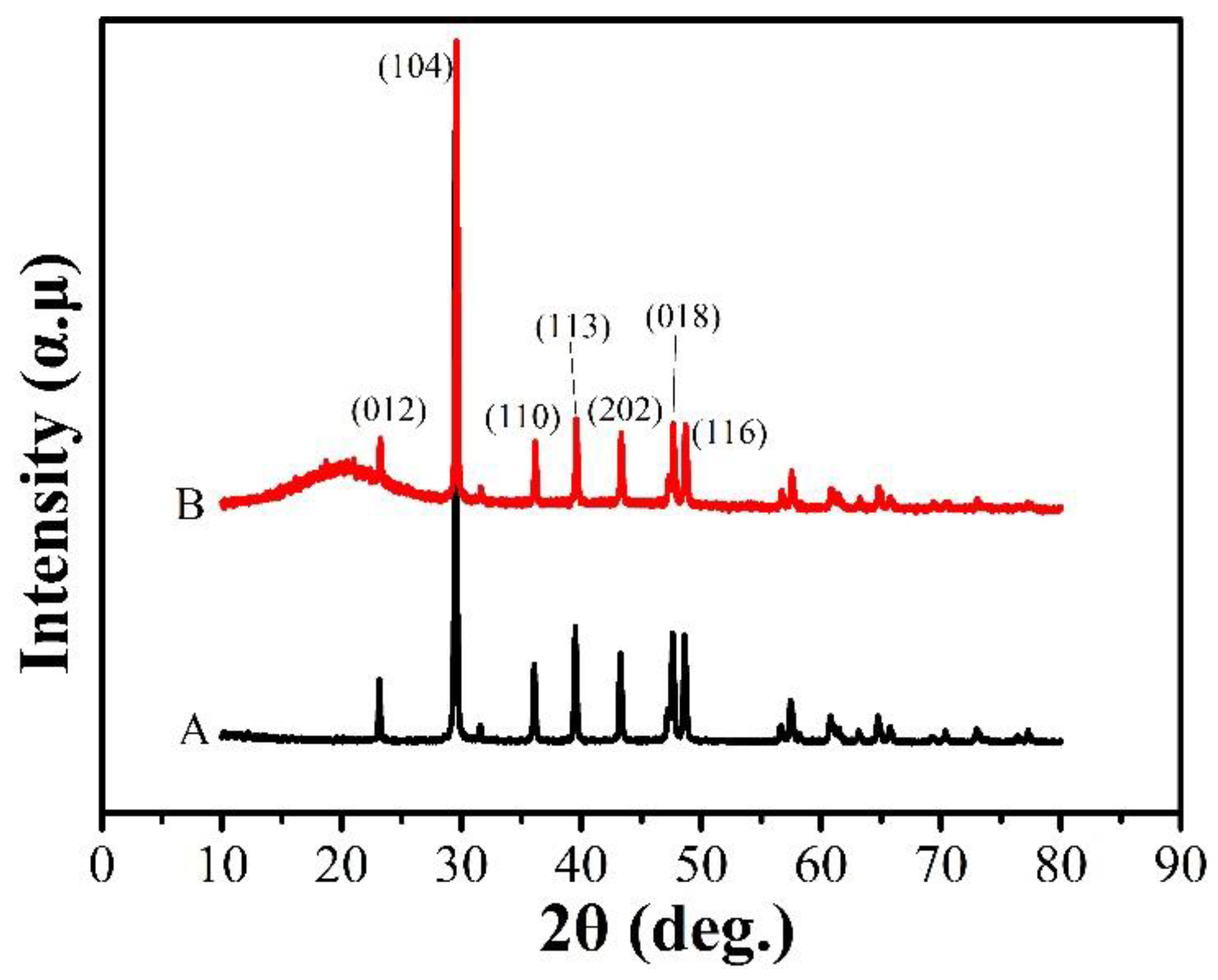
4.1.2. XRD Analysis
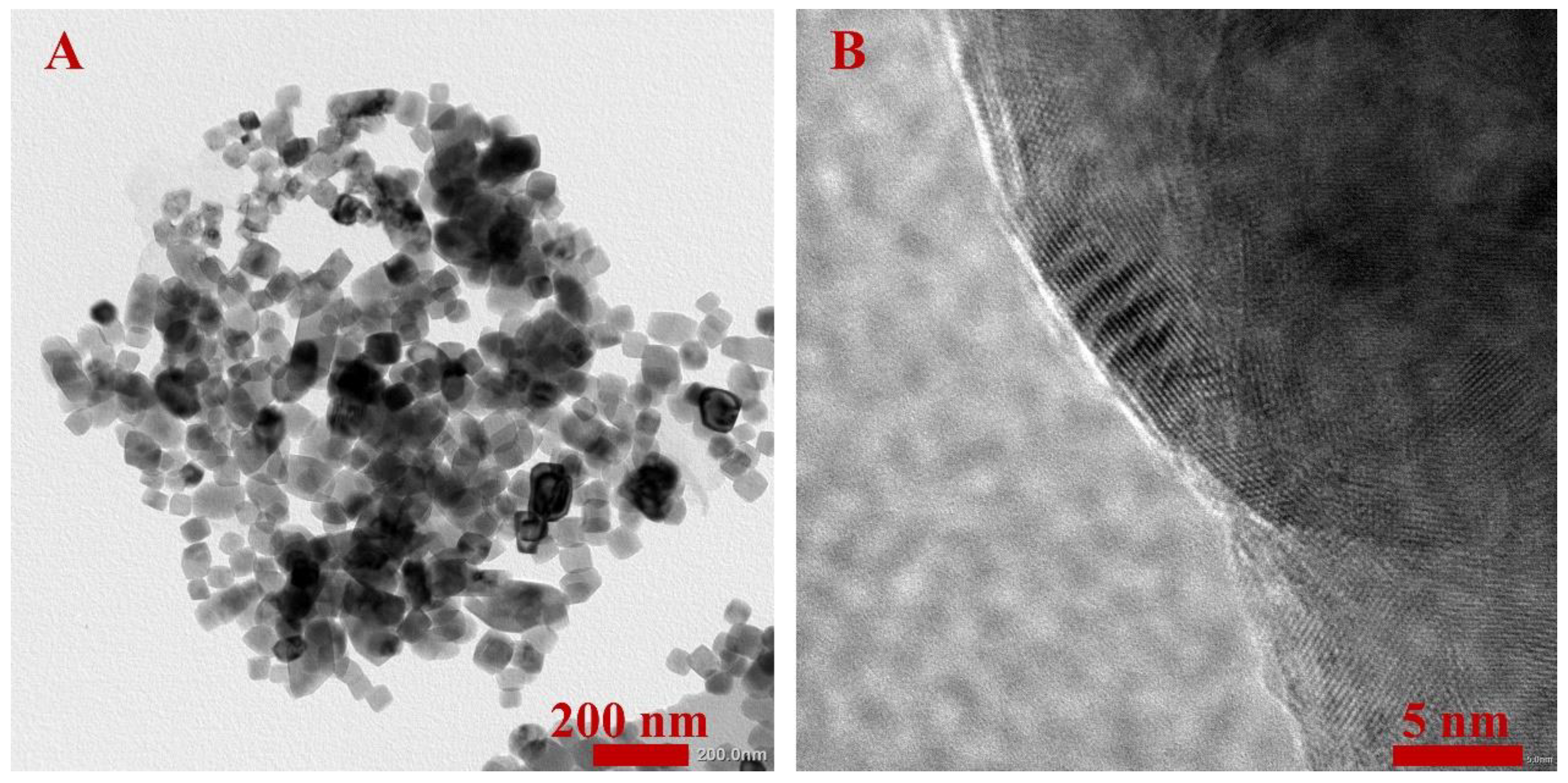
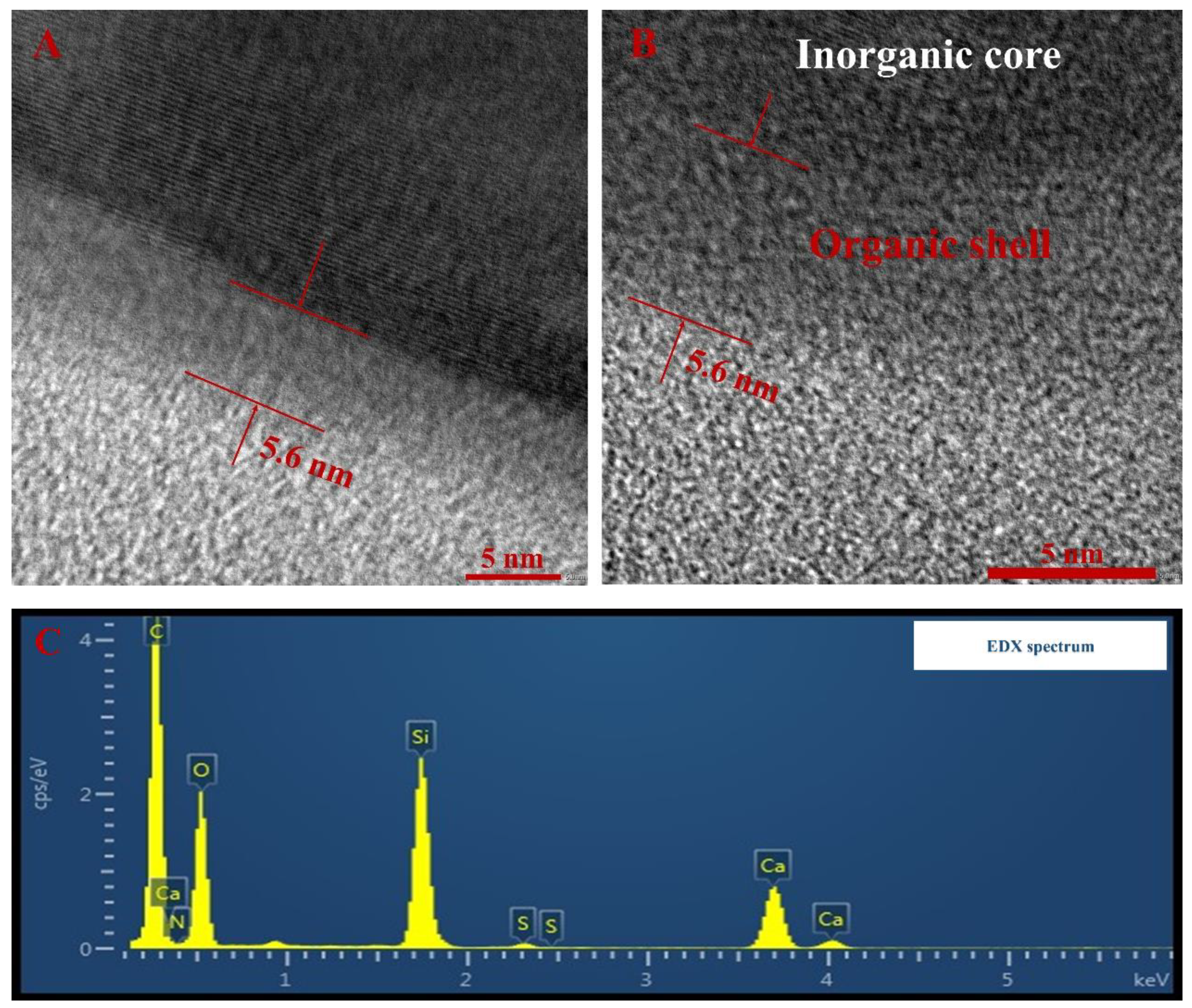
4.1.3. Morphological Analysis
4.1.4. Thermal Stability Analysis
4.2. Performance of Asphalt Binders
4.2.1. Polymer Phase Morphology
4.2.2. Conventional Properties Analysis
4.2.3. DSR Test
4.2.4. BBR Test
5. Conclusions
- (1)
- The results of microstructural characterization proved that the CaCO3 NFs were successfully prepared. Besides, the TG-DSC test confirmed that they were good enough to be applied in the asphalt binder at conventional temperatures due to their excellent thermal stability.
- (2)
- After the CaCO3 NFs addition, for modified asphalt binders, the penetration and softening point increased, and the ductility obviously increased, indicating that the high-temperature performance was negatively affected and the low-temperature performance was improved with the addition of CaCO3 NFs.
- (3)
- The effects of CaCO3 NFs addition on rheological properties were investigated by DSR tests. The addition had a slight negative effect on the high-temperature properties of asphalt binder. However, the positive influence at low temperatures was rather evident, especially at lower temperature (i.e., below −20 °C).
- (4)
- The BBR tests further confirmed that the addition of CaCO3 NFs enhanced the low-temperature cracking performance of the asphalt binder, and the influence gradually became more obvious with the increase of the modifier percent at temperatures below −12 °C. However, further research is needed to determine the optimal amount.
