Antioxidant and Antimicrobial Activity of Porcine Liver Hydrolysates Using Flavourzyme
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Chemicals, and Apparatus
2.2. Preparation of Enzymatic Extracts from Porcine Liver
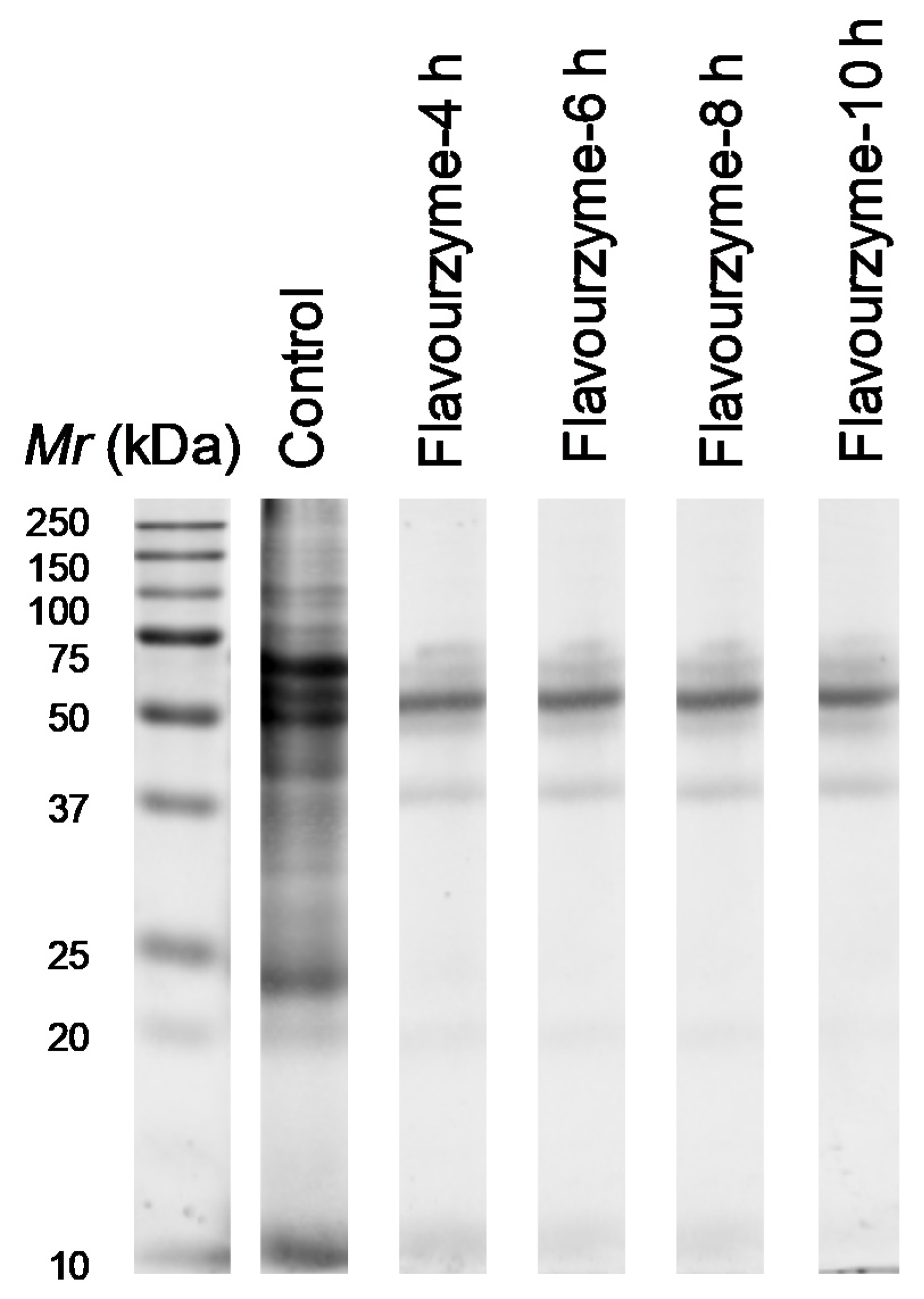
2.3. SDS-PAGE Analysis
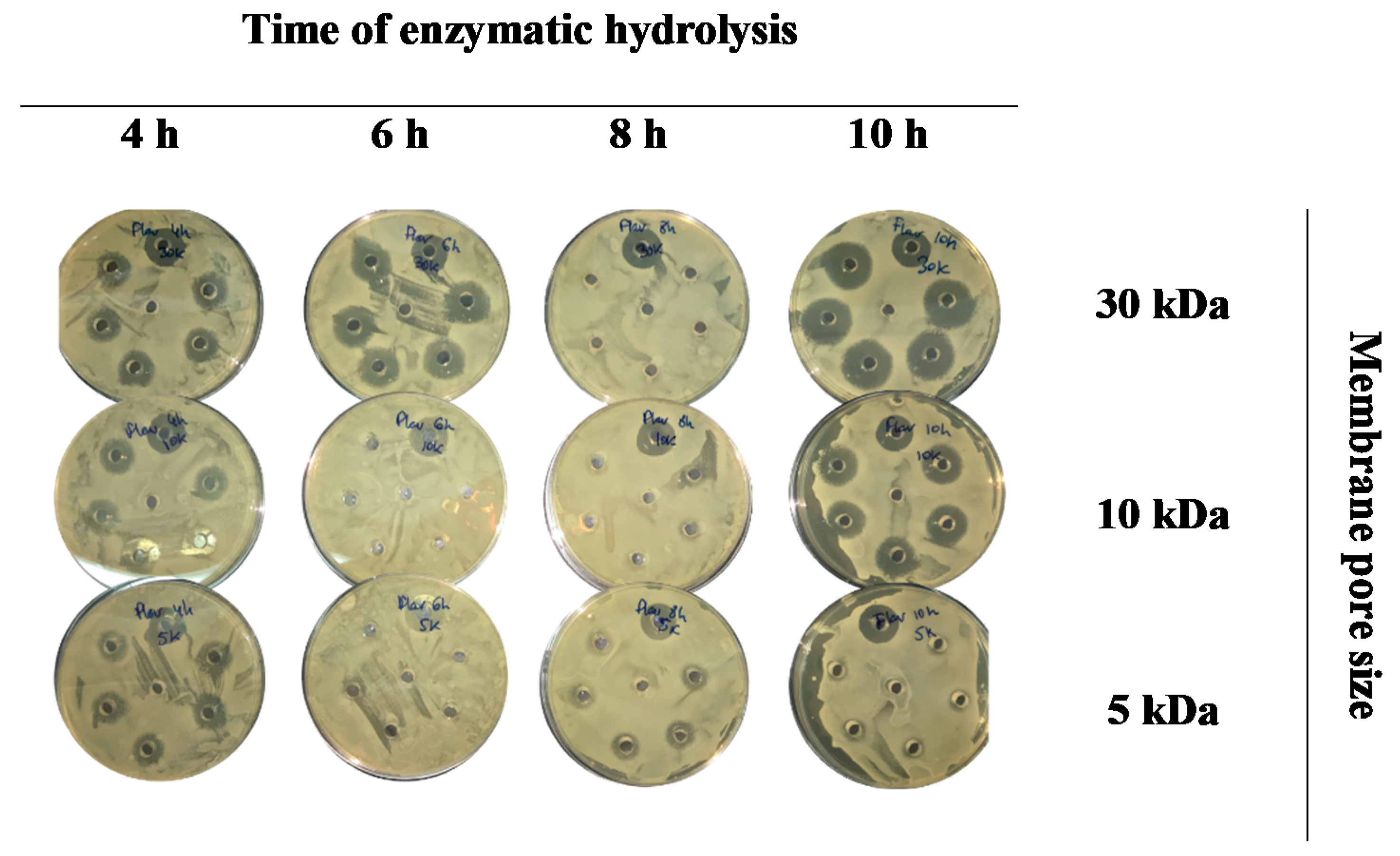
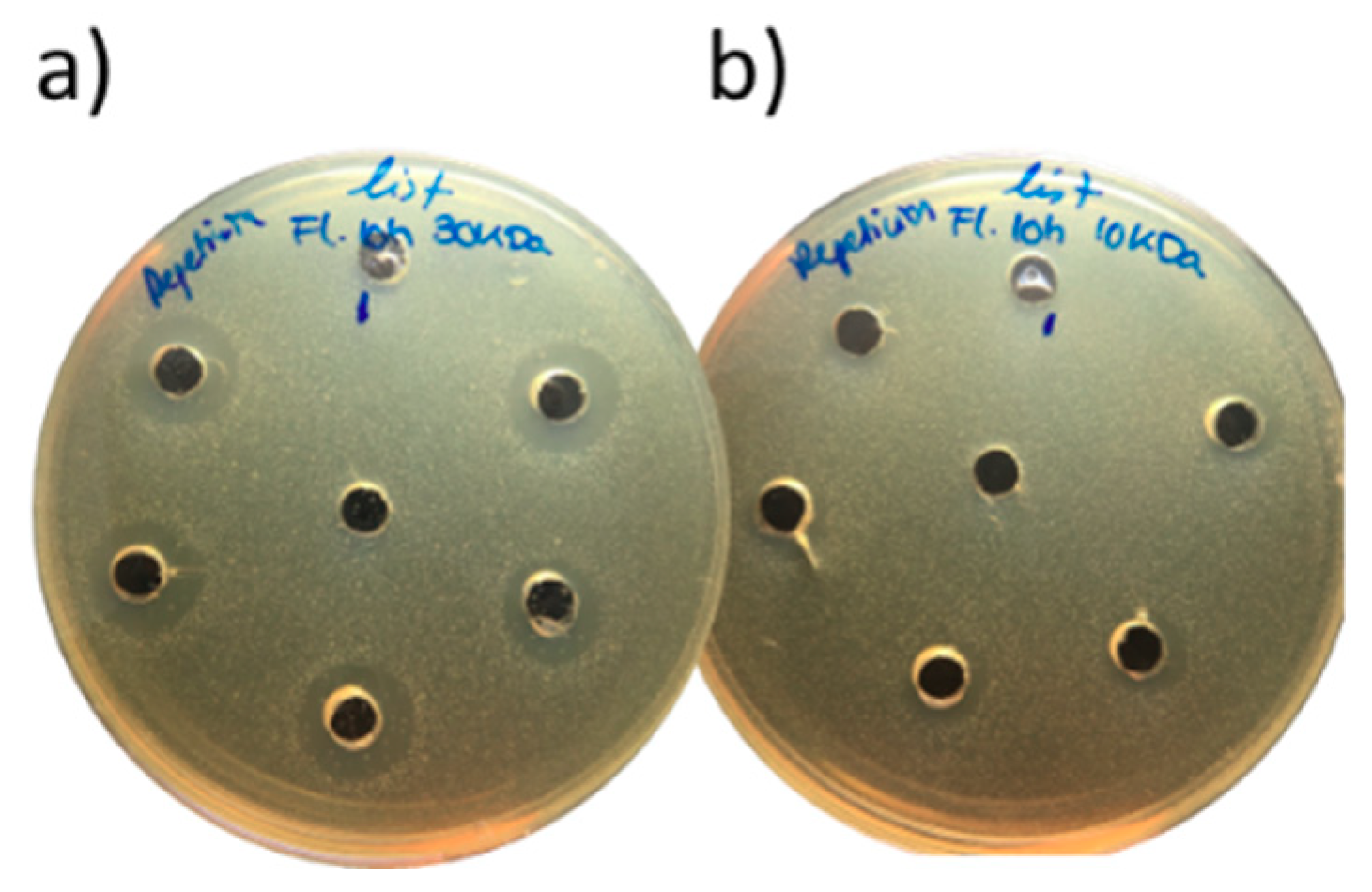
2.4. Antimicrobial Activity
2.5. Antioxidant Activity
2.5.1. ABTS Radical Scavenging Activity
2.5.2. Oxygen Radical Absorbance Capacity (ORAC)
2.5.3. DPPH Radical Scavenging Activity
2.5.4. Ferric Ion-Reducing Antioxidant Power (FRAP)
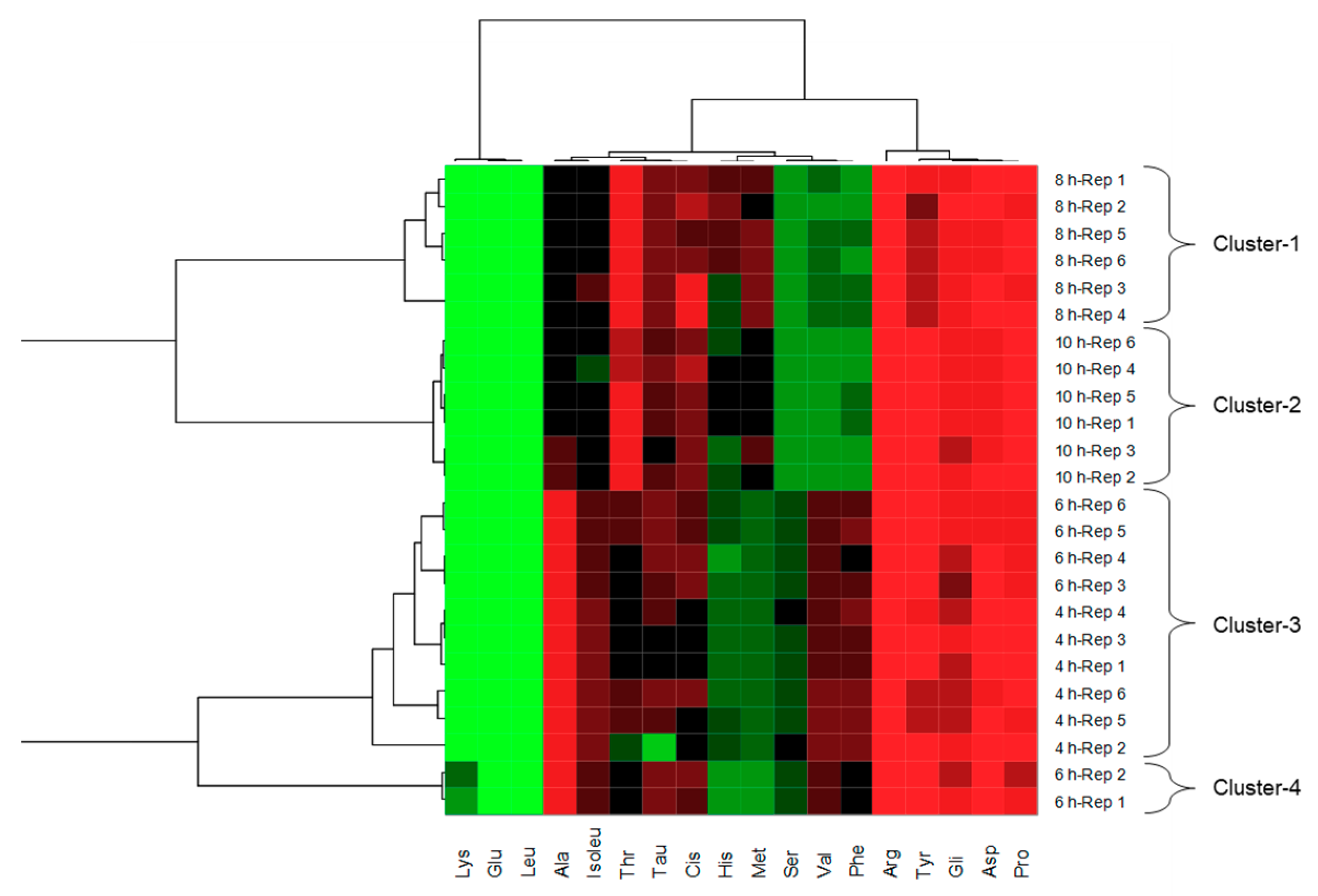
2.6. Free Amino Acid Profile
2.7. Statistical Analysis
3. Results and Discussion
3.1. Molecular Weight Profile and Amino Acid Profile of Pork Liver Hydrolysates
3.2. Antimicrobial Activity of Porcine Liver Hydrolysates
3.3. Antioxidant Activity of Extracts from Flavourzyme Hydrolysates
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, N.; Das, A.; Chaffee, S.; Roy, S.; Sen, C.K. Reactive Oxygen Species, Oxidative Damage and Cell Death. In Immunity and Inflammation in Health and Disease. Emerging Roles of Nutraceuticals and Functional Foods in Immune Support; Chatterjee, S., Jungraithmayr, W., Bagchi, D., Eds.; Academic Press: London, UK, 2018; pp. 45–55. ISBN 978-0-12-805417-8. [Google Scholar]
- Borrajo, P.; Pateiro, M.; Gagaoua, M.; Franco, D.; Zhang, W.; Lorenzo, J.M. Evaluation of the Antioxidant and Antimicrobial Activities of Porcine Liver Protein Hydrolysates Obtained Using Alcalase, Bromelain, and Papain. Appl. Sci. 2020, 10, 2290. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.-Y.; Libardo, M.D.J.; Angeles-Boza, A.M.; Pellois, J.-P. Membrane oxidation in cell delivery and cell killing applications. ACS Chem. Biol. 2017, 12, 1170–1182. [Google Scholar] [CrossRef] [Green Version]
