Abstract
Waste gases that cannot be released into the environment are generated in chemical industrial processes. There are various physico-chemical processes for the treatment of these gases, but in most cases, they present a major cost to the company. There is an EU directive for each industrial area describing the best available techniques (BAT) and the prescribed environmental limits for the maximum discharge of dangerous substances into the environment. The current process for the removal of formaldehyde and volatile organic compounds from waste industrial gases meets EU environmental regulations. However, expected new EU directives will require a significant reduction in formaldehyde and volatile organic compounds’ concentrations in industrial exhaust gases, thus necessitating a new technical solution for the removal of formaldehyde. This paper describes two methods of removing formaldehyde and volatile organic compounds from waste gases, generated by the metal oxide catalyst formaldehyde production processes. The first method involves upgrading existing processes of removing formaldehyde from waste gases with an additional absorption plant, with which emissions can be significantly reduced. The second method describes the co-incineration of waste gases generated by a metal oxide catalyst formaldehyde production process with natural gas in a gas turbine, where formaldehyde and volatile organic compounds are completely removed, while electricity is also produced. The second method is also useful for removing various concentrations of volatile organic compounds from waste gases generated in chemical industrial processes.
1. Introduction
Formalin, an aqueous solution of formaldehyde, is one of the most important base products in the production of high-volume products in the chemical industry [1]. The amount of international trade of formaldehyde today is less than 2% of the total volume of formaldehyde produced. The transport costs for formaldehyde aqueous solutions are very high because of the relatively high proportion of water in the product. Therefore, formaldehyde is usually used or processed at the site of production [2]. Taking into consideration the constant increases in energy expenses in the production of such a high volume of formaldehyde aqueous solutions, an optimal way of increasing efficiency is the use of by-product energy, based on energy released in the process, and on off-gases from the production of formaldehyde from methanol [3]. Energy management has become a very important branch of research in the chemical process industry [4]. New developments include trends toward annual emission coupon distribution affects, limiting time-related components in decision making, and executing the transition to processes based upon the latest technologies.
2. Production Processes of Formaldehyde
There are two basic production processes for formaldehyde:
- The metal oxide catalyst process, operating with air surplus [5],
- The silver catalyst process, operating with methanol surplus [6].
In the following sections, formaldehyde production plants using the two main process technologies are described. This is the outcome of the development and investment in energetic process optimization, including side streams of chemicals and energy, in various formaldehyde production plants over the course of several years.
At one site, PF (phenolic resin) and UF (urea resin) resins are manufactured, using the formaldehyde produced and the excess steam from formaldehyde production.
2.1. Formaldehyde Production Using Metal Oxide Catalyst Processes
During production with a metal oxide catalyst (molybdenum and iron oxide), formaldehyde is formed by partial oxidation of methanol with oxygen [5]. The reactions take place on a metal oxide catalyst in a reactor operating at a temperature of 250 to 500 °C with a static layer of catalyst for oxidation in the gas phase (fixed-bed vapor-phase oxidation reactor) and a cooling system:
CH3OH + 1/2 O2 → CH2O + H2O dH = −159 kJ/mol
To maintain the necessary oxidation atmosphere, the required ratio between methanol and air is low; the methanol content is approximately 4 to 10 vol.%. This high surplus of air is possible because a portion of the gas from the absorber is recycled, resulting in a sufficient reduction of oxygen to avoid the formation of explosive mixtures. The overall process yield of formaldehyde from methanol is 90–92 mol percent. The thermal energy released during the reaction is discharged from the reactor using thermal oil as a liquid medium for the energy transfer; the oil is then used for the production of steam.
2.2. Formaldehyde Production Using Silver Catalyst Processes
The first industrial formaldehyde production processes used silver as catalyst [7]. This process burdens the environment with gas emissions into the air, and with the generation of excess steam condensate containing up to 0.2% of formaldehyde, which cannot be recycled into the process.
The silver catalyst process partially converts methanol to formaldehyde with efficiency of up to 90%. The ternary mixture of formaldehyde/water/methanol must be separated via steam distillation. Afterwards, the steam condensate is contaminated with formaldehyde (up to 0.2%), creating extra costs for disposal or further treatment.
The synthesis is carried out using catalytic oxidation and dehydration of methanol in contact with a silver catalyst. Several reactions take place during the conversion process:
alternatively:
CH3OH + 1/2 O2 → CH2O + H2O dH = −159 kJ/mol
CH3OH → CH2O + H2 dH = +84 kJ/mol
H2 + 1/2 O2 → H2O dH = −243 kJ/mol
Reaction (4) is exothermic. The thermal energy released in this reaction maintains the temperature of the reactor and, at the same time, facilitates in reaction (3) the shift of the equilibrium to the right side. This increases the formation of formaldehyde. Parallel to the main reactions (2)–(4), some side reactions are carried out, reducing the efficiency of the process. The important side reactions are:
CH2O → CO + H2
CH2O + 1/2 O2 → HCOOH and CO + H2O
CH2O + O2 → CO2 + H2O
In this basic concept, no use of off-gases is included. Natural gas is purchased for the generation of steam, and external electric energy is required for the methanol conversion process and the connected UF and PF resin production.
In total, 89 kg of CO2 are created and emitted per ton of products manufactured (formaldehyde in 37% concentration, PF resin and UF resin).
3. Off-Gas Purification Process
Off-gases with high levels of carbon monoxide and formaldehyde must not be discharged into the environment.
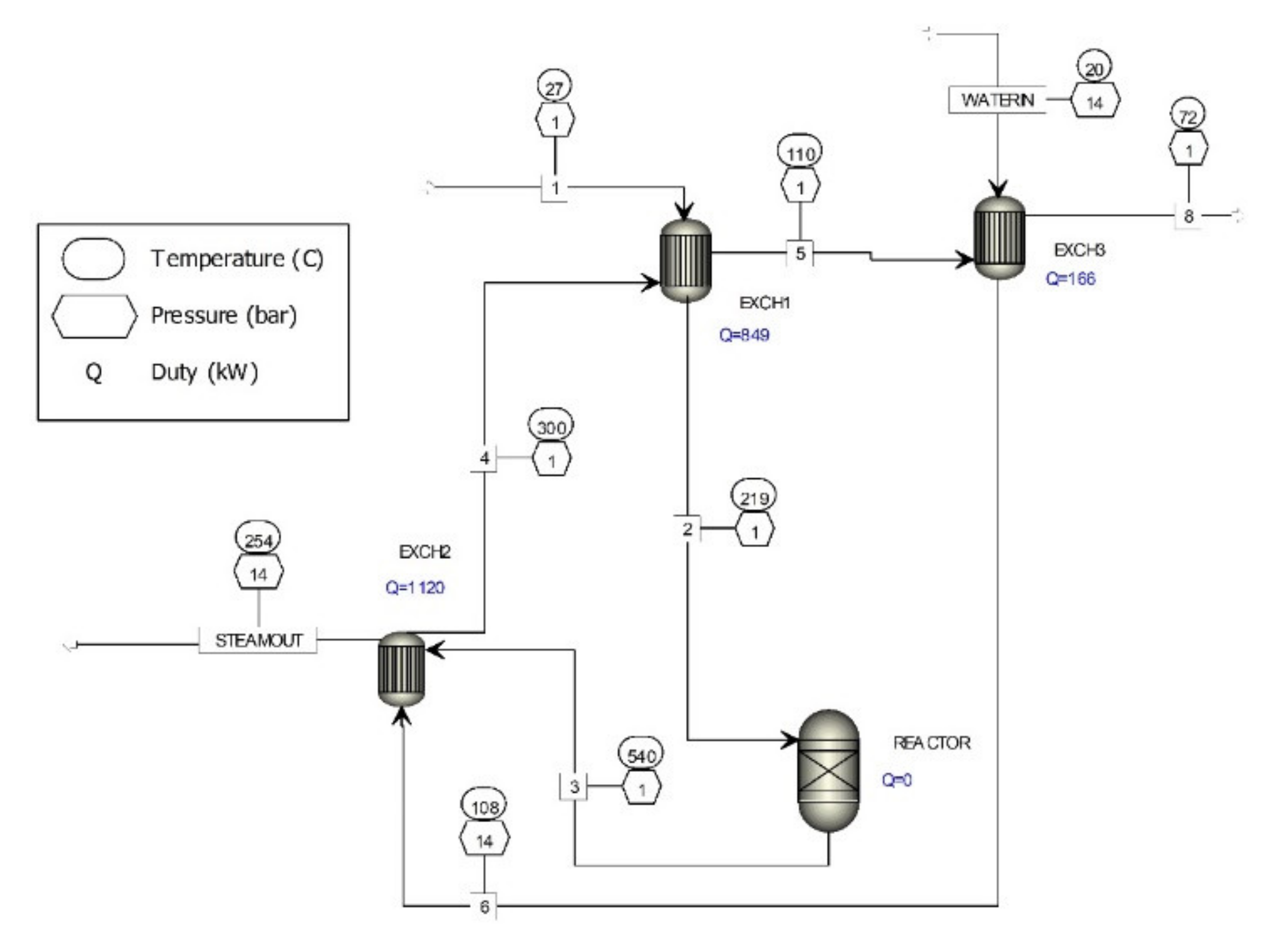
A computer simulation of the purification of waste gases generated by the metal oxide catalyst formaldehyde production processes was carried out with Aspen Plus software (Figure 1). The process of purification of waste gases from this process is exothermic and generates heat, which could be used for steam production. Steam, which can be used in other processes or used for electricity production, is generated in the purification of the waste gases. The average production is approximately 21.6 t/hr formaldehyde with 37% concentration. Table 1 shows the composition of waste off-gases (A) before the purification process and (B) after catalytic purification of waste gases. The data in Table 1 were obtained from gas chromatography measurements of existing metal oxide catalyst formaldehyde production processes. These data are the bases of further research to improve the waste off-gases cleaning process described in this article.
