Spectrophotometric Determination of Trace Concentrations of Copper in Waters Using the Chromogenic Reagent 4-Amino-3-Mercapto-6-[2-(2-Thienyl)Vinyl]-1,2,4-Triazin-5(4H)-One: Synthesis, Characterization, and Analytical Applications
Abstract
1. Introduction
2. Experimental
2.1. Chemicals and Reagents
2.2. Apparatus
2.3. Preparation of the AMT Ligand
2.4. Preparation of [Cu(L)(NO3)(H2O)2]•H2O
2.5. Estimation of Cu(II) in Waters Using Recommended Method
2.6. Evaluation of Proposed Method Using Certified Reference Materials
3. Results and Discussion
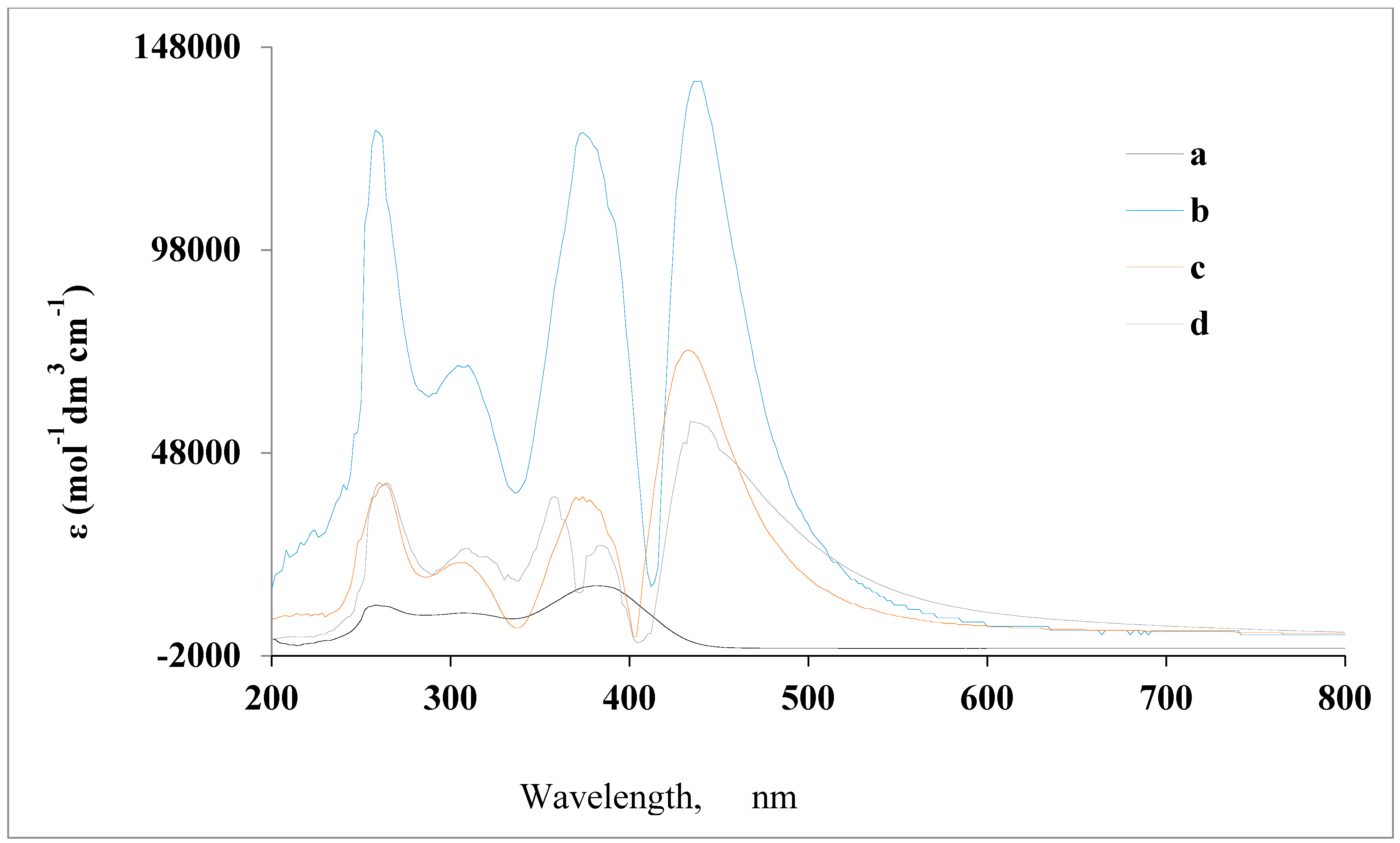
3.1. Characterization
3.1.1. FT-IR Technique
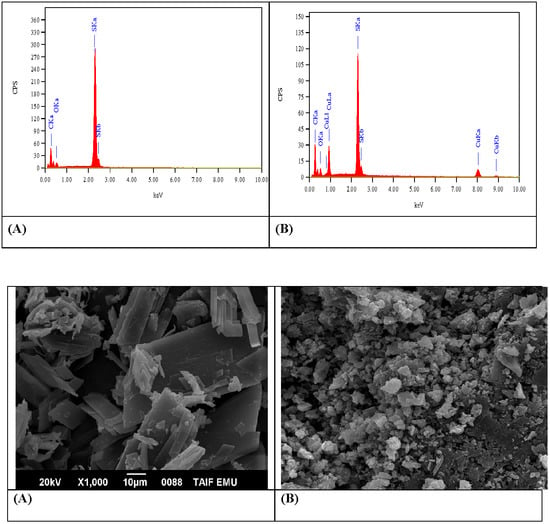
3.1.2. SEM and EDX Analysis
3.1.3. Thermal Analysis
3.1.4. Electron Spin Resonance of [Cu(L)(NO3)(H2O)2]•H2O
3.2. Analytical Application
3.2.1. Optimization of the Recommended Procedure
3.2.2. Investigation of Method Selectivity
3.2.3. Analytical Performance of the Recommended Procedure
3.2.4. Evaluation of the Recommended Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Veitía, M.S.I.; Dumas, F.; Morgant, G.; Sorenson, J.R.J.; Frapart, Y. Synthesis, structural analysis and anticonvulsant activity of a ternary Cu (II) mononuclear complex containing 1,10-phenanthroline and the leading antiepileptic drug valproic acid. Biochimie 2009, 91, 1286–1293. [Google Scholar] [CrossRef] [PubMed]
- Horstkotte, B.; Alexovic, M.; Maya, F.; Duarte, C.M.; Andruch, V.; Cerda, V. Automatic determination of copper by in-syringe dispersive liquid–liquid microextraction of its bathocuproine-complex using long path-length spectrophotometric detection. Talanta 2012, 99, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Kannan, D.; Arumugham, M.N. Synthesis, characterization, DNA-binding studies and antimicrobial activity of copper (II) complex with 1, 10-phenanthroline, L-tyrosine and urea as ligands. Int. J. Inorg. Bioinorg. Chem. 2013, 3, 8–15. [Google Scholar]
- Fornea, V.; Trupina, S. Spectrophotometric determination of Cu(II), Co(II) and Ni(II) ions in mono and multi-component systems. Bull. Polytech. Inst. Iasi Sect. Chem. Chem. Eng. 2016, 62, 9–20. [Google Scholar]
- Baghban, N.; Yilmaz, E.; Soylak, M. Nanodiamond/MoS2 nanorod composite as a novel sorbent for fast and effective vortex-assisted micro solid phase extraction of lead(II) and copper(II) for their flame atomic absorption spectrometric detection. J. Mol. Liq. 2017, 234, 260–267. [Google Scholar] [CrossRef]