- Zhao, K.; Zhao, G.-M.; Wu, D.; Soong, Y.; Birk, A.V.; Schiller, P.W.; Szeto, H.H. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J. Biol. Chem. 2004, 279, 34682–34690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ott, M.; Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Mitochondria, oxidative stress and cell death. Apoptosis 2007, 12, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Rinker, L.; Peng, J.; Chilian, W.M. Reactive oxygen species: The good and the bad. In Reactive Oxygen Species (ROS) in Living Cells; Filip, C., Albu, E., Eds.; IntechOpen: London, UK, 2018; pp. 7–20. ISBN 978-1-78923-134-2. [Google Scholar]
- Kothari, S.; Thompson, A.; Agarwal, A.; du Plessis, S.S. Free radicals: Their beneficial and detrimental effects on sperm function. Indian J. Exp. Biol. 2010, 48, 425–435. [Google Scholar]
- Ozougwu, J.C. The role of reactive oxygen species and antioxidants in oxidative stress. Int. J. Res. Pharm. Biosci. 2016, 3, 1–8. [Google Scholar]
- Fernandes, R.P.P.; Trindade, M.A.; Lorenzo, J.M.; de Melo, M.P. Assessment of the stability of sheep sausages with the addition of different concentrations of Origanum vulgare extract during storage. Meat Sci. 2018, 137, 244–257. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Batlle, R.; Gómez, M. Extension of the shelf-life of foal meat with two antioxidant active packaging systems. LWT Food Sci. Technol. 2014, 59, 181–188. [Google Scholar] [CrossRef]
- Nikmaram, N.; Budaraju, S.; Barba, F.J.; Lorenzo, J.M.; Cox, R.B.; Mallikarjunan, K.; Roohinejad, S. Application of plant extracts to improve the shelf-life, nutritional and health-related properties of ready-to-eat meat products. Meat Sci. 2018, 145, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amaral, A.B.; da Silva, M.V.; da Lannes, S.C.S. Lipid oxidation in meat: Mechanisms and protective factors—A review. Food Sci. Technol. 2018, 38, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.; Pickova, J.; Ahmad, T.; Liaquat, M.; Farid, A.; Jahangir, M. Oxidation of Lipids in Foods. Sarhad J. Agric. 2016, 32, 230–238. [Google Scholar] [CrossRef]