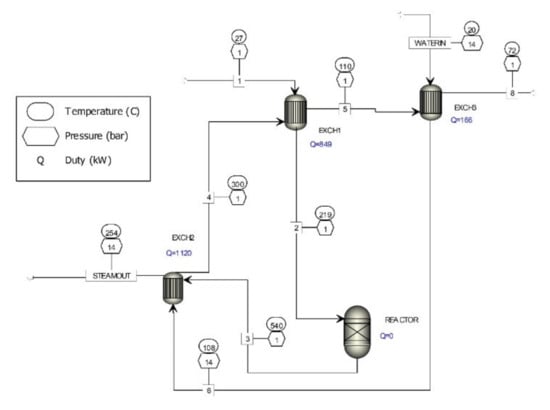
Figure 1.
Computer simulation of waste gas treatment process of metal oxide catalyst formaldehyde production.

Table 1.
The composition of waste off-gases (A) before the purification process and (B) after the catalytic process of purification.
The existing process for cleaning off-gases from metal oxide catalyst formaldehyde production processes consists of a heat exchanger (EXCH1) where the waste gases, having the composition given in Table 1, are heated to the temperature necessary for the oxidation of organic substances present in the flue gases and go to reactor (REACTOR) filled with ceramic filler. The hot gases with the composition, given in Table 1, from the reactor are fed to heat exchanger (EXCH2) where steam is produced. The cooled gases are then passed to a heat exchanger (EXCH1), thence, to a heat exchanger (EXCH3), and then discharged through the chimney to the environment. The chemical composition of the waste gases discharged into the environment is given in Table 1.
Energy Production
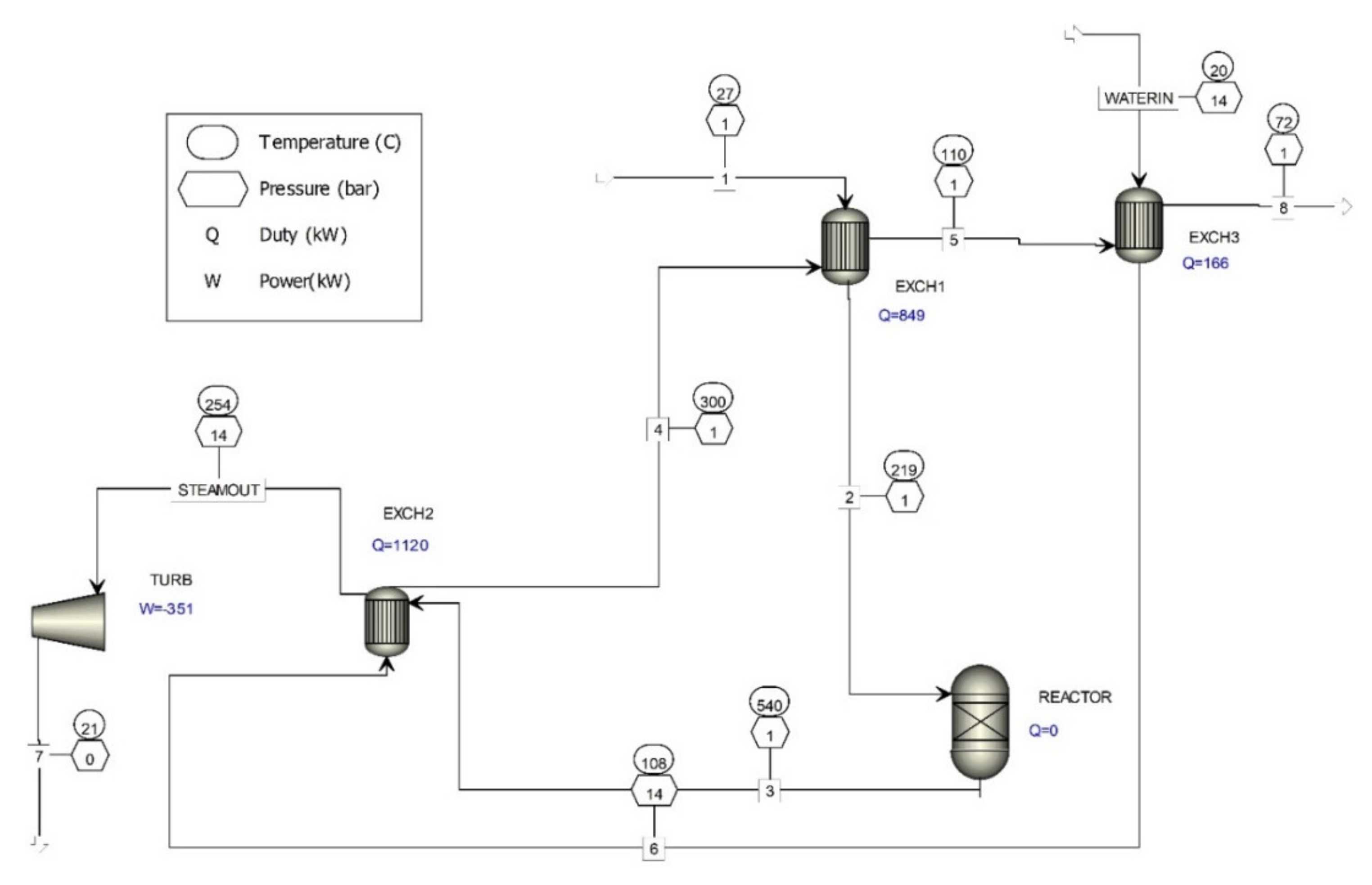
Currently, the steam produced is not used for electricity generation, so a computer simulation of electricity production by involving a steam turbine in the waste gas treatment process was done (Figure 2).
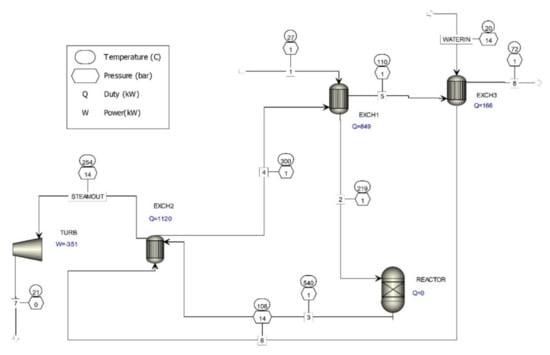
Figure 2.