- (5)
- SEM observation indicates that the compatibility with asphalt binder was improved after chemically grafting the surface of the CaCO3 NPs into CaCO3 NFs. Compared with the 5% CaCO3 NPs addition, the same dosage of CaCO3 NFs showed better compatibility with asphalt binder.
- (6)
- The CaCO3 NFs addition provides new ideas for the better application of original nanomaterials in asphalt binder. However, based on the content of each component in the CaCO3 NFs, the price required was about three times that of the original CaCO3 NPs at the same weight. Moreover, the energy consumption during the complicated preparation process also increased costs, so inexpensive production technology is required for further development.
Author Contributions
Funding
Conflicts of Interest
References
- Khattak, M.J.; Khattab, A.; Rizvi, H.R.; Zhang, P. The impact of carbon nano-fiber modification on asphalt binder rheology. Constr. Build. Mater. 2012, 30, 257–264. [Google Scholar] [CrossRef]
- Amirkhanian, A.N.; Xiao, F.P.; Amirkhanian, S.N. Evaluation of high temperature rheological characteristics of asphalt binder with carbon nano particles. J. Test Eval. 2016, 39, 1–9. [Google Scholar]
- Yang, Z.; Hollar, J.; Shi, X. Surface-sulfonated polystyrene microspheres improve crack resistance of carbon microfiber-reinforced Portland cement mortar. J. Mater. Sci. 2010, 45, 3497–3505. [Google Scholar] [CrossRef]
- Yao, H.; You, Z.; Li, L.; Goh, S.W.; Lee, C.H.; Yap, Y.K.; Shi, X.M. Rheological properties and chemical analysis of nanoclay and carbon microfiber modified asphalt with Fourier transform infrared spectroscopy. Constr. Build. Mater. 2013, 38, 327–337. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, C.; Tan, B.; Shi, C. Effect of organic layered silicate on microstructures and aging properties of styrene–butadiene–styrene copolymer modified bitumen. Constr. Build. Mater. 2014, 68, 31–38. [Google Scholar] [CrossRef]
- Abdelrahman, M.; Katti, D.R.; Ghavibazoo, A.; Upadhyay, H.B.; Katti, K.S. Engineering physical properties of asphalt binders through nanoclay-asphalt interactions. J. Mater. Civ. Eng. 2014, 26, 1–9. [Google Scholar] [CrossRef]
- Nazzal, M.D.; Kaya, S.; Gunay, T.; Ahmedzade, P. Fundamental characterization of asphalt clay nanocomposites. J. Nanomech. Micromech. 2013, 3, 1–8. [Google Scholar] [CrossRef]
- Goh, S.W.; Akin, M.; You, Z.; Shi, X. Effect of deicing solutions on the tensile strength of micro- or nano-modified asphalt mixture. Constr. Build. Mater. 2011, 25, 195–200. [Google Scholar] [CrossRef]
- Fang, C.; Yu, R.; Liu, S.; Li, Y. Nanomaterials applied in asphalt modification: A review. J. Mater. Sci. Technol. 2013, 29, 589–594. [Google Scholar] [CrossRef]
- Li, R.; Xiao, F.; Amirkhanian, S.; You, Z.; Huang, J. Developments of nano materials and technologies on asphalt materials—A review. Constr. Build. Mater. 2017, 143, 633–648. [Google Scholar] [CrossRef]
- Zare-Shahabadi, A.; Shokuhfar, A.; Ebrahimi-Nejad, S. Preparation and rheological characterization of asphalt binders reinforced with layered silicate nanoparticles. Constr. Build. Mater. 2010, 24, 1239–1244. [Google Scholar] [CrossRef]
- Li, R.; Pei, J.; Li, X.; Guo, Q. Preparation, mechanical and electric properties of polypropylene fiber reinforced lead zirconate titanate flexible materials. Ferroelectrics 2019, 540, 162–178. [Google Scholar] [CrossRef]
- Li, P.; Li, Y.L. Review on Nano Modified Asphalt. Appl. Mech. Mater. 2014, 587, 1220–1223. [Google Scholar] [CrossRef]