- Echegaray, N.; Gómez, B.; Barba, F.J.; Franco, D.; Estévez, M.; Carballo, J.; Marszałek, K.; Lorenzo, J.M. Chestnuts and by-products as source of natural antioxidants in meat and meat products: A review. Trends Food Sci. Technol. 2018, 82, 110–121. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Rocchetti, G.; Pateiro, M.; Lucini, L.; Domínguez, R.; Lorenzo, J.M. Addition of plant extracts to meat and meat products to extend shelf-life and health-promoting attributes: An overview. Curr. Opin. Food Sci. 2020, 31, 81–87. [Google Scholar] [CrossRef]
- Zamuz, S.; López-Pedrouso, M.; Barba, F.J.; Lorenzo, J.M.; Domínguez, H.; Franco, D. Application of hull, bur and leaf chestnut extracts on the shelf-life of beef patties stored under MAP: Evaluation of their impact on physicochemical properties, lipid oxidation, antioxidant, and antimicrobial potential. Food Res. Int. 2018, 112, 263–273. [Google Scholar] [CrossRef]
- Lorenzo, J.M.M.; Pateiro, M.; Domínguez, R.; Barba, F.J.F.J.; Putnik, P.; Kovačević, D.B.B.D.B.; Shpigelman, A.; Granato, D.; Franco, D. Berries extracts as natural antioxidants in meat products: A review. Food Res. Int. 2018, 106, 1095–1104. [Google Scholar] [CrossRef]
- Tkaczewska, J.; Bukowski, M.; Mak, P. Identification of antioxidant peptides in enzymatic hydrolysates of carp (Cyprinus Carpio) skin gelatin. Molecules 2019, 24, 97. [Google Scholar] [CrossRef] [Green Version]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-derived bioactive peptides in human health: Challenges and opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef] [Green Version]
- Mora, L.; Gallego, M.; Aristoy, M.C.; Reig, M.; Toldrá, F. Bioactive peptides. In Innovative Thermal and Non-Thermal Processing, Bioaccessibility and Bioavailability of Nutrients and Bioactive Compounds; Barba, F.J., Saraiva, J.M.A., Cravotto, G., Lorenzo, J.M., Eds.; Woodhead Publishing: Duxford, UK, 2019; pp. 333–345. ISBN 978-0-12-814174-8. [Google Scholar]
- Lorenzo, J.M.; Munekata, P.E.S.; Gómez, B.; Barba, F.J.; Mora, L.; Pérez-Santaescolástica, C.; Toldrá, F. Bioactive peptides as natural antioxidants in food products—A review. Trends Food Sci. Technol. 2018, 79, 136–147. [Google Scholar] [CrossRef]