Computer simulation of waste gas treatment in the oxide catalyst formaldehyde production process, utilizing heat to produce electricity.
Two basic kinds of steam turbines are available. One is a pulse design, in which a rotor turns as a result of the steam force on the blades. The second is a reaction design, in which the rotor produces its rotational force from steam when leaving the blades.
The primary type of steam turbine is a condensing steam turbine, which is used for high-power applications. The steam is discharged directly into one or more condensers that maintain vacuum conditions. Condensing steam turbine processes produce the highest mechanical power and efficiency; however, the output power of condensing steam turbines is sensitive to ambient temperature. Condensing steam turbines are expensive, large, and complex.
The second type is a back-pressure steam turbine, which is suitable for mechanical drive applications. The term back-pressure refers to turbines that release steam at a pressure higher than atmospheric pressure. Low pressures are often used in heating systems, while high pressures are often used in the supply of steam to industrial processes.
The third type is an extraction steam turbine, which has one or more openings in the casing for extracting a portion of the steam at a given intermediate pressure, which can be used for process purposes.
4. Upgrading the Waste Gas Purification Process
4.1. EU Directives on Industrial Emissions
The Treaty on the Functioning of the European Union and Directive 2010/75/EU of the European Parliament and of the Council of 24 November 2010 on industrial emissions (regarding integrated pollution prevention and control), in particular, Article 13 (5) on Best Available Techniques (BAT), constitutes the reference for setting permit conditions for installations referred to in Chapter II of Directive 2010/75/EU [8]. Many studies have been done on formaldehyde emissions from different processes [9,10,11].The competent authorities of an EU Member State should set emission limit values to ensure that, under normal operating conditions, emissions do not exceed the emission levels associated with the best available techniques as specified in BAT; the levels for total volatile organic carbon (TVOC) and formaldehyde (FA) are given in [12] and are for TVOC < 5–30 mg/m3 and for formaldehyde 2–5 mg/m3.
4.2. Kinetics and Chemical Equilibrium in the Hydration of Formaldehyde
Formaldehyde is highly reactive and, therefore, commonly handled in aqueous and/or methylene glycol solutions, where it forms different adducts with the solvents. Formaldehyde absorption by water is a mass-transfer process, complicated by a chemical reaction in the fluid phase [13]. In general, the basic resistance of mass transfer is represented by the Equation (8).
The basic design of such a process requires models of the phase equilibrium of aqueous and methylene glycol formaldehyde mixtures. Such models must consider not only differences in intermolecular forces but also a variety of chemical reactions [13].
Formaldehyde is unstable in its pure, gaseous state and is usually produced as an aqueous solution. In aqueous solutions, the most important chemical reaction product is methylene glycol (MG). In solution, formaldehyde is almost completely hydrated to methylene glycol.
CH2O + H2O ↔ CH2(OH)2
Methylene glycol, depending on the strength of the solution, can be polymerized and forms a series of polyoxymethylene glycols [14].
CH2(OH)2 + HO(CH2O)n−1H ↔ HO(CH2O)nH + H2O
These chemical reactions have a significant effect on the properties of formaldehyde-containing solutions and should be taken into account in each thermodynamic model of these systems. The physicochemical model developed by [15] combines these chemical reactions with physical interactions and has proved to be very successful in describing the balance of volatile liquids and enthalpy in mixtures containing formaldehyde.
This model is constantly updated by [16,17,18], and its application has been extended with new, reliable data.
The equilibrium distribution of formaldehyde and its reaction products with water (W) significantly affects not only the equilibrium properties, but also the kinetics of the chemical reaction and transport properties. Equilibrium distribution data are available from spectroscopic studies, which show that in aqueous solutions, formaldehyde is present as methylene glycol and polyoxymethylene glycol, but rarely as free molecular formaldehyde [9]. Using a NMR (high resonance frequency nuclear magnetic resonance spectrometer), it is possible to separate signals from -CH2- groups in different polyoxymethylene glycols.
Various models are proposed for describing the equilibrium of vapor-liquid in the formaldehyde water system. The model shown in [15] assumes that the vapors above the aqueous formaldehyde solution consist of three types: Water, formaldehyde monomers, and methylene glycol. These species are also present in the liquid phase. However, fluid with higher concentrations of formaldehyde also contains higher polyoxymethylene glycols. It is assumed that the vapor phase behaves as a mixture of ideal gases, but the chemical-reaction equilibrium for the formation of methylene glycol from formaldehyde and water is taken into account with the constant of the balance of chemical reactions [15].
The liquid phase is treated as a real, chemically reactive mixture of aqueous formaldehyde monomers, methylene glycols, and polyoxymethylene glycols at higher formaldehyde concentrations. The chemical potential of the components in the liquid phase is normalized according to Raoult’s law. The non-ideality of the liquid phase is taken into account, using the contribution method of the UNIFAC (Functional-group Activity Coefficients) group [19]. Chemical reaction equations are taken into account with the true reaction equilibrium constants [14].
One of the most important features of this model is its capacity to be expanded to other chemically reactive components, such as methanol, but also to nonreactive, chemically inert components such as trioxane and methylal.
The following information is needed to calculate the balance of vapors and liquids in the formaldehyde water system:
- Vapor pressure of pure components, water, formaldehyde, and methylene glycol;
- Constants of the chemical reaction equilibrium for the formation of methylene glycol in the vapor phase and the formation of polyoxymethylene glycols in the liquid phase; and
- UNIFAC parameters for size and surface, and binary parameters for interactions between all groups.
Along with this model, a complete set of parameters has been published [14] and, in [18], the model was constantly updated and expanded with new experimental data. The author [14] modified the structure of the model to reduce the time of calculation and included a simple extension to the non-equilibrium effects of transport and the reaction kinetics.
The main characteristic of these changes was the assumption that chemical equilibrium can be approximated with equivalent constants based on molar proportions, rather than on activities. This assumption has caused a significant reduction in computer time required, owing to the introduction of certain thermodynamic inconsistencies. These inconsistencies are particularly evident when the model is used to calculate azeotropic points. Given the lack of thermodynamic compliance, the calculated maximum pressure on the isothermal pressure diagram and the concentration of the binary formaldehyde-water system is incompatible with the pressure at which the boiling point line touches the dew point line.
The equations for the formaldehyde and water vapor pressure were adopted from previous versions of the model [14,15] and are given in Table 2.

Table 2.
Pure component vapor pressure.
The vapor pressure of the individual pure components is determined by Equation (11), the coefficients of which are given in Table 2.