- Li, R.; Dai, Y.; Wang, P.; Sun, C.; Zhang, J.; Pei, J. Evaluation of nano-ZnO dispersed state in bitumen with digital imaging processing techniques. J. Test. Eval. 2018, 46, 974–983. [Google Scholar] [CrossRef]
- Wang, H.P.; Gong, M.H.; Yang, J.; Niu, X.W. Advances in Nanometer Modified Asphalt. Pet. Pitch 2015, 29, 51–58. [Google Scholar]
- Zhang, J.; Li, Z.; Li, M.; Xu, J.; Yin, W.; Liu, L. A study on mutual adaptability and mechanism of dispersion and stability of nanometer-modified asphalt. Highway 2005, 8, 142–146. [Google Scholar]
- Ma, F.; Zhang, C.; Fu, Z. Performance & Modification Mechanism of Nano-CaCO3 Modified Asphalt. J. Wuhan Univ. Technol. 2007, 31, 88–91. [Google Scholar]
- Peplow, M. Nanoparticles go with the flow. Nature 2004, 432, 688. [Google Scholar]
- Bourlinos, A.B.; Herrera, R.; Chalkias, N.; Jiang, D.D.; Zhang, Q.; Archer, L.A.; Giannelis, E.P. Surface-Functionalized Nanoparticles with Liquid-Like Behavior. Adv. Mater. 2005, 17, 234–237. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Chowdhury, S.R.; Jiang, D.D.; Zhang, Q. Weakly solvated PEG-functionalized silica nanoparticles with liquid-like behavior. J. Mater. Sci. 2005, 40, 5095–5097. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Giannelis, E.P.; Zhang, Q.; Archer, L.A.; Floudas, G.; Fytas, G. Surface-functionalized nanoparticles with liquid-like behavior: The role of the constituent components. Eur. Phys. J. 2006, 20, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Dong, L.; Deng, W.; Zhu, Q.; Liu, Y.; Xiong, C. Solvent-free Fluids Based on Rhombohedral Nanoparticles of Calcium Carbonate. J. Am. Chem. Soc. 2009, 26, 9148–9149. [Google Scholar] [CrossRef] [PubMed]
- Stulirova, J.; Pospisil, K. Observation of bitumen microstructure changes using scanning electron microscopy. Road. Mater. Pavement 2008, 9, 745–754. [Google Scholar] [CrossRef]
- Puente-Lee, I.; Schabes-Retchkiman, P.S.; Rojas-García, J.; Ríos Guerrero, L.; Herrera-Nájera, R. Morphology of SBS-modified asphalt using low vacuum SEM. Microsc. Microanal. 2003, 9, 446–447. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Chen, Z.; Xu, G.; Shi, C. Physical, rheological and chemical characterization of aging behaviors of thermochromic asphalt binder. Fuel 2018, 211, 850–858. [Google Scholar] [CrossRef]
- Wei, J.; Liu, Z.; Zhang, Y. Rheological properties of amorphous poly alpha olefin (APAO) modified asphalt binders. Constr. Build. Mater. 2013, 48, 533–539. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Z.; Xu, G. Evaluation of aging behaviors of asphalt binders through different rheological indices. Fuel 2018, 221, 78–88. [Google Scholar] [CrossRef]
- Yang, X.; You, Z. High temperature performance evaluation of bio-oil modified asphalt binders using the DSR and MSCR tests. Constr. Build. Mater. 2015, 76, 380–387. [Google Scholar] [CrossRef]
- Xing, X.Y.; Pei, J.Z.; Li, R.; Tan, X.Y. Effect and mechanism of calcium carbonate whisker on asphalt binder. Mater. Res. Express. 2019, 6, 055306. [Google Scholar] [CrossRef]
- Cai, J.; Song, C.; Zhou, B.; Tian, Y.; Li, R.; Zhang, J.; Pei, J. Investigation on high-viscosity asphalt binder for permeable asphalt concrete with waste materials. J. Clean. Prod. 2019, 228, 40–51. [Google Scholar] [CrossRef]
- Yao, H.; You, Z.; Li, L.; Goh, S.W.; Mills-Beale, J.; Shi, X.; Wingard, D. Evaluation of asphalt blended with low percentage of carbon micro-fiber and nanoclay. J. Test. Eval. 2013, 41, 278–288. [Google Scholar] [CrossRef]