- Pateiro, M.; Borrajo, P.; Campagnol, P.C.B.; Domínguez, R.; Tomasevic, I.; Munekata, P.E.S.; Barba, F.J.; Lorenzo, J.M. Extraction of valuable compounds from meat by-products. In Green Extraction and Valorization of By-Products from Food Processing; Barba, F.J., Roselló-Soto, E., Brncic, M., Lorenzo, J.M., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 55–90. ISBN 9781138544048. [Google Scholar]
- Yu, H.C.; Hsu, J.L.; Chang, C.I.; Tan, F.J. Antioxidant properties of porcine liver proteins hydrolyzed using Monascus purpureus. Food Sci. Biotechnol. 2017, 26, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Liu, J.; Hansen, E.T.; Bredie, W.L.P.; Lametsch, R. Structural characteristics of low bitter and high umami protein hydrolysates prepared from bovine muscle and porcine plasma. Food Chem. 2018, 257, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Ramírez Aristizabal, L.S.; Marín, D. Evaluación de la actividad antibacteriana de aceites esenciales y extractos etanólicos utilizando métodos de difusión en agar y dilución en pozo. Sci. Tech. 2012, 17, 152–157. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C.; Proteggenete, A.; Pannala, A.; Yang, M.; Rice-Evans, C.; et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Huan, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of ‘“Antioxidant Power”’: The FRAP Assay Iris. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo, J.M.; Cittadini, A.; Bermúdez, R.; Munekata, P.E.; Domínguez, R. Influence of partial replacement of NaCl with KCl, CaCl 2 and MgCl 2 on proteolysis, lipolysis and sensory properties during the manufacture of dry-cured lacón. Food Control 2015, 55, 90–96. [Google Scholar] [CrossRef]
- Chang, C.Y.; Wu, K.C.; Chiang, S.H. Antioxidant properties and protein compositions of porcine haemoglobin hydrolysates. Food Chem. 2007, 100, 1537–1543. [Google Scholar] [CrossRef]