- Lima, R.T.; Raposo, J.L.; Virgílio, A.; Neto, J.A.G. Determination of copper at wide range concentrations using instrumental features of high-resolution continuum source flame atomic absorption spectrometry. Eclét. Quím. 2010, 35, 86–92. [Google Scholar] [CrossRef]
- Bagheri, H.; Shirzadmehr, A.; Rezaei, M. Determination of copper ions in foodstuff products with a newly modified potentiometric carbon paste electrode based on a novel nano-sensing layer. Ionics 2016, 22, 1–12. [Google Scholar] [CrossRef]
- Issa, Y.M.; Ibrahim, H.; Shehab, O.R. New copper(II)-selective chemically modified carbon paste electrode based on etioporphyrin I dihydrobromide. J. Electroanal. Chem. 2012, 666, 11–18. [Google Scholar] [CrossRef]
- El-Raheem, H.A.; Hassan, R.Y.A.; Khaled, R.; Farghali, A.; El-Sherbiny, I.M. Polyurethane-doped platinum nanoparticles modified carbon paste electrode for the sensitive and selective voltammetric determination of free copper ions in biological samples. Microchem. J. 2020, 155, 104765. [Google Scholar] [CrossRef]
- Reddy, S.K.; Reddy, C.S.; Reddy, G.V.S. Bioaccumulation of trace metals in selected vegetable crops around tummalapalle uranium mine kadapa district, Andhra Pradesh. Orient. J. Chem. 2018, 34, 1078–1090. [Google Scholar] [CrossRef]
- Peng, G.; He, Q.; Zhou, G.; Li, Y.; Su, X.; Liu, M.; Fan, L. Determination of heavy metals in water samples using dual-cloud point extraction coupled with inductively coupled plasma mass spectrometry. Anal. Methods 2015, 7, 6732–6739. [Google Scholar] [CrossRef]
- Huo, F.J.; Yin, C.X.; Yang, Y.T.; Su, J.; Chao, J.B.; Liu, D.S. Ultraviolet-Visible Light (UV-Vis)-Reversible but fluorescence-irreversible chemosensor for copper in water and its application in living cells. Anal. Chem. 2012, 84, 2219–2223. [Google Scholar] [CrossRef] [PubMed]
- Lutfullah; Sharma, S.; Rahman, N.; Azmi, S.N.H.; Iqbal, B.; Amburkb, M.I.B.B.; AlBarwanib, Z.M.H. UV spectrophotometric determination of Cu(II) in synthetic mixture and water samples. J. Chin. Chem. Soc. 2010, 57, 622–631. [Google Scholar] [CrossRef]
- Hashem, E.Y.; Seleim, M.M.; El-Zohry, A.M. Spectrophotometric determination of copper(II) in pharmaceutical, biological and water samples by 4-(2′-benzothiazolylazo)-salicylic acid. J. Appl. Spectrosc. 2011, 78, 586–593. [Google Scholar] [CrossRef]