- Colbert, B.; You, Z. The properties of asphalt binder blended with variable quantities of recycled asphalt using short term and long term aging simulations. Constr. Build. Mater. 2012, 26, 552–557. [Google Scholar] [CrossRef]
- Sui, C.; Farrar, M.J.; Tuminello, W.H.; Turner, T.F. New Technique for Measuring Low-Temperature Properties of Asphalt Binders with Small Amounts of Material. Transp. Res. Rec. J. Transp. Res. Board 2010, 2179, 23–28. [Google Scholar] [CrossRef]
- Das, P.K.; Tasdemir, Y.; Birgisson, B. Low temperature cracking performance of WMA with the use of the Superpave indirect tensile test. Constr. Build. Mater. 2012, 30, 643–649. [Google Scholar] [CrossRef]
- Yildirim, Y. Polymer modified asphalt binders. Constr. Build. Mater. 2007, 21, 66–72. [Google Scholar] [CrossRef]
- Shen, J.; Amirkhanian, S.; Tang, B. Effects of rejuvenator on performance-based properties of rejuvenated asphalt binder and mixtures. Constr. Build. Mater. 2007, 21, 958–964. [Google Scholar] [CrossRef]
- Lee, S.J.; Amirkhanian, S.N.; Park, N.W.; Kim, K.W. Characterization of warm mix asphalt binders containing artificially long-term aged binders. Constr. Build. Mater. 2009, 23, 2371–2379. [Google Scholar] [CrossRef]
- ASTM. Standard Test Method for Determining the Flexural Creep Stiffness of Asphalt Binder Using the Bending Beam Rheometer (BBR); ASTM Committee D04; ASTM: Madison, WI, USA, 2012. [Google Scholar]
- Lei, Y.A.; Xiong, C.X.; Dong, L.J.; Guo, H.; Su, X.H.; Yao, J.L.; You, Y.J.; Tian, D.M.; Shang, X.M. Ionic liquid of ultralong carbon nanotubes. Small 2007, 3, 1889–1893. [Google Scholar] [CrossRef]
- Du, Z.; Jia, Z.; Rao, G.; Chen, J. Study on surface properties of modified nanometer calcium carbonate. Mod. Chem. Ind. 2001, 21, 42–44. [Google Scholar]
- Wang, Y.Z.; Chen, M.; Zeng, Z.Q. Modification Research Development on Nanometer Calcium Carbonate Surface. Guangdong Chem. Ind. 2008, 35, 42–45. [Google Scholar]





| Properties | Test Values |
|---|---|
| Exterior | White paste |
| Moisture (wt%) | 50 |
| Solid content (wt%) | 50 |
| Average particle size (nm) | 50–70 |
| Specific surface area (m2/g) | 19.2 |
| pH | 9.52 |
| Technical Index | Values | Requirement (MOT, 2004) |
|---|---|---|
| Penetration (25 °C, 0.1 mm) | 81.8 | 80–100 |
| Ductility (15 °C, cm) | 128.1 | ≥100 |
| Softening Point (°C) | 46.2 | ≥45 |
| Density (15 °C, g/cm3) | 1.002 | ≥0.995 |
| Solubility (trichlorethylene)/% | 0.16 | ≤±0.8 |
| Test Items | Dosage (%) | |||
|---|---|---|---|---|
| 0 | 1 | 3 | 5 | |
| Penetration (25 °C, 0.1 mm) | 81.8 | 82.1 | 82.9 | 83.0 |
| Ductility (5 °C, cm) | 7.4 | 8.9 | 10.8 | 12.0 |
| Softening Point (°C) | 46.2 | 45.9 | 45.8 | 45.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, C.; Li, R.; Pei, J.; Cai, J.; Liu, T.; Li, Y. Preparation and the Effect of Surface-Functionalized Calcium Carbonate Nanoparticles on Asphalt Binder. Appl. Sci. 2020, 10, 91. https://doi.org/10.3390/app10010091
Shen C, Li R, Pei J, Cai J, Liu T, Li Y. Preparation and the Effect of Surface-Functionalized Calcium Carbonate Nanoparticles on Asphalt Binder. Applied Sciences. 2020; 10(1):91. https://doi.org/10.3390/app10010091
Chicago/Turabian StyleShen, Chenchen, Rui Li, Jianzhong Pei, Jun Cai, Tao Liu, and Yang Li. 2020. "Preparation and the Effect of Surface-Functionalized Calcium Carbonate Nanoparticles on Asphalt Binder" Applied Sciences 10, no. 1: 91. https://doi.org/10.3390/app10010091