- Bah, C.S.F.; Carne, A.; McConnell, M.A.; Mros, S.; Bekhit, A.E.D.A. Production of bioactive peptide hydrolysates from deer, sheep, pig and cattle red blood cell fractions using plant and fungal protease preparations. Food Chem. 2016, 202, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, C.; Qiao, Y.; Xing, L.; Kang, D.; Khan, I.A.; Huang, M.; Zhou, G. Effect of Flavourzyme on proteolysis, antioxidant activity and sensory qualities of Cantonese bacon. Food Chem. 2017, 237, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Xing, L.; Fu, Q.; Zhou, G.H.; Zhang, W.G. A review of antioxidant peptides derived from meat muscle and by-products. Antioxidants 2016, 5, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, A.K.; Chatli, M.K.; Kumar, P.; Mehta, N. Antioxidant and Antimicrobial Efficacy of Peptidic Hydrolysate Obtained from Porcine Blood. Agric. Res. 2019, 8, 116–124. [Google Scholar] [CrossRef]
- Galili, G.; Amir, R.; Fernie, A.R. The Regulation of Essential Amino Acid Synthesis and Accumulation in Plants. Annu. Rev. Plant Biol. 2016, 67, 153–178. [Google Scholar] [CrossRef]
- Bah, C.S.F.; Bekhit, A.E.D.A.; Carne, A.; Mcconnell, M.A. Slaughterhouse blood: An emerging source of bioactive compounds. Compr. Rev. Food Sci. Food Saf. 2013, 12, 314–331. [Google Scholar] [CrossRef]
- Sun, Y.; Pan, D.; Guo, Y.; Li, J. Purification of chicken breast protein hydrolysate and analysis of its antioxidant activity. Food Chem. Toxicol. 2012, 50, 3397–3404. [Google Scholar] [CrossRef]
- de Queiroz, A.L.M.; Bezerra, T.K.A.; de Freitas Pereira, S.; da Silva, M.E.C.; de Almeida Gadelha, C.A.; Gadelha, T.S.; Pacheco, M.T.B.; Madruga, M.S. Functional protein hydrolysate from goat by-products: Optimization and characterization studies. Food Biosci. 2017, 20, 19–27. [Google Scholar] [CrossRef]
- Verma, A.K.; Chatli, M.K.; Kumar, P.; Mehta, N. Antioxidant and antimicrobial activity of protein hydrolysate extracted from porcine liver. Indian J. Anim. Sci. 2017, 87, 711–717. [Google Scholar]
- Stanborough, T.; Fegan, N.; Powell, S.M.; Tamplin, M.; Chandry, P.S. Insight into the genome of Brochothrix thermosphacta, a problematic meat spoilage bacterium. Appl. Environ. Microbiol. 2017, 83, e02786-16. [Google Scholar] [CrossRef] [Green Version]