- Kamble, G.S.; Kolekar, S.S.; Anuse, M.A. Synergistic extraction and spectrophotometric determination of copper(II) using 1-(2,4-dinitro aminophenyl)-4,4,6-trimethyl-1,4-dihydropyrimidine-2-thiol: Analysis of alloys, pharmaceuticals and biological samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 1455–1466. [Google Scholar] [CrossRef]
- Uddin, M.N.; Salam, M.A.; Hossain, M.A. Spectrophotometric measurement of Cu(DDTC)2 for the simultaneous determination of zinc and copper. Chemosphere 2013, 90, 366–373. [Google Scholar] [CrossRef]
- Uddin, M.N.; Shah, N.M.; Hossain, M.A.; Islam, M.M. Copper and Mercury in Food, Biological and Pharmaceutical Samples: Spectrophotometric Estimation as Cu(DDTC)2. Am. J. Anal. Chem. 2014, 5, 838–850. [Google Scholar] [CrossRef]
- Gouda, A.A.; Amin, A.S. Cloud-point extraction, preconcentration and spectrophotometric determination of trace quantities of copper in food, water and biological samples. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 120, 88–96. [Google Scholar] [CrossRef]
- Madan, P.U.; Barhate, V.D. Extractive spectrophotometric determination of copper (II) using 2-(5-bromo-2-oxoindolin-3-ylidene) hydrazine carbothioamide as an analytical reagent. Eur. J. Biomed. Pharma. Sci. 2016, 3, 392–396. [Google Scholar]
- Shaikh, B.; Barache, U.B.; Anuse, M.A.; Gaikwad, S.H. 4-(4′-nitrobenzylideneimino)-3-methyl-5-mercapto-1, 2, 4-triazole, a new chromogenic reagent for extractive spectrophotometric determination of copper (II) in pharmaceutical and alloy samples. S. Afr. J. Chem. 2016, 69, 157–165. [Google Scholar] [CrossRef]
- Admasu, D.; Reddy, D.N.; Mekonnen, K.N. Spectrophotometric determination of Cu(II) in soil and vegetable samples collected from Abraha Atsbeha, Tigray, Ethiopia using heterocyclic thiosemicarbazone. Springer Plus 2016, 1169, 2–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Babayeva, K.; Demir, S.; Andac, M. A novel spectrophotometric method for the determination of copper ion by using a salophen ligand, N,N′-disalicylidene-2,3-diaminopyridine. J. Taibah Univ. Sci. 2017, 11, 808–814. [Google Scholar] [CrossRef]
- Subudhi, K.S.; Sreevani, D.; Rao, K.A. Spectrophotometric determination of trace copper in hair, milk, banana, rice and pharm products with 5-{ά–methyl-3-hydroxybenzylidene} rhodamine [5M, 3H-Br] reagent. Int. J. Res. Anal. Rev. 2019, 6, 809–817. [Google Scholar]