The chemical equilibrium constant Kh was written as Kh = kh/kd, where kd was taken from [20]. The reaction rate constant of hydration at reciprocal temperature is:
The average absolute relative deviation between data from individual experiments and from Equation (12) is 9.6% [21]. With the rate of hydration according to Equation (12) and the rate of dehydration [21], the constant of equilibrium for formaldehyde hydration [13] was obtained as
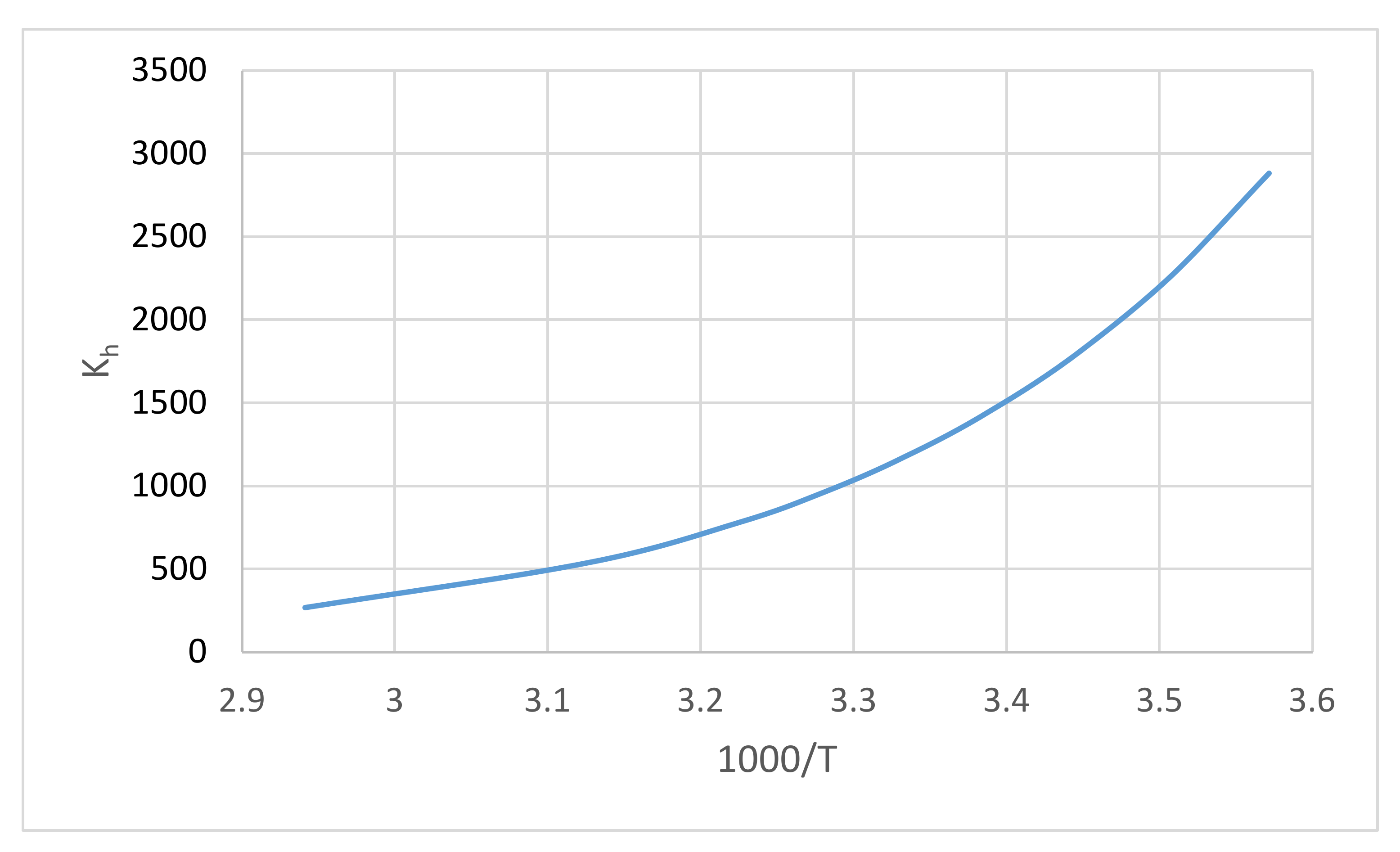
A diagram of the formaldehyde hydration chemical equilibrium constant Equation (13) is shown in Figure 3. From the temperature coefficient in Equation (13), the enthalpy of the hydration reaction obtained was ΔH = −31.4 kJ/mol.
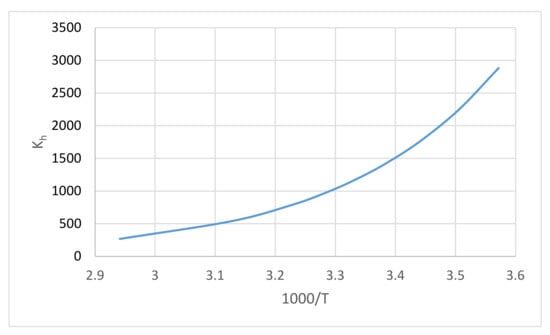
Figure 3.
Diagram of the formaldehyde hydration chemical equilibrium constant Equation (13).
At temperatures of 293–333 K and at pH values between 5 and 7, the rate of the dehydration reaction kd is determined as Equation (12).
The rate constant of the dehydration reaction, kd, obtained earlier under similar conditions, is the chemical equilibrium constant for hydration shown in Equation (13).
4.3. The Waste Gas Purification Process with Absorbers
The procedure described in Figure 1 sufficiently reduces formaldehyde concentrations in waste gases to levels prescribed in the new EU directives. However, as seen in Table 1, approximately 280 kg of formaldehyde per year are still released after catalytic purification of waste gases. Given increasing levels of environmental concern, more formaldehyde should be removed from waste gases produced by metal oxide catalyst formaldehyde production processes.
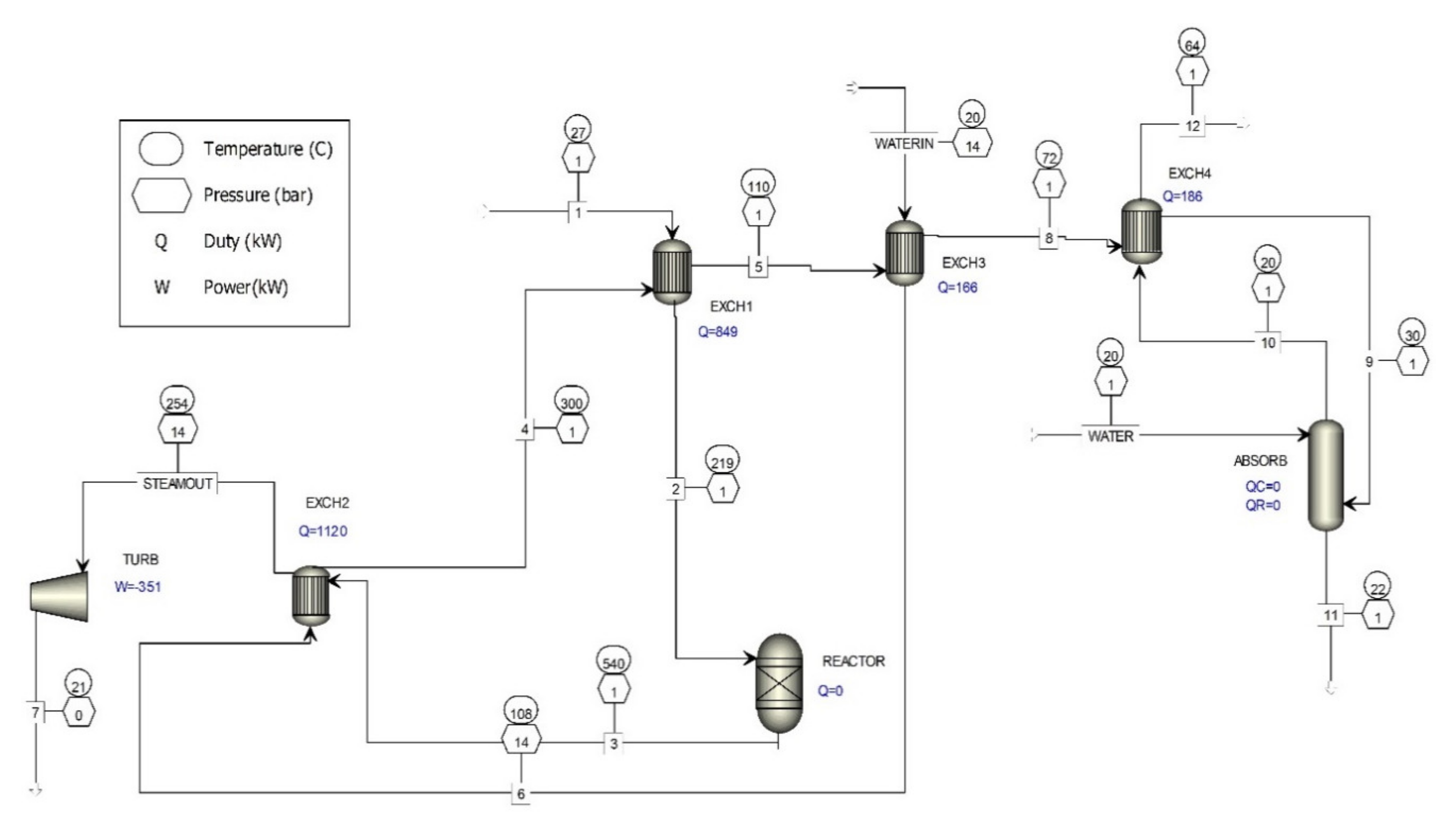
Figure 1 shows the existing catalytic process for the removal of formaldehyde from waste gases. The existing catalytic process for the removal of formaldehyde from waste gases is complemented by new flue gas cleaning technology with the implementation of a heat exchanger (EXCH4) and an absorber (ABSORB).