- Epand, R.M.; Walker, C.; Epand, R.F.; Magarvey, N.A. Molecular mechanisms of membrane targeting antibiotics. Biochim. Biophys. Acta Biomembr. 2016, 1858, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Delcour, A.H. Outer membrane permeability and antibiotic resistance. Biochim. Biophys. Acta Proteins Proteom. 2009, 1794, 808–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denyer, S.P.; Maillard, J.-Y. Cellular impermeability and uptake of biocides and antibiotics in Gram-negative bacteria. J. Appl. Microbiol. 2002, 92, 35S–45S. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Koh, J.J.; Liu, S.; Lakshminarayanan, R.; Verma, C.S.; Beuerman, R.W. Membrane active antimicrobial peptides: Translating mechanistic insights to design. Front. Neurosci. 2017, 11, 73. [Google Scholar] [CrossRef] [Green Version]
- Jamdar, S.N.; Rajalakshmi, V.; Sharma, A. Antioxidant and ace inhibitory properties of poultry viscera protein hydrolysate and its peptide fractions. J. Food Biochem. 2012, 36, 494–501. [Google Scholar] [CrossRef]
- Guija-Poma, E.; Inocente-Camones, M.Á.; Ponce-Pardo, J.; Zarzosa-Norabuena, E. Evaluación de la técnica 2,2-Difenil-1-Picrilhidrazilo (DPPH) para determinar capacidad antioxidante. Horiz. Med. 2015, 15, 57–60. [Google Scholar] [CrossRef] [Green Version]
- Sowndhararajan, K.; Kang, S.C. Free radical scavenging activity from different extracts of leaves of Bauhinia vahlii Wight & Arn. Saudi J. Biol. Sci. 2013, 20, 319–325. [Google Scholar]
- Liu, Q.; Kong, B.; Xiong, Y.L.; Xia, X. Antioxidant activity and functional properties of porcine plasma protein hydrolysate as influenced by the degree of hydrolysis. Food Chem. 2010, 118, 403–410. [Google Scholar] [CrossRef]
- Sun, Q.; Shen, H.; Luo, Y. Antioxidant activity of hydrolysates and peptide fractions derived from porcine hemoglobin. J. Food Sci. Technol. 2011, 48, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.S.; Huang, J.C.; Chen, Y.L.; Huang, M.; Zhou, G.H. Identification and Characterization of Antioxidant Peptides from Enzymatic Hydrolysates of Duck Meat. J. Agric. Food Chem. 2015, 63, 3437–3444. [Google Scholar] [CrossRef]