- Lin, Q.; Chen, P.; Liu, J.; Fu, Y.P.; Zhang, Y.M.; Wei, T.B. Colorimetric chemosensor and test kit for detection copper(II) cations in aqueous solution with specific selectivity and high sensitivity. Dyes Pigm. 2013, 98, 100–105. [Google Scholar] [CrossRef]
- Al-Juwaiser, I.A.; Ibrahim, M.R.; Al-Awadi, N.A.; Ibrahim, Y.A. An improved direct synthetic approach to anhydronucleosides. Tetrahedron 2008, 64, 8206–8212. [Google Scholar] [CrossRef]
- Saad, H.A.; Youssef, M.M.; Mosselhi, M.A. Microwave assisted synthesis of some new fused 1,2,4-triazines bearing thiophene moieties with expected pharmacological activitymosselhi. Molecules 2011, 16, 4937–4957. [Google Scholar] [CrossRef]
- Saad, H.A.; Moustafa, A.H. Synthesis and anticancer activity of some new s-glycosyl and s-alkyl 1,2,4-triazinone derivatives. Molecules 2011, 16, 5682–5700. [Google Scholar] [CrossRef]
- El-Nahass, M.M.; Ashour, A.; Atta, A.A.; Saad, H.; Hassanien, A.M.; Al-Baradi, A.M.; El-zaidia, E.F.M. Dielectric relaxation and optical properties of 4-amino-3-mercapto-6-(2-(2-thienyl)vinyl)-1,2,4-triazin-5(4H)-one Donor. Pramana 2017, 88. [Google Scholar] [CrossRef]
- Korostelev, P.P. Preparation of Solutions for Chemical Analysis Works; Publishing House of Academy of Sciences of the USSR: Moscow, Russia, 1964; p. 401. [Google Scholar]
- Lever, A.B.P. Inorganic Electronic Spectroscopy, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Nakamoto, K. Infrared Spectra of Inorganic and Coordination Compounds; Willey: New York, NY, USA, 1970. [Google Scholar]
- Lund, A.; Shiotani, M.; Shimada, S. Principles and Applications of ESR Spectroscopy; Springer: New York, NY, USA, 2011. [Google Scholar]
- Cotton, F.A.; Wilkinson, G. Advanced Inorganic Chemistry, A Comprehensive Text, 4th ed.; John Wiley & Sons: New York, NY, USA, 1986. [Google Scholar]
- Geaey, W.J. The Use of Conductivity Measurements in Organic Solvents for the Characterisation of Coordination Compounds. Coord. Chem. Rev. 1971, 7, 81–122. [Google Scholar]
- Hamza, A.; Bashammakh, A.S.; Al-Sibaai, A.A.; Al-Saidi, H.M.; El-Shahawi, M.S. Part 1. Spectrophotometric determination of trace mercury (II) in dental-unit wastewater and fertilizer samples using the novel reagent 6-hydroxy-3-(2-oxoindolin-3-ylideneamino)-2-thioxo-2H-1,3-thiazin-4(3H)-one and the dual-wavelength β- correction spectrophotometry. J. Hazard. Mater. 2010, 178, 287–292. [Google Scholar]