Aspen Plus computer simulator does not have absorption kinetics for low concentrations of formaldehyde in water in the database, so the kinetics of formaldehyde absorption in water were added to the computer program described in Section 4.2.
The results of the computer simulation shown in Figure 4 are given in Table 3, where it is evident that the concentration of formaldehyde decreased by more than five-fold.
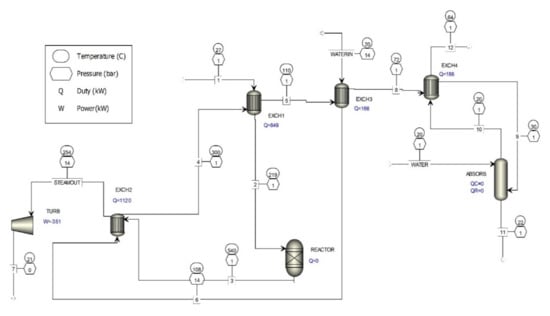
Figure 4.
Computer simulation of additional waste gas treatment of metal oxide catalyst formaldehyde production processes.

Table 3.
The results of the computer simulation for a heat exchanger (EXCH4) and an absorber (ABSORB).
The waste gases (Figure 4) that go from heat exchanger 3 (EXCH3) to heat exchanger 4 (EXCH4) are cooled to a temperature near the dew point of the aqueous vapors present in the gases and then fed into the absorber (scrubber). In the absorber, the waste gases are rinsed with the addition of water, and FA, MG, and TVOC are absorbed in water. The waste gases go back to heat exchanger 4, where they are heated and fed to the chimney, where they are discharged into the atmosphere. The temperature of the waste gases fed from the absorber to heat exchanger 4 can be regulated by regulating the temperature of the gases leaving heat exchanger 3, because they must be sufficiently heated so that they can be discharged into higher layers of the atmosphere.
With additional cleaning of the waste gases with water, the amount of FA, MG, and TVOC present is reduced, depending on the amount of water used. For example, if the gases in the absorber are washed with 22.2 kg/s of water (Table 3), the concentration of FA is reduced to 0.41 mg/m3 and TVOC to 23.4 mg/m3, which is substantially less than the values given in [12].
Wastewater leaving the absorber contains small quantities of FA, MG, and TVOC. The rate of cleaning FA, MG, and TVOC from waste industrial gases depends on the amount of fresh water supplied to the absorber.
5. Co-Incineration of Waste Industrial Gases
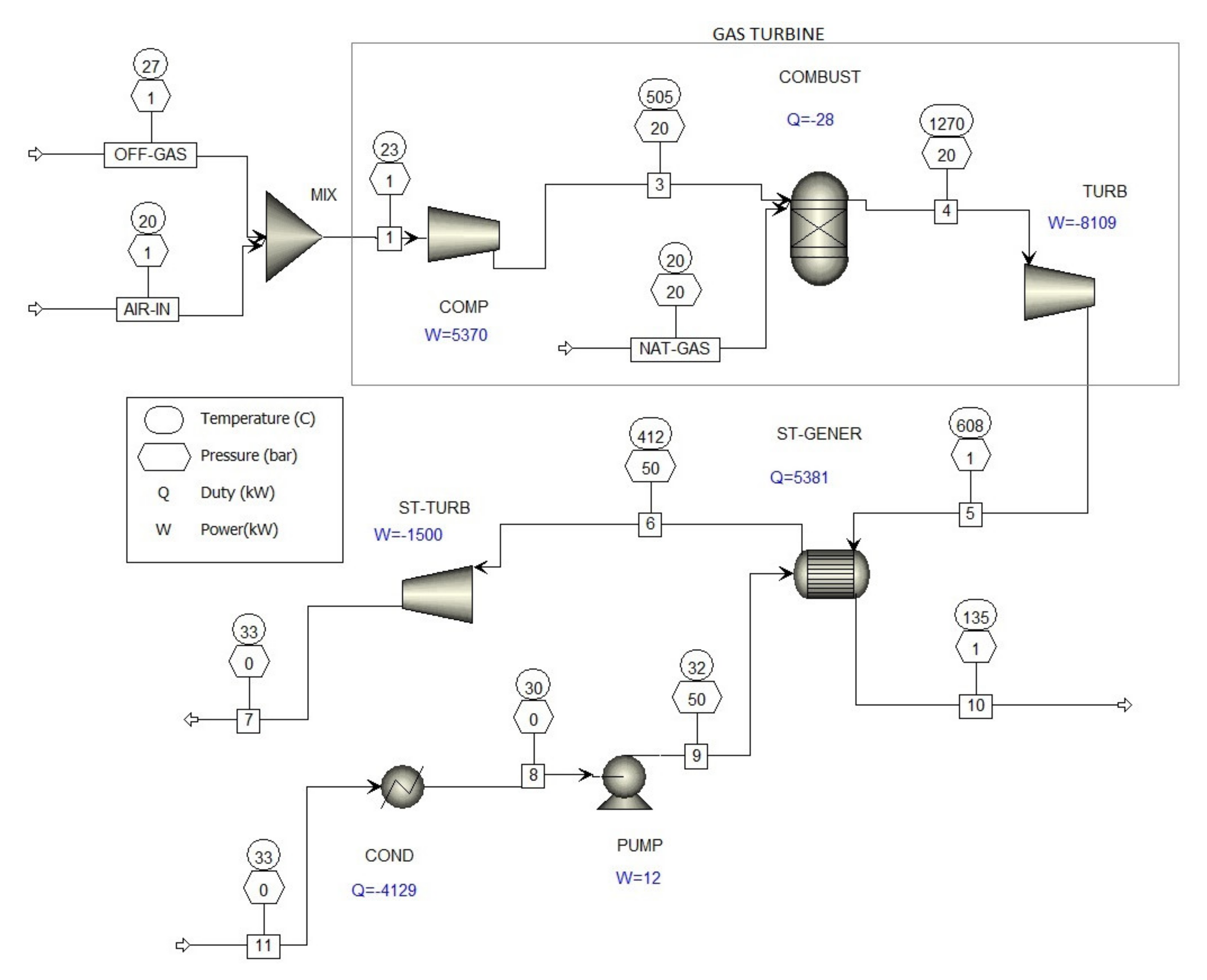
The waste gas treatment process described in Figure 1 is in accordance with existing EU directives and sufficiently reduces the concentration of formaldehyde in waste gases to comply with current standards, but it still releases about 280 kg of formaldehyde per year. By purifying the waste gases with water absorption, as shown in Figure 4, formaldehyde emission is reduced from 0.033 kg/h to about 0.0056 kg/h; about 0.027 kg/h formaldehyde is absorbed in water, which is then used as process water. In order to completely remove formaldehyde from waste gases, which contain other combustible organic substances, co-incineration with natural gas is recommended, with the resulting energy used to generate electricity. Proposed new technological process for off-gases’ co-incineration from the metal oxide catalyst formaldehyde production processes reduces the levels of formaldehyde, carbon monoxide (CO), and volatile organic compounds (VOCs) to practically zero. The co-incineration process would use the combined-cycle gas turbine (CCGT) power system shown in Figure 5.
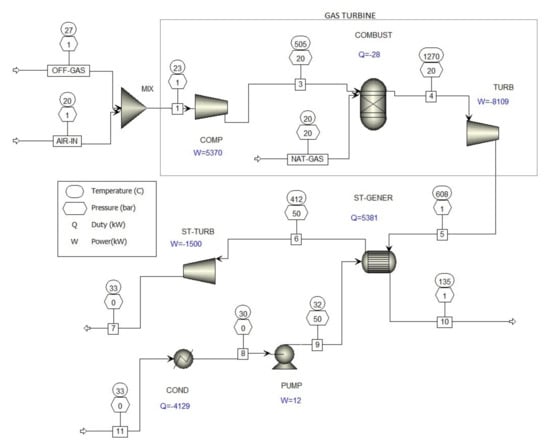
Figure 5.