- Verma, A.K.; Chatli, M.K.; Kumar, P.; Mehta, N. In-vitro assessment of antioxidant and antimicrobial activity of whole porcine-liver hydrolysates and its fractions. Anim. Prod. Sci. 2019, 59, 641–646. [Google Scholar] [CrossRef]
| Hydrolysis Time | Sig. | ||||
|---|---|---|---|---|---|
| Amino Acid | 4 h | 6 h | 8 h | 10 h | |
| Aspartic acid | 118.00 ± 5.83 a | 158.16 ± 31.35 b | 211.42 ± 6.42 c | 246.85 ± 21.22 d | *** |
| Serine | 282.14 ± 17.61 a | 384.66 ± 75.67 b | 505.00 ± 15.07 c | 579.71 ± 52.93 d | *** |
| Glutamic acid | 471.42 ± 19.25 a | 648.66 ± 131.99 c | 607.28 ± 15.26 b | 721.71 ± 70.69 d | *** |
| Glycine | 178.57 ± 19.86 a | 238.50 ± 28.31 b | 232.57 ± 21.46 b | 279.00 ± 42.38 c | *** |
| Histidine | 303.28 ± 24.47 a | 417.66 ± 48.58 b | 391.57 ± 34.36 b | 476.00 ± 63.67 c | *** |
| Arginine | 22.57 ± 7.25 a | 29.16 ± 8.75 ab | 36.28 ± 11.20 b | 25.14 ± 9.37 a | * |
| Threonine | 267.28 ± 35.57 b | 341.83 ± 48.61 d | 252.00 ± 6.27 a | 301.57 ± 22.21 c | *** |
| Alanine | 165.00 ± 11.47 a | 219.00 ± 35.92 b | 398.14 ± 9.68 c | 435.85 ± 36.97 d | *** |
| Proline | 108.57 ± 19.44 a | 200.83 ± 18.85 b | 207.71 ± 12.95 b | 201.28 ± 20.65 b | *** |
| Cysteine | 261.57 ± 32.17 a | 301.33 ± 66.45 b | 303.14 ± 46.82 b | 355.71 ± 44.61 c | *** |
| Tyrosine | 136.85 ± 29.43 a | 157.33 ± 18.38 c | 298.85 ± 25.50 d | 115.00 ± 14.44 a | *** |
| Valine | 228.00 ± 20.17 a | 318.66 ± 49.65 b | 480.57 ± 25.15 b | 568.17 ± 54.17 c | *** |
| Methionine | 320.57 ± 23.48 a | 433.83 ± 68.65 c | 356.74 ± 23.00 a | 436.71 ± 45.28 c | *** |
| Lysine | 498.85 ± 34.43 a | 573.50 ± 202.12 b | 737.28 ± 44.58 c | 751.71 ± 72.00 c | *** |
| Isoleucine | 224.57 ± 17.17 a | 308.67 ± 51.22 b | 397.57 ± 19.07 c | 468.42 ± 46.56 d | *** |
| Leucine | 477.14 ± 48.02 a | 658.66 ± 102.68 b | 706.42 ± 39.21 c | 825.57 ± 82.36 d | *** |
| Phenylalanine | 232.42 ± 22.70 a | 326.00 ± 28.94 b | 491.28 ± 24.10 c | 550.14 ± 60.03 d | *** |
| Total FAA | 4562.42 ± 322.24 a | 6010.00 ± 1031.88 b | 6936.85 ± 208.28 c | 7751.85 ± 753.67 d | *** |
| Hydrolysis Time | Pore Size of Membrane | Sig. | ||
|---|---|---|---|---|
| 5 kDa | 10 kDa | 30 kDa | ||