- Barache, U.B.; Shaikh, A.B.; Lokhande, T.N.; Kamble, G.S.; Anuse, M.A.; Gaikwad, S.H. An efficient, cost effective, sensing behavior liquid-liquid extraction and spectrophotometric determination of copper(II) incorporated with 4-(4’-chlorobenzylideneimino)-3-methyl-5-mercapto-1, 2, 4-triazole: Analysis of food samples, leafy vegetables, fertilizers and environmental samples. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 189, 443–453. [Google Scholar] [PubMed]
- Jain, A.P.; Puri, B.K. Atomic absorption spectrometric determination of copper after adsorption of its sodiumdiethyldithiocarbamate complex on a new polymeric adsorbent. Indian J. Chem. 2006, 40, 409–411. [Google Scholar]
- Ghaedi, M.; Montazerozohori, K.M.M.; Shokrollahi, A.; Soylak, M. Flame atomic absorption spectrometric (FAAS) determination of copper, iron and zinc in food samples after solid-phase extraction on Schiff base-modified duolite XAD 761. Mater. Sci. Eng. C 2013, 33, 2338–2344. [Google Scholar] [CrossRef] [PubMed]
- Al-Saidi, H.S. Biosorption using chitosan thiourea polymer as an extraction and preconcentration technique for copper prior to its determination in environmental and food samples by flame atomic absorption spectrometry: Synthesis, characterization and analytical applications. Int. J. Biol. Macromol. 2016, 93, 390–401. [Google Scholar]














| AMT Reagent | Tentative Assignments | Cu(II)-AMT Complex | Tentative Assignments |
|---|---|---|---|
| 3291, 3195 strong | ν(NH2) | 3142 weak | ν(NH) |
| 3064–3091 weak | ν(aromatic C–H) | 3066 weak | ν(aromatic C–H) |
| 2936 weak | ν(aliphatic C–H) | 2901 weak | ν(aliphatic C–H) |
| 2665 weak | ν(SH) | 2667 weak | ν(SH) |
| 1660 very strong | ν(C=O) | 1705 very strong | ν(C=O) |
| 1593 strong | ν(C=C) | 1554 strong | ν(C=C) |
| 1618 strong | ν(C=N) | 1618 strong | ν(C=N) |
| 1401 strong | δ(N–C–S) | 1421 strong | δ(N-C-S) |
| 1420 medium | δ(aliphatic C–H) | 1412 medium | δ(aliphatic C–H) |
| 530 medium | ν(Cu–N) | ||
| 518 medium | ν(Cu–O) | ||
| 1545, 1353, 820 | Nitrate group |
| Element | Weight % Calculated | Weight % Found | |
| AMT | C | 42.85 | 42.90 |
| H | 3.21 | 3.16 | |
| N | 22.22 | 22.03 | |
| O | 6.34 | 6.56 | |
| S | 25.42 | 25.81 | |
| Weight % Calculated | Weight % Found | ||
| Cu(II)-AMT | C | 25.06 | 25.76 |
| H | 3.27 | 2.45 | |
| N | 16.22 | 15.36 | |
| O | 25.93 | 24.50 | |
| S | 14.81 | 14.44 | |
| Cu | 14.59 | 14.36 * |
| Coexisting Ion | Diverse Ion/Copper(II) | Coexisting Ion | Diverse Ion/Copper (II) |
