The Potential Effectiveness of Biochar Application to Reduce Soil Cd Bioavailability and Encourage Oak Seedling Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant and Soil Preparation
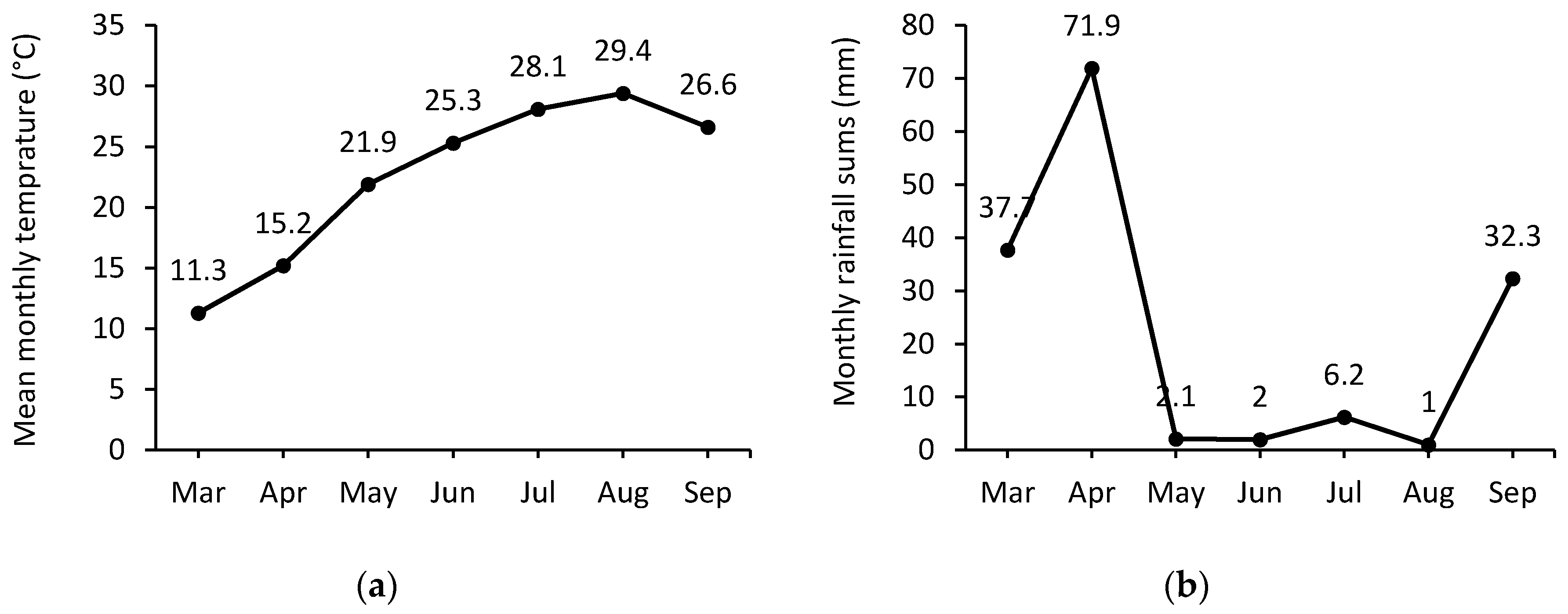
2.2. Climatic Conditions and Irrigation
2.3. Feedstock and Properties of Biochar
2.4. Seedling Height, Growth, and Biomass Analysis
2.5. Selected Soil Properties and Heavy Metal Detection
2.6. Data Analysis
3. Results
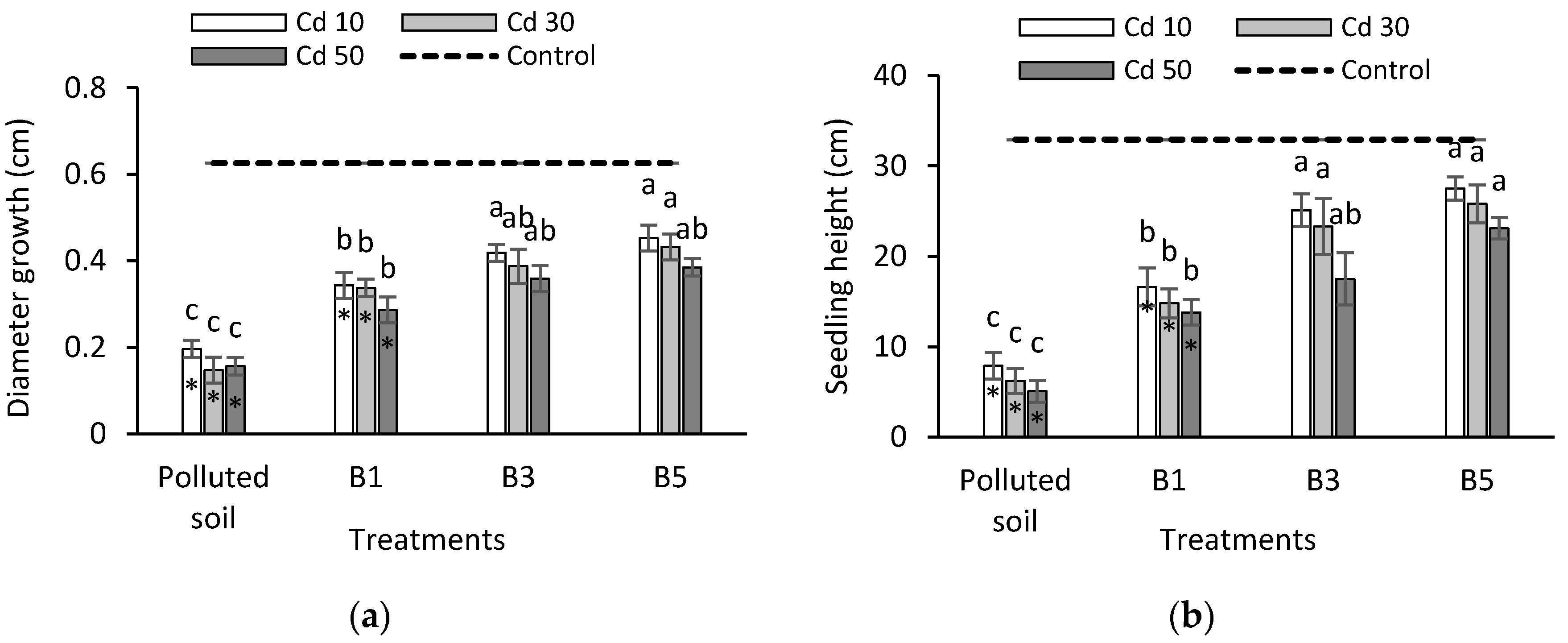
3.1. Seedling Growth
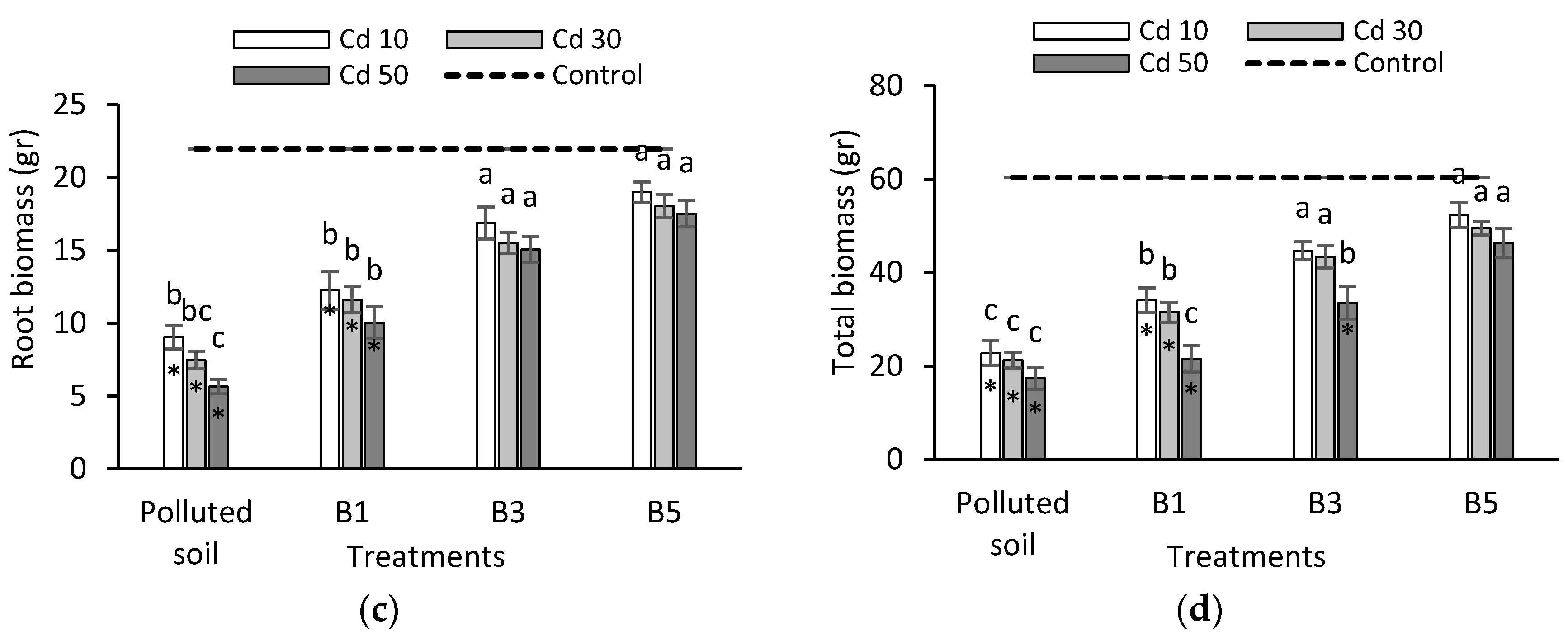
3.2. Seedling Biomass
3.3. Chemical Soil Properties
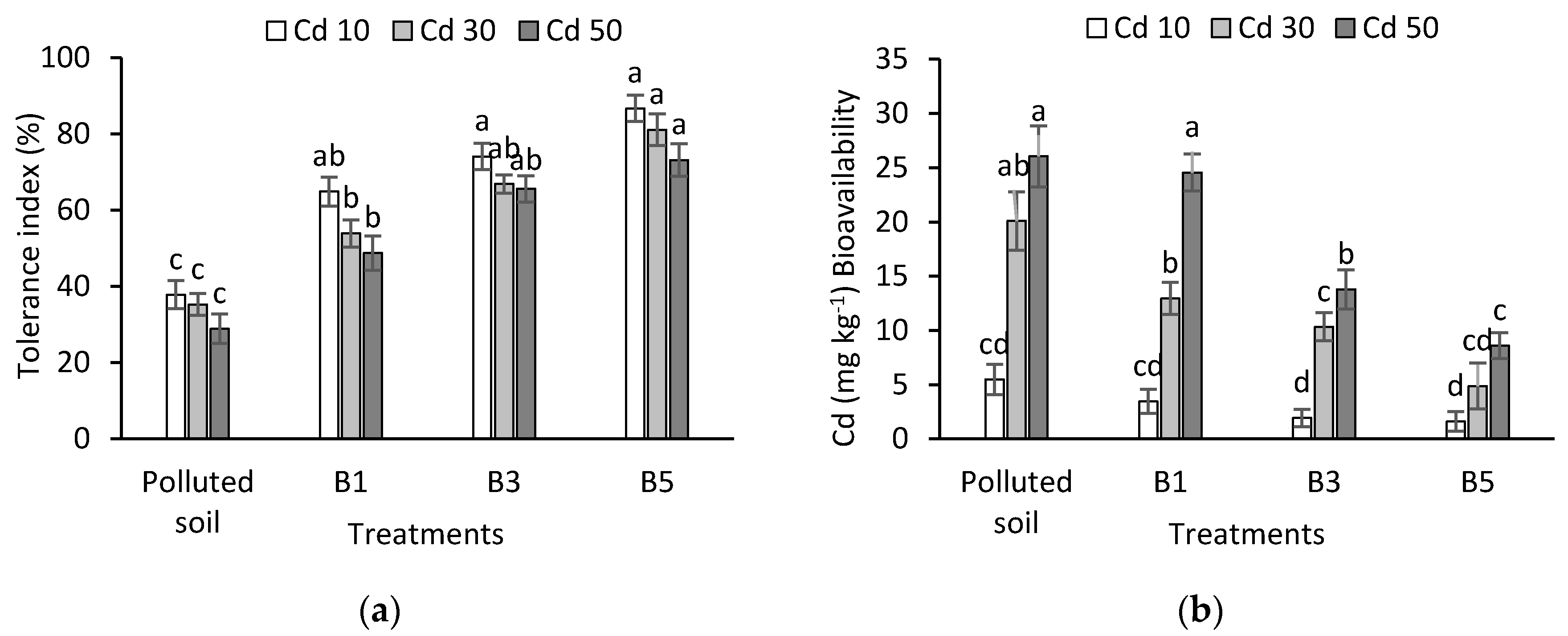
3.4. Tolerance Index and Bioavailability
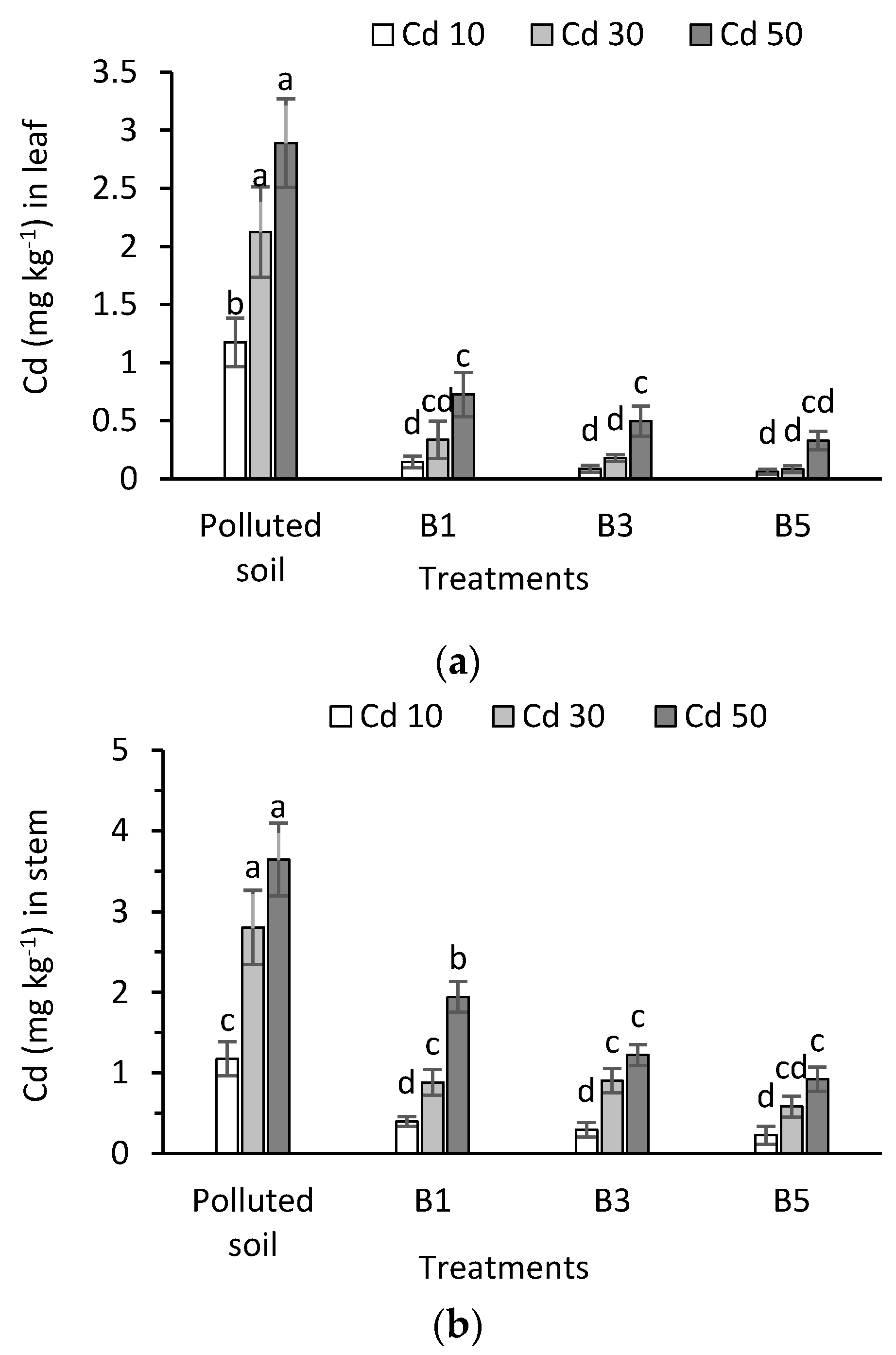
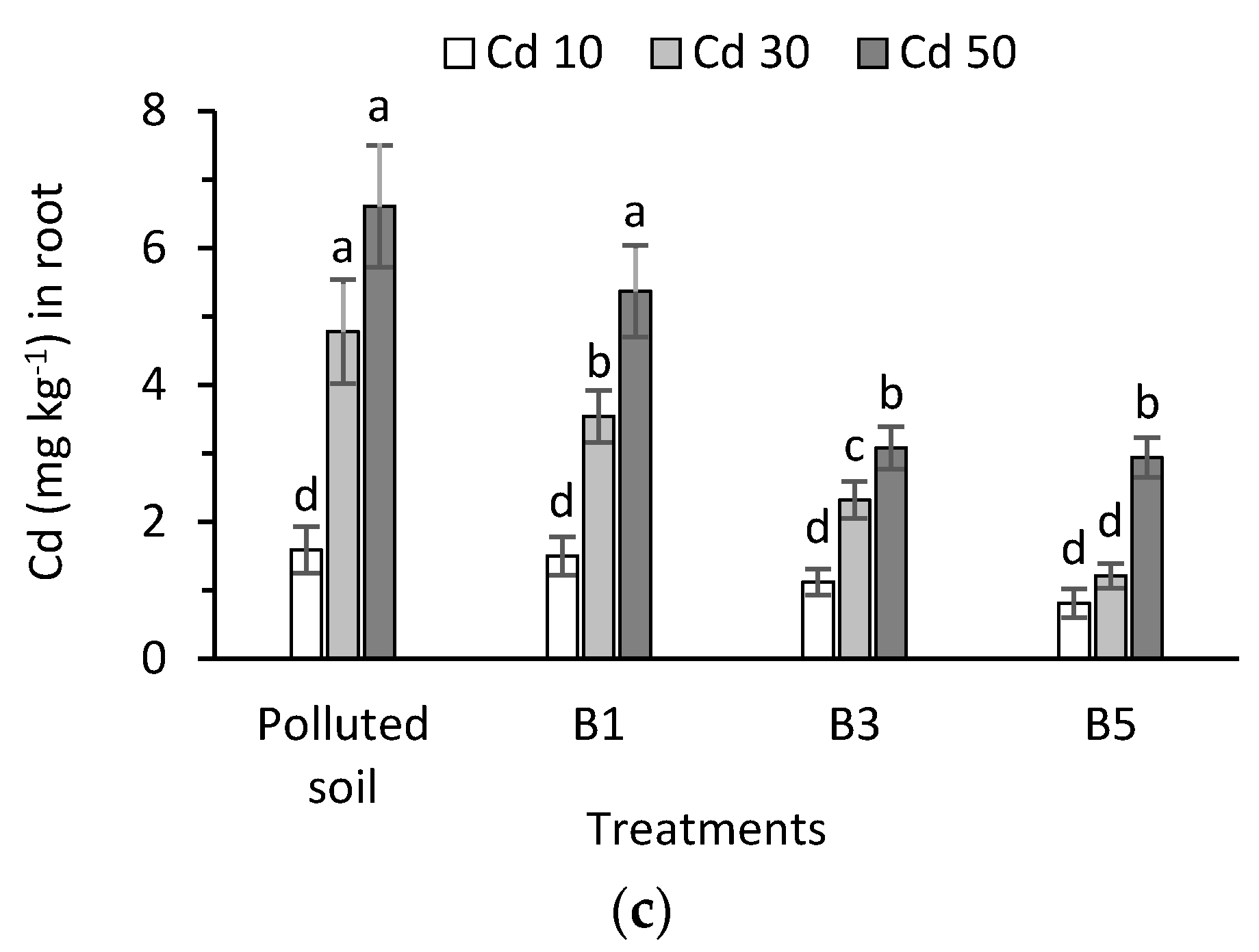
3.5. Cadmium in Plant Tissues
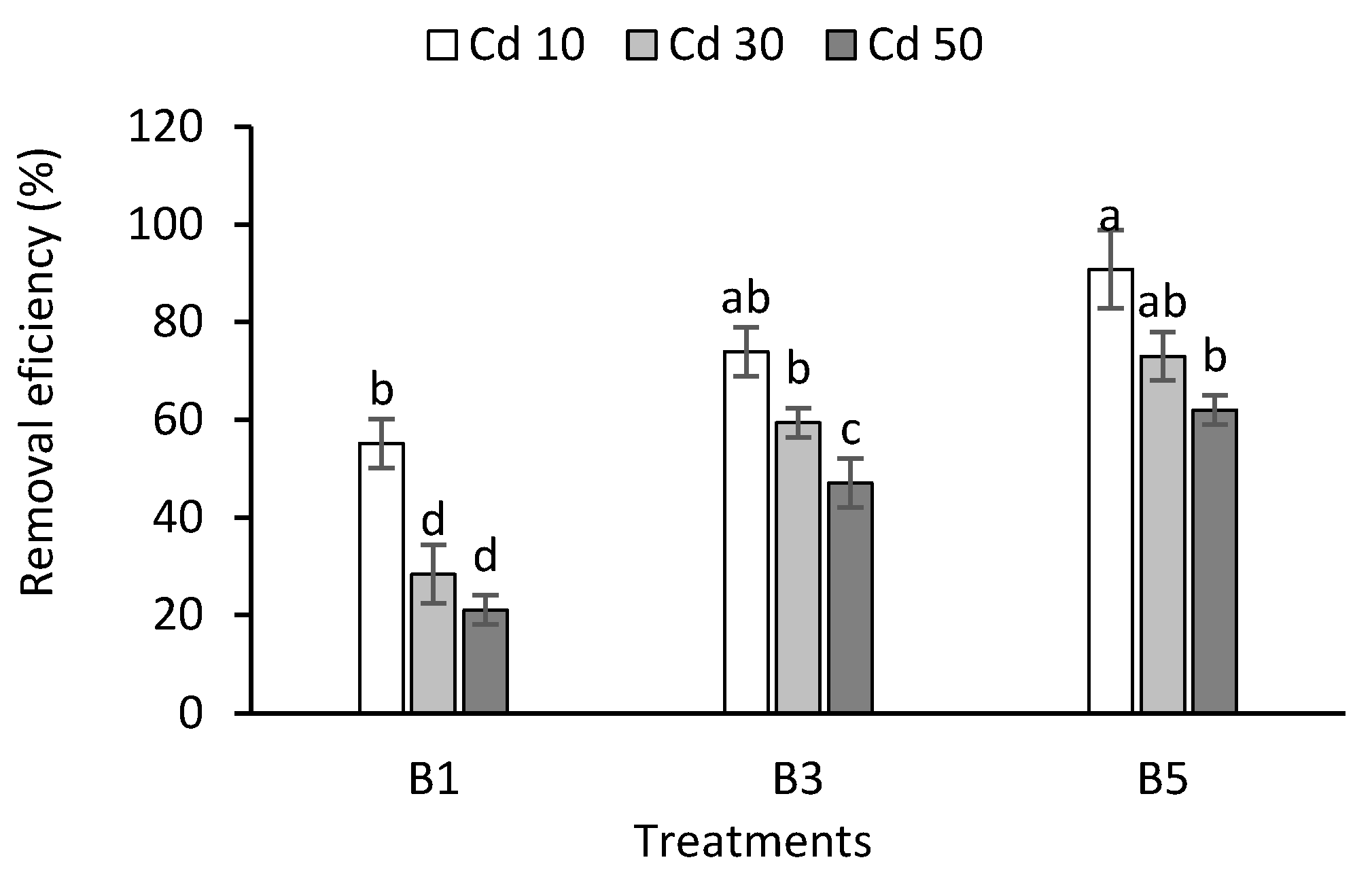
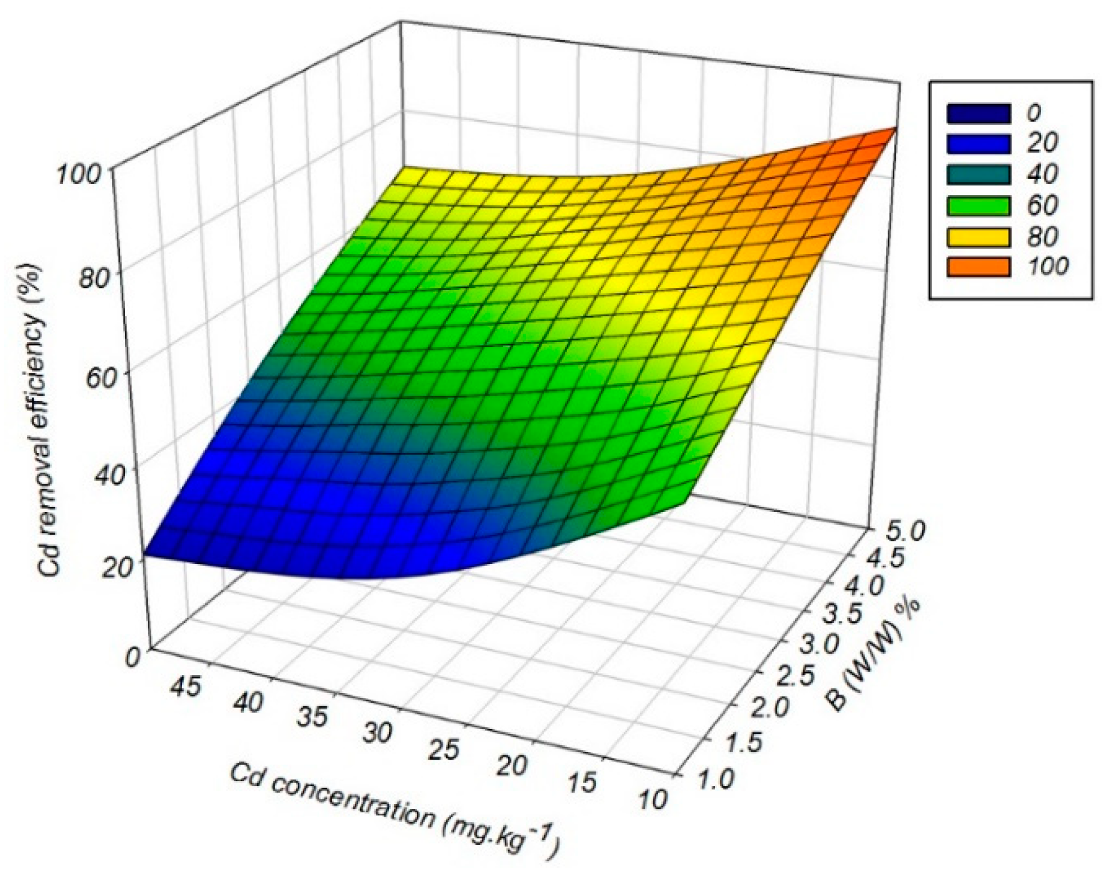
3.6. Cadmium Removal Efficiency
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seneviratne, M.; Weerasundara, L.; Ok, Y.S.; Rinklebe, J.; Vithanage, M. Phytotoxicity attenuation in Vigna radiata under heavy metal stress at the presence of biochar and N fixing bacteria. J. Environ. Manag. 