Computer simulation of the co-incineration of waste industrial gases from metal oxide catalyst formaldehyde production processes in a combined-cycle gas turbine (CCGT).
A combined-cycle gas turbine power system consists of the following components: Gas turbine, steam turbine, waste heat recovery units for steam generation, condenser, and other auxiliary equipment [22]. A combined-cycle gas turbine power system typically uses a gas turbine to drive an electrical generator and recovers waste heat from the turbine exhaust gas to generate steam, which drives a steam turbine to provide supplemental electricity. The overall electrical efficiency of a combined-cycle gas turbine power system is typically in the range of 50–60% [23].
Figure 5 shows the co-combustion of waste gases in the metal oxide catalytic formaldehyde production process; the waste gases (OFF-GAS) are mixed with air (AIR-IN) in a static mixer (MIX) before going into the compressor (COMP). The lower explosion limit of the carbon monoxide/air mixture is 12.5 vol.%, so no explosion will occur when compressing a very diluted off-gas/air mixture in the gas turbine compressor.
The gas turbine is comprised of a compressor (COMP), a combustion chamber (COMBUST), which supplies natural gas (NAT-GAS) to the burners, and a turbine (TURB) that drives the generator. The gas turbine emits flue gases into heat recovery units for steam generation (ST-GENER), and the steam then goes into the steam turbine (ST-TURB) to provide supplemental electricity.
The results of the computer simulation co-incineration of waste gases from formaldehyde production processes in combined cycle gas turbines (Figure 5) are given in Table 4, showing that the concentration of formaldehyde CO and VOC in the waste gases decreased practically to zero.

Table 4.
Results of computer simulation of co-incineration of waste industrial gases from metal oxide catalyst formaldehyde production processes in combined-cycle gas turbines (CCGT).
The theoretical net power (Figure 5) of co-incineration of waste industrial gases from metal oxide catalyst formaldehyde production processes in a combined-cycle gas turbine is 4239 kW. According to the low heat value (LHV) of the natural gas, at the gas consumption of 577.5 kg/h the efficiency is 52.6%.
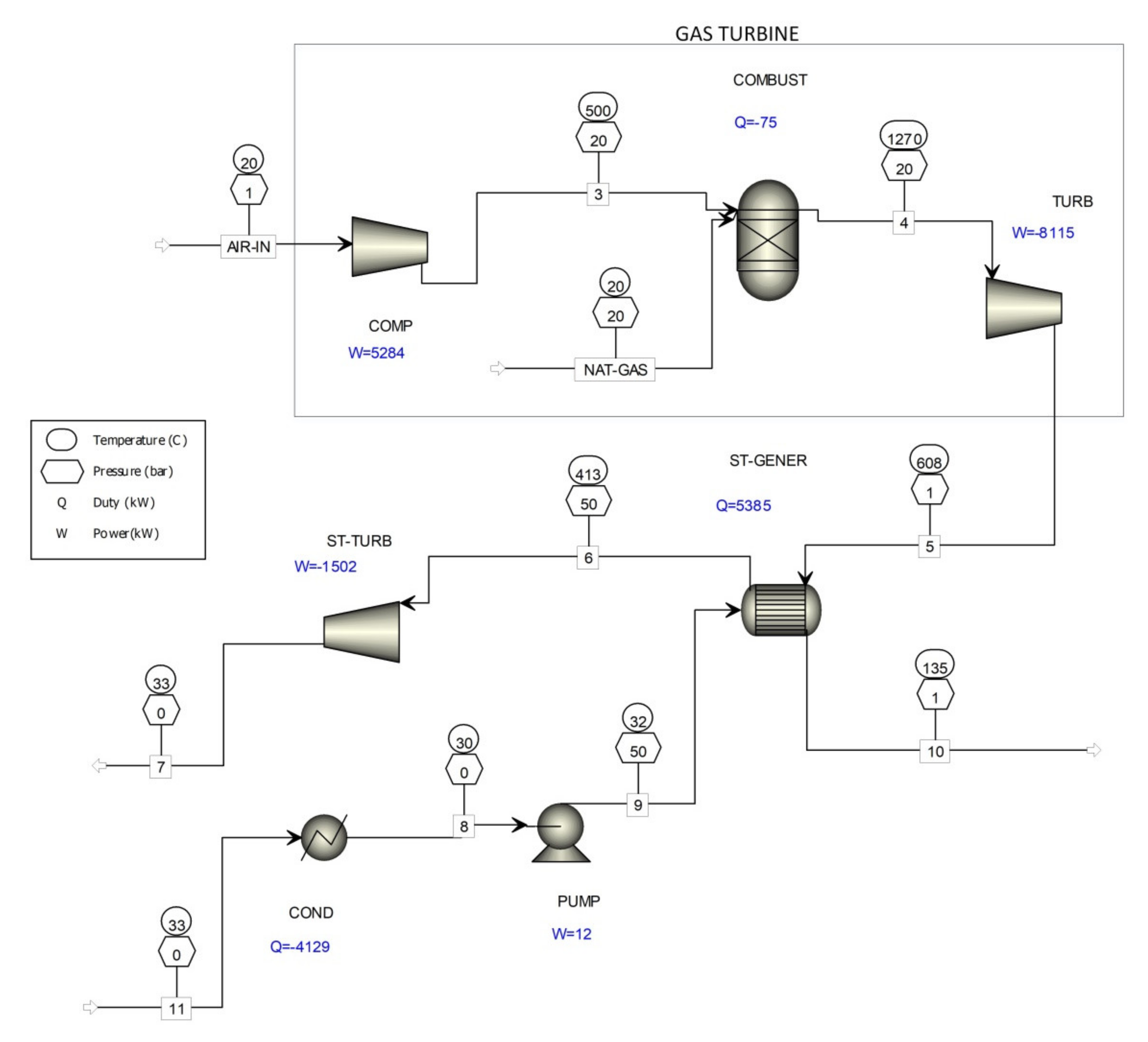
To compare how much less natural gas is consumed to produce the same amount of electricity as in the process shown in Figure 5, a computer simulation of combined cycle gas turbines (CCGT) is shown in Figure 6 for electricity production using only natural gas. The results of the computer simulation of combined cycle gas turbines (Figure 6) are given in Table 5.
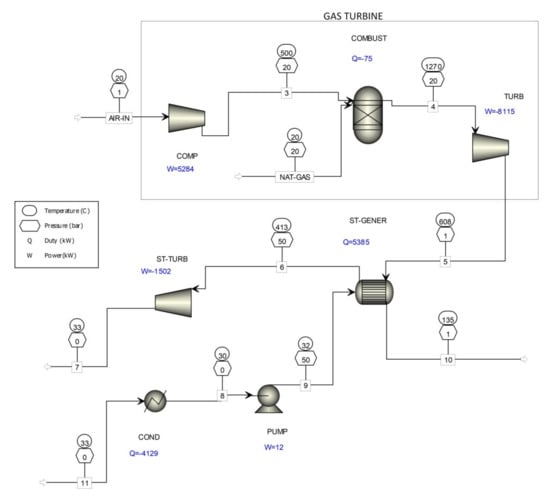
Figure 6.
Computer simulation of the combined-cycle gas turbine.

Table 5.
Results of computer simulation of the combined-cycle gas turbines.
According to the data of the waste off-gases’ composition from the existing metal oxide catalyst formaldehyde production processes given in Table 1, the device for co-incineration of waste industrial gases with a combined-cycle gas turbine is small.
This makes it difficult to optimize its performance in terms of maximizing efficiency. Newer, large CCGTs can achieve efficiencies of more than 55%, some even close to 60% [23].
The theoretical net power of a combined-cycle gas turbine, presented in Figure 6, is 4333 kW. According to the (LHV) used natural gas, the efficiency is 43.3% at the natural gas consumption of 716.2 kg/h.
By comparing the results of the computer simulation shown in Table 5, it is evident that the co-incineration of waste gases from the existing formaldehyde production processes (Figure 5) with combined cycle gas turbines (Figure 6) consumed up to 20% less natural gas.