| ABTS (mg Ascorbic Acid/100 g) | ||||
| 4 h | 337.27 ± 6.39 aα | 399.07 ± 3.69 β | 433.77 ± 2.93 aγ | *** |
| 6 h | 388.91 ± 5.93 cα | 407.53 ± 5.15 β | 497.26 ± 2.93 cγ | *** |
| 8 h | 379.60 ± 8.34 bcα | 410.92 ± 11.45 β | 474.40 ± 5.29 bγ | *** |
| 10 h | 366.05 ± 0.85 bα | 410.07 ± 8.34 β | 426.15 ± 2.54 aβ | *** |
| Sig. | ** | ns | *** | |
| DPPH (µg trolox/g) | ||||
| 4 h | 395.62 ± 1.29 aα | 446.87 ± 4.64 cα | 970.06 ± 57.66 bβ | *** |
| 6 h | 414.19 ± 3.93 cα | 430.53 ± 1.49 bβ | 484.00 ± 16.74 aβ | ** |
| 8 h | 411.96 ± 1.96 bcα | 426.07 ± 3.71 bβ | 441.67 ± 4.87 aγ | ** |
| 10 h | 400.82 ± 5.80 ab | 397.85 ± 1.29 a | 403.05 ± 2.68 a | ns |
| Sig. | * | *** | *** | |
| ORAC (mg trolox/g) | ||||
| 4 h | 20.93 ± 1.25 a | 18.40 ± 3.60 | 16.26 ± 3.31 a | ns |
| 6 h | 30.48 ± 2.05 cβ | 21.72 ± 2.24 α | 33.71 ± 2.39 bβ | * |
| 8 h | 24.74 ± 0.13 abγ | 9.80 ± 1.51 α | 15.60 ± 1.58 aβ | *** |
| 10 h | 30.02 ± 2.36 bcβ | 18.06 ± 3.62 α | 26.49 ± 1.58 bαβ | * |
| Sig. | * | ns | ** | |
| FRAP (µmol Fe+2/100 g) | ||||
| 4 h | 34.09 ± 0.62 aα | 40.97 ± 0.36 cβ | 41.81 ± 0.58 β | *** |
| 6 h | 35.93 ± 0.42 b | 38.34 ± 0.34 b | 48.43 ± 5.33 | ns |
| 8 h | 36.08 ± 0.55 bα | 39.29 ± 0.34 bβ | 42.54 ± 0.72 γ | *** |
| 10 h | 33.19 ± 0.37 aα | 36.03 ± 0.68 aβ | 36.77 ± 0.23 β | ** |
| Sig. | ** | *** | ns | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borrajo, P.; López-Pedrouso, M.; Franco, D.; Pateiro, M.; Lorenzo, J.M. Antioxidant and Antimicrobial Activity of Porcine Liver Hydrolysates Using Flavourzyme. Appl. Sci. 2020, 10, 3950. https://doi.org/10.3390/app10113950
Borrajo P, López-Pedrouso M, Franco D, Pateiro M, Lorenzo JM. Antioxidant and Antimicrobial Activity of Porcine Liver Hydrolysates Using Flavourzyme. Applied Sciences. 2020; 10(11):3950. https://doi.org/10.3390/app10113950
Chicago/Turabian StyleBorrajo, Paula, María López-Pedrouso, Daniel Franco, Mirian Pateiro, and José M. Lorenzo. 2020. "Antioxidant and Antimicrobial Activity of Porcine Liver Hydrolysates Using Flavourzyme" Applied Sciences 10, no. 11: 3950. https://doi.org/10.3390/app10113950