|---|---|---|---|
| Na+ | 1200 | Co2+ | 100 |
| K+ | 1000 | Ni2+ | 100 |
| Li+ | 1000 | Ba2+ | 100 |
| Zn2+ | 1000 | Cr3+ | 100 |
| Ca2+ | 1000 | Pb2+ | 100 |
| Ag+ | 1000 | Sr2+ | 100 |
| F− | 500 | NO2− | 200 |
| Cl− | 500 | Mn2+ * | 5 |
| Bi3+ | 300 | Al3+ * | 5 |
| Variable | Value |
|---|---|
| Wavelength, λmax (nm) | 434 |
| pH | 4.5 |
| Temperature (°C) | 25 ± 2 |
| Linear dynamic range (µg/mL) | 0.7–25 |
| LOD, µg/mL | 0.011 |
| RSD,% (n = 10) | 1.4 |
| RE,% (n = 10) | 1.2 |
| Slope | 0.18 |
| Intercept | 0.009 |
| ε, (L/mol.cm) | 1.9 × 104 |
| Sandell’s factor (μg cm−2) | 0.003 |
| Correlation factor (R2) | 0.999 |
| Chromogenic Reagent | λmax (nm) | pH | Molar Absorptivity, L/mol.cm | Linear Dynamic Range μg/mL | Detection Limit, (LOD), (μg/mL) | Interferences | Ref. |
|---|---|---|---|---|---|---|---|
| Cefixime | 336 | 4.68 | 8.29 × 103 | 1.015–8.122 | 0.032 | Hg2+,Al3+,Zn2+,Fe3+,Mn2+ | [13] |
| BTAS | 485 | 5.0 | 2.35 × 104 | 0.63–5.04 | 7 × 10−3 | Sn2+,Cd2+,Co2+ | [14] |
| 2,4-dinitro APTPT | 445 | 8.7 | 0.87 × 103 | 10–80 | 1.72 | Sb3+,Mn2+,Ag+,Pb2+,Co2+,Hg2+ | [15] |
| DDTC | 435 | 5 | 2.86 × 105 | 0.2–12 | 0.2 | Fe2+,Ni2+,Pb2+,Mn2+ | [16] |
| DDTC | 435 | 5 | 3.61 × 105 | 0.02–12.0 | 0.029 | Ag+,Pb2+ | [17] |
| ATAP | 608 | 4.5 | 4.37 × 105 | 4 × 10−3–0.115 | 1.2 × 10−3 | Fe3+,Sb3+,Mo5+,NO2- | [18] |
| HBITSC | 510 | 4 | 2.5 × 103 | 1–8 | NM | Co2+,Fe2+,Fe3+,Ni2+,Cr3+ | [19] |
| NBIMMT | 470 | 6.2 | 2.8 × 103 | 4.75–16.13 | NM | Pb2+,Cd2+,Sn2+,Fe2+,Zn2+,Ag+,Al3+ | [20] |
| 2-APT | 370 | 8 | 2.14 × 104 | 0.16–1.3 | 0.053 | NM | [21] |
| H2IF | 414 | 4.8 | 1.46 × 105 | 6.35 × 10−3–0.318 | 6.38 × 10−3 | There is no interference at1:10 ratio | [22] |
| 5M, 3H-BR | 430 | 5.5 | 0.603 × 104 | 0.05–13 | NM | There is no interference at1:10 ratio | [23] |
| Rubeanic acid | 380 | 3.5 | 1.01 × 104 | 0.65–2.65 | NM | Co2+,Ni2+ | [4] |
| CBIMMT | 414 | 4.2 | 3.38 × 103 | 5–17.5 | NM | Ni2+,Pb2+,Al3+,Bi3+ | [37] |
| AMT | 434 | 4–6 | 1.9 × 104 | 0.7–25 | 0.011 | Mn2+,Al3+ | PW |
| Analysis Technique | pH | LOD, μg/mL | Concentration Range, μg/mL | Interferences | Ref. |
|---|---|---|---|---|---|
| Micro SPE-AAS | 3 | 0.022 | 0.05–1 | Fe3+,Ni2+,Co2+,Zn2+ | [5] |
| AAS with high resolution continuum source | Acidic medium | 0.021–1.4 * | 0.07–100 * | NM | [6] |
| Potentiometry using modified carbon paste electrode | 3 | 9.2 × 10−5 | 2.22 × 10−4–635 | Hg2+,Cd2+,Ag+ | [7] |