A gas turbine requires a large amount of air for its operations. When mixing air with the off-gases produced by the metal oxide catalyst formaldehyde production processes, the concentration of off-gas/air mixture is very low. Increasing the off-gas/air mixture concentration would reduce the wasteful amount of natural gas at the co-incineration of waste industrial gases with a combined-cycle gas turbine, but further research is required to avoid an explosion in the gas turbine compressor.
6. Economics of Co-Incineration of Waste Gases
Manufacturing processes in the chemical process industry require a lot of energy for their operation. The electricity required to operate the chemical process can be obtained from the electricity grid or can be produced by our own production. The heat required for operation of the chemical process could be produced or utilize the heat flows of the production process through thermal integration. When consuming electricity from the electricity grid, except for the cost of energy consumed, it is necessary to pay the connection power, transport, distribution, CO2 emissions, and other taxes, which differ from one country to another.
For own production of electricity for the purposes of the chemical process, apart from the cost of consuming primary energy to produce electricity, there are no other taxes than CO2 tax. Of course, it is necessary to take into account the investment costs in the power plant for electricity production. Electricity generation for transmission to the electricity grid is not cost effective due to the low purchase price, except in the case of subsidies for the use of renewable sources or the combination of heat and power.
If the heat required for the operation of the chemical production process is taken from the CCGT power plant, steam is withdrawn before entering the steam turbine. In this case, the power plant is called CHP (Combined Heat and Power). Some countries promote CHP through subsidized energy production, where electricity can be supplied to the electricity grid or consumed for its own production process, which is also economically most favorable.
The research for removing formaldehyde and other VOCs from the off-gases produced by the metal oxide catalyst formaldehyde production process presented in this article was not intended to investigate the economics of the various methods of electricity generation but with a view to reducing the cost of the ecological problem posed by removing formaldehyde from off-gases.
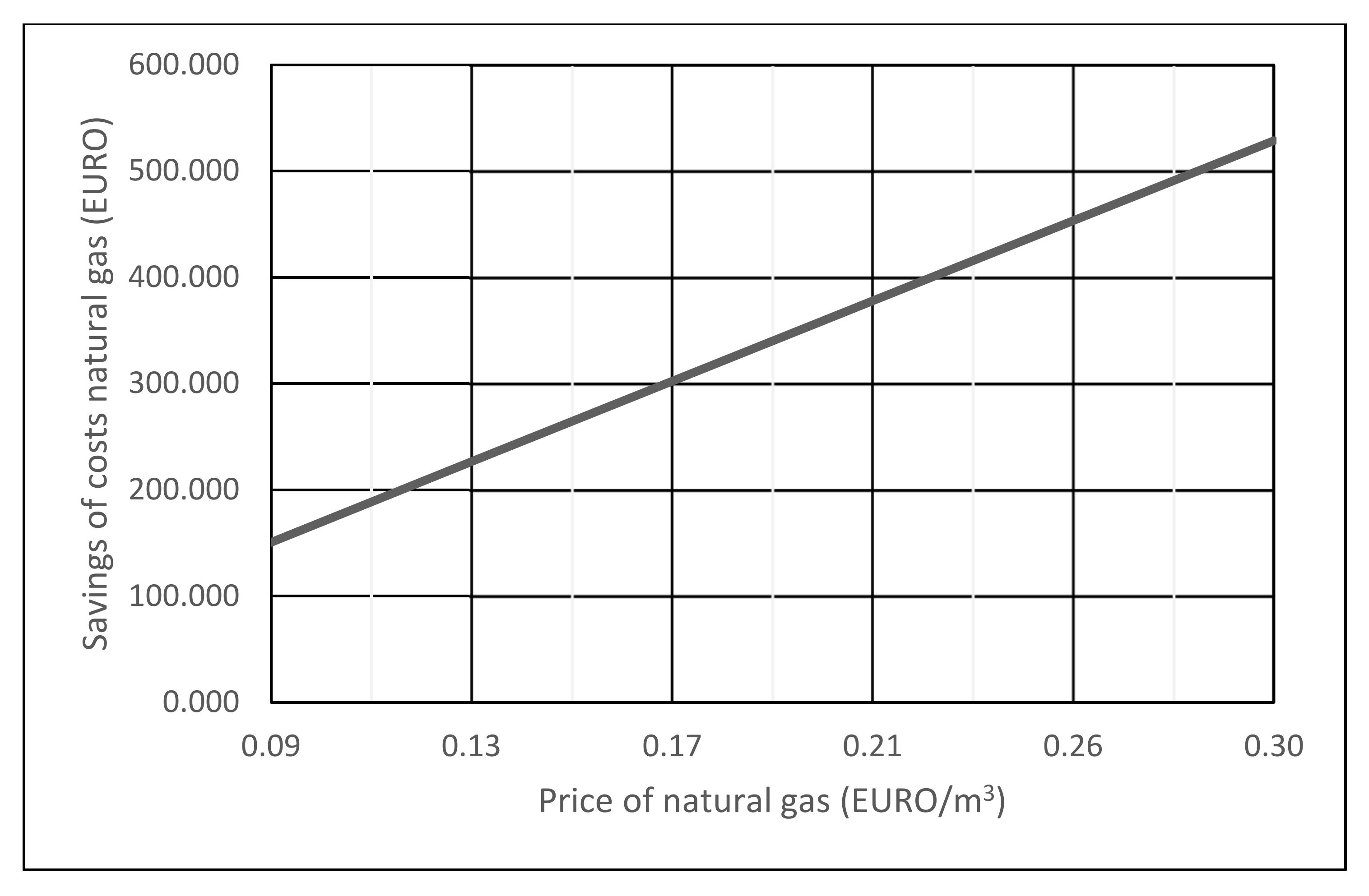
A comparison of the savings for natural gas costs in electricity production with the co-incineration of waste industrial gases from metal oxide catalyst formaldehyde production process with CCGT (Figure 5) and CCGT using only natural gas as energy (Figure 6) is shown in Figure 7.
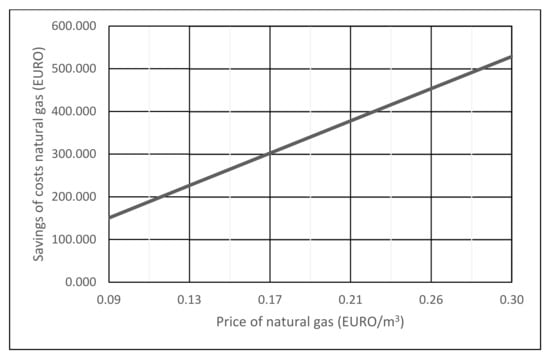
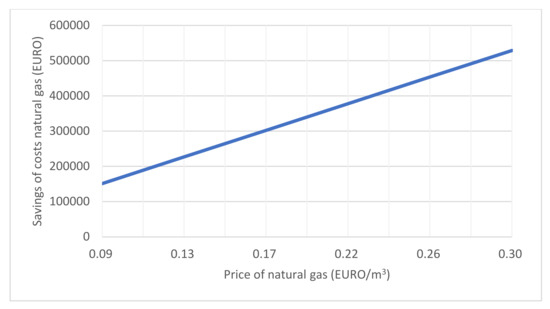
Figure 7.
Savings of natural gas costs when co-incineration of waste gases operating 8500 h/year.
Due to changes in the market price of natural gas, Figure 7 shows the dependence of the cost savings of natural gas when CCGT co-incineration of waste gases operating 8500 h/year and CCGT operation of the same power on natural gas only.
7. Conclusions
The current process of removing formaldehyde and VOCs from waste gas produced by metal oxide catalyst formaldehyde production processes will no longer meet the expected new EU environmental directives. Therefore, a study of possible new technical solutions was conducted to significantly reduce the concentration of formaldehyde and VOCs in the waste gases generated by the formaldehyde production process with metal oxide.
Computer simulations of the proposed new technical solutions for the removal of formaldehyde and VOCs from waste gases were performed with the Aspen Plus software package.
The first proposed technical solution for the removal of formaldehyde and VOCs from waste gases produced by metal oxide catalyst formaldehyde production processes (Figure 1) is an upgrade of the existing process by the additional absorption of formaldehyde present in flue gases in water (Figure 2). In the computer simulation, the equilibrium absorption constants and reactions of low concentrations of formaldehyde in water were taken into account.