2017, 186, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Al-Wabel, M.I.; Usman, A.R.; El-Naggar, A.H.; Aly, A.A.; Ibrahim, H.M.; Elmaghraby, S.; Al-Omran, A. Conocarpus biochar as a soil amendment for reducing heavy metal availability and uptake by maize plants. Saudi J. Biol. Sci. 2015, 22, 503–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Li, J.; Yue, F.; Yan, X.; Wang, F.; Bloszies, S.; Wang, Y. Effects of arbuscular mycorrhizal inoculation and biochar amendment on maize growth, cadmium uptake and soil cadmium speciation in Cd-contaminated soil. Chemosphere 2018, 194, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Soudek, P.; Valseca, I.R.; Petrová, Š.; Song, J.; Vaněk, T. Characteristics of different types of biochar and effects on the toxicity of heavy metals to germinating sorghum seeds. J. Geochem. Explor. 2017, 182, 157–165. [Google Scholar] [CrossRef]
- Abbas, T.; Rizwan, M.; Ali, S.; Adrees, M.; Mahmood, A.; Zia-ur-Rehman, M.; Ibrahim, M.; Arshad, M.; Qayyum, M.F. Biochar application increased the growth and yield and reduced cadmium in drought stressed wheat grown in an aged contaminated soil. Ecotoxicol. Environ. 2018, 148, 825–833. [Google Scholar] [CrossRef]
- Tekdal, D.; Cetiner, S. Investigation of the effects of salt (NaCl) stress and cadmium (cd) toxicity on growth and mineral acquisition of Vuralia turcica. S. Afr. J. Bot. 2018, 118, 274–279. [Google Scholar] [CrossRef]
- Cui, L.; Pan, G.; Li, L.; Bian, R.; Liu, X.; Yan, J.; Quan, G.; Ding, C.; Chen, T.; Liu, Y.; et al. Continuous immobilization of cadmium and lead in biochar amended contaminated paddy soil: A five-year field experiment. Ecol. Eng. 2016, 93, 1–8. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota: A review. Soil. Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Mohamed, B.A.; Ellis, N.; Kim, C.S.; Bi, X. The role of tailored biochar in increasing plant growth, and reducing bioavailability, phytotoxicity, and uptake of heavy metals in contaminated soil. Environ. Pollut. 2017, 230, 329–338. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Laird, D.A.; Busscher, W.J. Environmental benefits of biochar. J. Environ. Qual. 2012, 41, 967–972. [Google Scholar] [CrossRef] [Green Version]
- Ramzani, P.M.A.; Coyne, M.S.; Anjum, S.; Iqbal, M. In situ immobilization of Cd by organic amendments and their effect on antioxidant enzyme defense mechanism in mung bean (Vigna radiata L.) seedlings. Plant. Physiol. Bioch. 2017, 118, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Amirahmadi, E. Effect of rice husk Biochar (RHB) on some of chemical properties of an acidic soil and the absorption of some nutrients. J. Appl. Sci. Environ. Manag. 2018, 22, 313–317. [Google Scholar] [CrossRef] [Green Version]
- Alobwede, E.; Leake, J.R.; Pandhal, J. Circular economy fertilization: Testing micro and macro algal species as soil improvers and nutrient sources for crop production in greenhouse and field conditions. Geoderma 2019, 334, 113–123. [Google Scholar] [CrossRef]
- Fellet, G.; Marmiroli, M.; Marchiol, L. Elements uptake by metal accumulator species grown on mine tailings amended with three types of biochar. Sci. Total Environ. 2014, 468, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Sabeti, H. Forests, Trees and Shrubs of Iran; Ministry of Agriculture and Natural Resources: Iran, Tehran, 1976.
- Tafazoli, M.; Hojjati, S.M.; Biparva, P.; Kooch, Y.; Lamersdorf, N. Reduction of soil heavy metal bioavailability by nanoparticles and cellulosic wastes improved the biomass of tree seedlings. J. Plant. Nutr. Soil. Sci. 2017, 180, 683–693. [Google Scholar] [CrossRef]
- Zhang, Z.; Solaiman, Z.M.; Meney, K.; Murphy, D.V.; Rengel, Z. Biochars immobilize soil cadmium, but do not improve growth of emergent wetland species Juncus subsecundus in cadmium-contaminated soil. J. Soil. Sediment. 2013, 13, 140–151. [Google Scholar] [CrossRef]
- Gamage, D.N.V.; Mapa, R.B.; Dharmakeerthi, R.S.; Biswas, A. Effect of rice-husk biochar on selected soil properties in tropical Alfisols. Soil Res. 2016, 54, 302. [Google Scholar] [CrossRef]
- Ghorbani, M.; Asadi, H.; Abrishamkesh, S. Effects of rice husk biochar on selected soil properties and nitrate leaching in loamy sand and clay soil. Int. Soil Water Conserv. Res. 2019, 7, 258–265. [Google Scholar] [CrossRef]
- Rajkovich, S.; Enders, A.; Hanley, K.; Hyland, C.; Zimmerman, A.R.; Lehmann, J. Corn growth and nitrogen nutrition after additions of biochars with varying properties to a temperate soil. Biol. Fertil. Soils 2012, 48, 271–284. [Google Scholar] [CrossRef]
- Chan, Y.C.; Vowles, P.D.; McTainsh, G.H.; Simpson, R.W.; Cohen, D.D.; Bailey, G.M. Use of a modified Walkley-Black method to determine the organic and elemental carbon content of urban aerosols collected on glass fibre filters. Chemosphere 1995, 31, 4403–4411. [Google Scholar] [CrossRef]
- Sumner, M.E.; Miller, W.P. Cation exchange capacity and exchange coefficients. Methods Soil Anal. Part 3 Chem. Methods 1996, 5, 1201–1229. [Google Scholar]
- Helmke, P.; Sparks, D. Lithium, sodium, potassium, rubidium, and cesium. Methods Soil Anal. Part 3 Chem. Methods 1996, 551–740. [Google Scholar] [CrossRef]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; United States Department of Agriculture: Washington, DC, USA, 1954.