| Potentiometry using modified carbon paste electrode | 4.5–8.5 | 0.057 | 0.081–812 | Fe3+,Cd2+,Al3+,Ca2+,Cr3+ | [8] |
| Voltammetry using modified carbon paste electrode | 1.4 | 0.017 | 0.1–1 | Hg2+,Cd2+,Pb2+ | [9] |
| AAS combined with SPE | 5 | NM | 0.15–2 | Al3+,Sn2+,Ni2+,Mn2+ | [37] |
| AAS combined with SPE | 5 | 0.01 | 0.01–0.34 | There is no interference at1Cu:10M ratio | [38] |
| AAS combined with SPE | 5.6 | 0.3 | NM | Fe3+,Co2+ | [39] |
| Direct spectrophotometry | 4–6 | 0.011 | 0.7–25 | Mn2+,Al3+ | PW |
| Sample | Spectrophotometric Method | ICP-OES | ||||
|---|---|---|---|---|---|---|
| Amount of Added Cu(II) (µg mL−1) | Found (µg mL−1) | Recovery % | Amount of Added Cu(II) (µg mL−1) | Found (µg mL−1) | Recovery % | |
| Red Sea water | ___ | ND | ___ | ___ | ND | ___ |
| Red Sea water | 4 | 3.79 ± 0.31 | 95.0 | 4 | 3.62 ± 0.22 | 90.5 |
| Tap water | ___ | ND | ___ | ___ | ND | ___ |
| Tap water | 4 | 3.95 ± 0.23 | 98.7 | 4 | 3.78 ± 0.15 | 95.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharthi, S.S.; Al-Saidi, H.M. Spectrophotometric Determination of Trace Concentrations of Copper in Waters Using the Chromogenic Reagent 4-Amino-3-Mercapto-6-[2-(2-Thienyl)Vinyl]-1,2,4-Triazin-5(4H)-One: Synthesis, Characterization, and Analytical Applications. Appl. Sci. 2020, 10, 3895. https://doi.org/10.3390/app10113895
Alharthi SS, Al-Saidi HM. Spectrophotometric Determination of Trace Concentrations of Copper in Waters Using the Chromogenic Reagent 4-Amino-3-Mercapto-6-[2-(2-Thienyl)Vinyl]-1,2,4-Triazin-5(4H)-One: Synthesis, Characterization, and Analytical Applications. Applied Sciences. 2020; 10(11):3895. https://doi.org/10.3390/app10113895
Chicago/Turabian StyleAlharthi, Salman. S., and Hamed. M. Al-Saidi. 2020. "Spectrophotometric Determination of Trace Concentrations of Copper in Waters Using the Chromogenic Reagent 4-Amino-3-Mercapto-6-[2-(2-Thienyl)Vinyl]-1,2,4-Triazin-5(4H)-One: Synthesis, Characterization, and Analytical Applications" Applied Sciences 10, no. 11: 3895. https://doi.org/10.3390/app10113895
APA StyleAlharthi, S. S., & Al-Saidi, H. M. (2020). Spectrophotometric Determination of Trace Concentrations of Copper in Waters Using the Chromogenic Reagent 4-Amino-3-Mercapto-6-[2-(2-Thienyl)Vinyl]-1,2,4-Triazin-5(4H)-One: Synthesis, Characterization, and Analytical Applications. Applied Sciences, 10(11), 3895. https://doi.org/10.3390/app10113895