By describing an additional new process for absorbing low concentrations of formaldehyde from waste gases (Figure 2) and comparing the data obtained with the measurements of the real production process (Figure 1) given in Table 1 and the results of computer simulation given in [12], formaldehyde emissions into the environment can be reduced by more than five times. The heat generated by the process of removing formaldehyde and VOCs from waste gases produced by metal oxide catalyst formaldehyde production processes (Figure 1 and Figure 2) can be used to produce steam used for the production process or to generate electricity.
The second proposed technical solution in the conducted study is the co-combustion of waste gases produced by metal oxide catalyst formaldehyde production processes with CCGT (Figure 5) or CHP if the steam produced is used for heat production instead of electricity production. The results of a computer simulation of co-incineration of waste gases generated by metal oxide catalyst formaldehyde production processes with CCGT or CHP are given in Table 4, which shows that the concentration of formaldehyde and VOCs in the waste gases decreased to practically zero.
In the performed research of the removal of formaldehyde and VOCs by co-incineration of waste gases produced by metal oxide catalyst formaldehyde production processes with CCGT or CHP power plants, we removed formaldehyde and VOCs from the waste gas and saved the cost of electricity or heat required to operate the production process, which is in line with the expected new EU environmental directives, sustainable development, and the company’s global competitiveness.
Author Contributions
J.M. is PhD student at the University of Maribor and it is an expert in the area of formaldehyde and forlamdehyde resins used wood industry. He developed the model for formaldehyde elimination from waste gases. D.G. is a full professor at the University of Maribor. He developed the model in Aspen Plus together with the assistant professor D.U. All authors worked together in the results interpretation and paper preparation. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| K | mass-transfer coefficient, m/s |
| Kh | chemical equilibrium constant of the formaldehyde hydration, dimensionless number |
| kh | reaction rate constant of hydration, s−1 |
| kd | reaction rate constant of dehydration, s−1 |
| LP | liquid phase |
| m | equilibrium constant in gas–liquid system |
| ps | pure component vapor pressure, kPa |
| VP | vapor phase |
| βg | mass-transfer coefficients in a gas phase, m/s |
| βl | mass-transfer coefficients in a liquid phase, m/s |
References
- Walker, J.F. Formaldehyde, 3rd ed.; Robert. E. Krieger Publishing Co.: Huntington, NY, USA, 1975. [Google Scholar]
- Aspen, J.; Gao, S.; Hawari, Y.; Lam, E.; Larsson, T. Optimization of Formaldehyde Plant, Feasibility Studies KET050; Haldor Topsøes: Lyngby, Denmark, 2015. [Google Scholar]
- Shawabkeh, R. Production of Formaldehyde from Methanol; Integrated Final Report; King Fahd University of Petroleum & Minerals: Dhahran, Saudi Arabia, 2012. [Google Scholar]
- Bahmanpour, A.M.; Hoadley, A.; Tanksale, A. Critical review and exergy analysis of formaldehyde production processes. Rev. Chem. Eng. 2014. [Google Scholar] [CrossRef]
- Lunev, N.K.; Shmyrko, Y.I.; Pavlenko, N.V.; Norton, B. Selective formation of formaldehyde from carbon dioxide and hydrogen over PtCu/SiO2. Proc. Appl. Organomet. Chem. 2001, 15, 148–150. [Google Scholar]
- Qian, M.; Liauw, M.A.; Emig, G. Formaldehyde synthesis from methanol over silver catalysts. Appl. Catal. A Gen. 2003. [Google Scholar] [CrossRef]
- Millar, G.J.; Collins, M. Industrial Production of Formaldehyde Using Polycrystalline Silver Catalyst. Ind. Eng. Chem. Res. 2017, 56, 9247–9265. [Google Scholar] [CrossRef]
- Directive Council. European Commission Establishing Best Available Techniques (BAT) Conclusions, under Directive 2010/75/EU of the European Parliament and of the Council, for the production of large volume organic chemicals. Off. J. Eur. Union 2004, 334, 17–119. Available online: http://eur-lex.europa.eu/pri/en/oj/dat/2003/l_285/l_28520031101en00330037.pdf (accessed on 10 December 2019).
- Zhou, X.; Liu, Y.; Song, C.; Wang, X.; Wang, F.; Liu, J. A novel method to determine the formaldehyde emission characteristic parameters of building materials at multiple temperatures. Build. Environ. 2019. [Google Scholar] [CrossRef]
- Chen, W.; Mendell, M.; Li, N.; Kumagai, K. Formaldehyde emissions from seams and cut edges of laminate flooring: Implications for emission testing protocols and exposure estimation. Build. Environ. 2018. [Google Scholar] [CrossRef]
- Li, B.; Cheng, Z.; Yao, R.; Wang, H.; Yu, W.; Bu, Z.; Xiong, J.; Zhang, T.; Essah, E.; Luo, Z.; et al. An investigation of formaldehyde concentration in residences and the development of a model for the prediction of its emission rates. Build. Environ. 2019. [Google Scholar] [CrossRef]
- Kuramshina, K.S.; Pavlova, K.A. Physical and chemical analysis of formaldehyde absorption process. J. Eng. Appl. Sci. 2016, 11, 9655–9663. [Google Scholar]
- Winkelman, J.G.M.; Voorwinde, O.K.; Ottens, M.; Beenackers, A.A.C.M.; Janssen, L.P.B.M. Kinetics and chemical equilibrium of the hydration of formaldehyde. Chem. Eng. Sci. 2002. [Google Scholar] [CrossRef]
- Albert, M.; Garcia, B.G.; Kreiter, C.; Maurer, G. Vapor-liquid and chemical equilibria of formaldehyde-water mixtures. AIChE J. 1999. [Google Scholar] [CrossRef]
- Maurer, G. Vapor-Liquid Equilibrium of Formaldehyde- and Water-Containing Multicomponent Mixtures. AIChE J. 1986, 32, 932–948. [Google Scholar] [CrossRef]
- Hahnenstein, I.; Albert, M.; Hasse, H.; Kreiter, C.G.; Maurer, G. NMR Spectroscopic and Densimetric Study of Reaction Kinetics of Formaldehyde Polymer Formation in Water, Deuterium Oxide, and Methanol. Ind. Eng. Chem. Res. 1995. [Google Scholar] [CrossRef]
- Hahnenstein, I.; Hasse, H.; Kreiter, C.G.; Maurer, G. 1H- and 13C-NMR-Spectroscopic Study of Chemical Equilibria in Solutions of Formaldehyde in Water, Deuterium Oxide, and Methanol. Ind. Eng. Chem. Res. 1994. [Google Scholar] [CrossRef]
- Hahnenstein, I.; Hasse, H.; Liu, Y.Q.; Maurer, G. Thermodynamic Properties of Formaldehyde Containing Mixtures for Separation Process Design. In AIChE Symposium Series; American Institute of Chemical Engineers: New York, NY, USA, 1994; p. 141. [Google Scholar]
- Fredenslund, A. Vapor—Liquid Equilibria Using UNIFAC, a Group Contribution Method; Elsevier: Amsterdam, The Netherlands, 1977. [Google Scholar]
- Kogan, L.V. State of the Vapor Phase above Solutions of Formaldehyde in Water and Methanol. Zhur. Prikl. Khim. 1979, 52, 2576–2578. [Google Scholar]
- Winkelman, J.G.M.; Beenackers, A.A.C.M. Correlations for the density and viscosity of aqueous formaldehyde solutions. Ind. Eng. Chem. Res. 2000. [Google Scholar] [CrossRef]
- Seyedan, B.; Dhar, P.L.; Gaur, R.R.; Bindra, G.S. Computer simulation of a combined cycle power plant. Heat Recover. Syst. CHP 1995. [Google Scholar] [CrossRef]
- Colmenar-Santos, A.; Gómez-Camazón, D.; Rosales-Asensio, E.; Blanes-Peiró, J.J. Technological improvements in energetic efficiency and sustainability in existing combined-cycle gas turbine (CCGT) power plants. Appl. Energy 2018. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).