- Quevauviller, P.; Rauret, G.; López-Sánchez, J.F.; Rubio, R.; Ure, A.; Muntau, H. Certification of trace metal extractable contents in a sediment reference material (CRM 601) following a three-step sequential extraction procedure. Sci. Total. Environ. 1997, 205, 223–234. [Google Scholar] [CrossRef]
- Landberg, T.; Greger, M. Differences in oxidative stress in heavy metal resistant and sensitive clones of Salix viminalis. J. Plant Physiol. 2002, 159, 69–75. [Google Scholar] [CrossRef]
- Poo, K.M.; Son, E.B.; Chang, J.S.; Ren, X.; Choi, Y.J.; Chae, K.J. Biochars derived from wasted marine macro-algae (Saccharina japonica and Sargassum fusiforme) and their potential for heavy metal removal in aqueous solution. J. Environ. Manag. 2018, 206, 364–372. [Google Scholar] [CrossRef]
- Kukier, U.; Chaney, R.L. In situ remediation of nickel phytotoxicity for different plant species. J. Plant Nutr. 2004, 27, 465–495. [Google Scholar] [CrossRef]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of heavy metal (loid) s contaminated soils–to mobilize or to immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef]
- Quintela-Sabaris, C.; Marchand, L.; Kidd, P.S.; Friesl-Hanl, W.; Puschenreiter, M.; Kumpiene, J.; Mueller, I.; Neu, S.; Janssen, J.; Vangronsveld, J.; et al. Assessing phytotoxicity of trace element-contaminated soils phytomanaged with gentle remediation options at ten European field trials. Sci. Total. Environ. 2017, 599, 1388–1398. [Google Scholar] [CrossRef]
- Ramzani, P.M.A.; Iqbal, M.; Kausar, S.; Ali, S.; Rizwan, M.; Virk, Z.A. Effect of different amendments on rice (Oryza sativa L.) growth, yield, nutrient uptake and grain quality in Ni-contaminated soil. Environ. Sci. Pollut. Res. 2016, 23, 18585–18595. [Google Scholar] [CrossRef]
- Nahar, K.; Rahman, M.; Hasanuzzaman, M.; Alam, M.M.; Rahman, A.; Suzuki, T.; Fujita, M. Physiological and biochemical mechanisms of spermine-induced cadmium stress tolerance in mung bean (Vigna radiata L.) seedlings. Environ. Sci. Pollut. Res. 2016, 23, 21206–21218. [Google Scholar] [CrossRef]
- Xu, G.; Shao, H.B.; Sun, J.N. What is more important for enhancing nutrient bioavailability with biochar application into a sandy soil: Direct or indirect mechanism. Ecol. Eng. 2013, 52, 119–124. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; He, L.; Lu, K.; Sarmah, A.; Li, J.; Huang, H. Using biochar for remediation of soils contaminated with heavy metals and organic pollutants. Environ. Sci. Pollut. Res. 2013, 20, 8472–8483. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.L.; Zhang, Q.; Hu, N.; Li, Z.; Lou, Y.; Li, Y.; Xue, D.; Chen, Y.; Wu, C.; et al. Long-term effects of nitrogen fertilization on aggregation and localization of carbon, nitrogen and microbial activities in soil. Sci. Total. Environ. 2018, 624, 1131–1139. [Google Scholar] [CrossRef]
- Cao, X.; Ma, L.; Gao, B.; Harris, W. Dairy-manure derived biochar effectively sorbs lead and atrazine. Environ. Sci. Technol. 2009, 43, 3285–3291. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Rajput, T.B.S.; Kumar, R.; Patel, N. Water and nitrate dynamics in baby corn (Zea mays L.) under different fertigation frequencies and operating pressures in semiarid region of India. Agric. Water Manag. 2016, 163, 263–274. [Google Scholar] [CrossRef]
- Chen, D.; Liu, X.; Bian, R.; Cheng, K.; Zhang, X.; Zheng, J.; Joseph, S.; Crowley, D.; Pan, G.; Li, L. Effects of biochar on availability and plant uptake of heavy metals–A meta-analysis. J. Environ. Manag. 2018, 222, 76–85. [Google Scholar] [CrossRef]
- Weil, R.R.; Brady, N.C. The Nature and Properties of Soils, 15th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2016. [Google Scholar]
- Lončarić, Z.; Karalić, K.; Popović, B.; Rastija, D.; Vukobratović, M. Total and plant available micronutrients in acidic and calcareous soils in Croatia. Cereal. Res. Commun. 2008, 36, 331–334. [Google Scholar]
- Wu, S.; Zhang, Y.; Tan, Q.; Sun, X.; Wei, W.; Hu, C. Biochar is superior to lime in improving acidic soil properties and fruit quality of Satsuma mandarin. Sci. Total. Environ. 2020, 714, 136722. [Google Scholar] [CrossRef]
- Masulili, A.; Utomo, W.H.; Syechfani, M. Rice husk biochar for rice based cropping system in acid soil 1. The characteristics of rice husk biochar and its influence on the properties of acid sulfate soils and rice growth in West Kalimantan, Indonesia. J. Agric. Sci. 2010, 2, 39. [Google Scholar] [CrossRef] [Green Version]
- Singh, C.; Tiwari, S.; Gupta, V.K.; Singh, J.S. The effect of rice husk biochar on soil nutrient status, microbial biomass and paddy productivity of nutrient poor agriculture soils. Catena 2018, 171, 485–493. [Google Scholar] [CrossRef]
- Namgay, T.; Singh, B.; Singh, B.P. Influence of biochar application to soil on the availability of As, Cd, Cu, Pb, and Zn to maize (Zea mays L.). Soil Res. 2010, 48, 638–647. [Google Scholar] [CrossRef]
- Buss, W.; Kammann, C.; Koyro, H.W. Biochar reduces copper toxicity in Chenopodium quinoa Willd. in a sandy soil. J. Environ. Qual. 2012, 41, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Beesley, L.E.; Moreno-Jiménez, L.; Jose, J.L.; Gomez-Eyles, E.; Harris, B.; Robinson, B.; Sizmur, T. A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ. Pollut. 2011, 159, 3269–3282. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.; Shackley, S.; Sohi, S.; Suy, T.B.; Haefele, S. The Impact of Biochar Application on Soil Properties and Plant Growth of Pot Grown Lettuce (Lactuca sativa) and Cabbage (Brassica chinensis). Agronomy 2013, 3, 404–418. [Google Scholar] [CrossRef] [Green Version]
- Lucchini, P.; Quilliam, R.S.; DeLuca, T.H.; Vamerali, T.; Jones, D.L. Does biochar application alter heavy metal dynamics in agricultural soil? Agric. Ecosyst. Environ. 2014, 184, 149–157. [Google Scholar] [CrossRef]
- Lindsay, W.L. Chemical Equilibria in Soils; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 1979. [Google Scholar]
- McBride, M.; Sauve, S.; Hendershot, W. Solubility control of Cu, Zn, Cd and Pb in contaminated soils. Eur. J. Soil Sci. 1997, 48, 337–346. [Google Scholar] [CrossRef]
- Ma, L.; Xu, R.; Jiang, J. Adsorption and desorption of Cu (II) and Pb (II) in paddy soils cultivated for various years in the subtropical China. Int. J. Environ. Sci. 2010, 22, 689–695. [Google Scholar] [CrossRef]
- Shen, X.; Huang, D.Y.; Ren, X.F.; Zhu, H.H.; Wang, S.; Xu, C.; He, Y.B.; Luo, Z.C.; Zhu, Q.H. Phytoavailability of Cd and Pb in crop straw biochar-amended soil is related to the heavy metal content of both biochar and soil. J. Environ. Manag. 2016, 168, 245–251. [Google Scholar] [CrossRef]
- Jien, S.H.; Wang, C.S. Effects of biochar on soil properties and erosion potential in a highly weathered soil. Catena 2013, 110, 225–233. [Google Scholar] [CrossRef] [Green Version]
- Li, J.H.; Lv, G.H.; Bai, W.B.; Liu, Q.; Zhang, Y.C.; Song, J.Q. Modification and use of biochar from wheat straw (Triticum aestivum L.) for nitrate and phosphate removal from water. Desalin. Water Treat 2016, 57, 4681–4693. [Google Scholar]
- Gong, J.; David, A.; Julian, I. Longdistance root-to-shoot transport of phytochelatins and cadmium in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 10118–10123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozano-Rodriguez, E.; Hernandez, L.E.; Bonay, P.; Carpena-Ruiz, R.O. Distribution of cadmium in shoot and root tissues of maize and pea plants: Physiological disturbances. J. Exp. Bot. 1997, 48, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B.; Lin, Y.; Mbog, J.C. Biochar derived from swine manure digestate and applied on the removals of heavy metals and antibiotics. Bioresour. Technol. 2018, 270, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, K.; Baquy, M.A.A.; Xu, R.K. Influence of nitrogen fertilizer forms and crop straw biochars on soil exchange properties and maize growth on an acidic Ultisol. Arch. Agron. Soil. Sci. 2018, 64, 834–849. [Google Scholar] [CrossRef]

| Treatments | Cd10 | Cd30 | Cd50 |
|---|---|---|---|
| Polluted soil | Cd10 | Cd30 | Cd50 |
| Control | - | - | - |
| B1 | B1 + Cd10 | B1 + Cd30 | B1 + Cd50 |
| B3 | B3 + Cd10 | B3 + Cd30 | B3 + Cd50 |
| B5 | B5 + Cd10 | B5 + Cd30 | B5 + Cd50 |
| Physicochemical Parameter | Amount |
|---|---|
| pH | 6.67 ± 0.01 |
| EC (dS/m) | 819 ± 1.08 |
| OC (%) | 6.53 ± 0.23 |
| N (%) | 0.082 ± 0.003 |
| P (mg kg−1) | 29.17 ± 0.66 |
| K (mg kg−1) | 550.39 ± 6.73 |
| CEC (cmol(+) kg−1) | 7.97 ± 0.25 |
| Saturation Fluid Moisture (%) | 53.65 ± 0.48 |
| FC (%) | 32 ± 0.52 |
| (% Sand:Silt:Clay) | 50.1:31.8:18.1 |
| Soil Texture | Loamy |
| Indicators | Value | Unit | Characterizations | Value | Unite |
|---|---|---|---|---|---|
| pH | 8.14 | - | Oxygen | 0.001 | % |
| EC | 359 | dS m−1 | Phosphorous | 412 | mg kg−1 |
| H/C | 0.36 | Molar ratio | Sodium | 76.1 | mg kg−1 |
| C/N ratio | 101.7 | - | Potassium | 595 | mg kg−1 |
| CEC | 18.28 | cmol(+) kg−1 | Calcium | 609 | mg kg−1 |
| Carbon | 68.03 | % | Magnesium | 163 | mg kg−1 |
| Nitrogen | 0.64 | % | Iron | 65 | mg kg−1 |
| Hydrogen | 25.12 | % | Zinc | 11.5 | mg kg−1 |
| Percent of Biochar | pH | EC (dS m−1) | OC (%) | CEC (cmol(+) kg−1) | N (mg kg−1) | P (mg kg−1) | K (mg kg−1) |
|---|---|---|---|---|---|---|---|
| 0 (Control) | 6.4 ± 0.02 b | 0.31 ± 0.02 a | 1.1 ± 0.02 b | 8.11 ± 1.02 c | 0.15 ± 0.01 c | 12.1 ± 1.2 c | 8.3 ± 1.2 c |
| 1% | 6.5 ± 0.01 b | 0.27 ± 0.05 a | 1.2 ± 0.04 b | 12.31 ± 1.01 b | 0.4 ± 0.03 b | 21.1 ± 2.1 b | 15.4 ± 1.1 b |
| 3% | 7.17 ± 0.03 a | 0.26 ± 0.02 a | 1.57 ± 0.01 a | 13.35 ± 1.03 ab | 0.5 ± 0.05 b | 35.5 ± 3.3 a | 29.5 ± 1.3 a |
| 5% | 7.45 ± 0.02 a | 0.23 ± 0.04 a | 1.92 ± 0.03 a | 14.68 ± 0.44 a | 0.91 ± 0.08 a | 39.6 ± 3.9 a | 32.7 ± 0.4 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amirahmadi, E.; Mohammad Hojjati, S.; Kammann, C.; Ghorbani, M.; Biparva, P. The Potential Effectiveness of Biochar Application to Reduce Soil Cd Bioavailability and Encourage Oak Seedling Growth. Appl. Sci. 2020, 10, 3410. https://doi.org/10.3390/app10103410
Amirahmadi E, Mohammad Hojjati S, Kammann C, Ghorbani M, Biparva P. The Potential Effectiveness of Biochar Application to Reduce Soil Cd Bioavailability and Encourage Oak Seedling Growth. Applied Sciences. 2020; 10(10):3410. https://doi.org/10.3390/app10103410
Chicago/Turabian StyleAmirahmadi, Elnaz, Seyed Mohammad Hojjati, Claudia Kammann, Mohammad Ghorbani, and Pourya Biparva. 2020. "The Potential Effectiveness of Biochar Application to Reduce Soil Cd Bioavailability and Encourage Oak Seedling Growth" Applied Sciences 10, no. 10: 3410. https://doi.org/10.3390/app10103410
APA StyleAmirahmadi, E., Mohammad Hojjati, S., Kammann, C., Ghorbani, M., & Biparva, P. (2020). The Potential Effectiveness of Biochar Application to Reduce Soil Cd Bioavailability and Encourage Oak Seedling Growth. Applied Sciences, 10(10), 3410. https://doi.org/10.3390/app10103410







