Comparative Activity of Six Recombinant Stilbene Synthases in Yeast for Resveratrol Production
Abstract
:Featured Application
Abstract
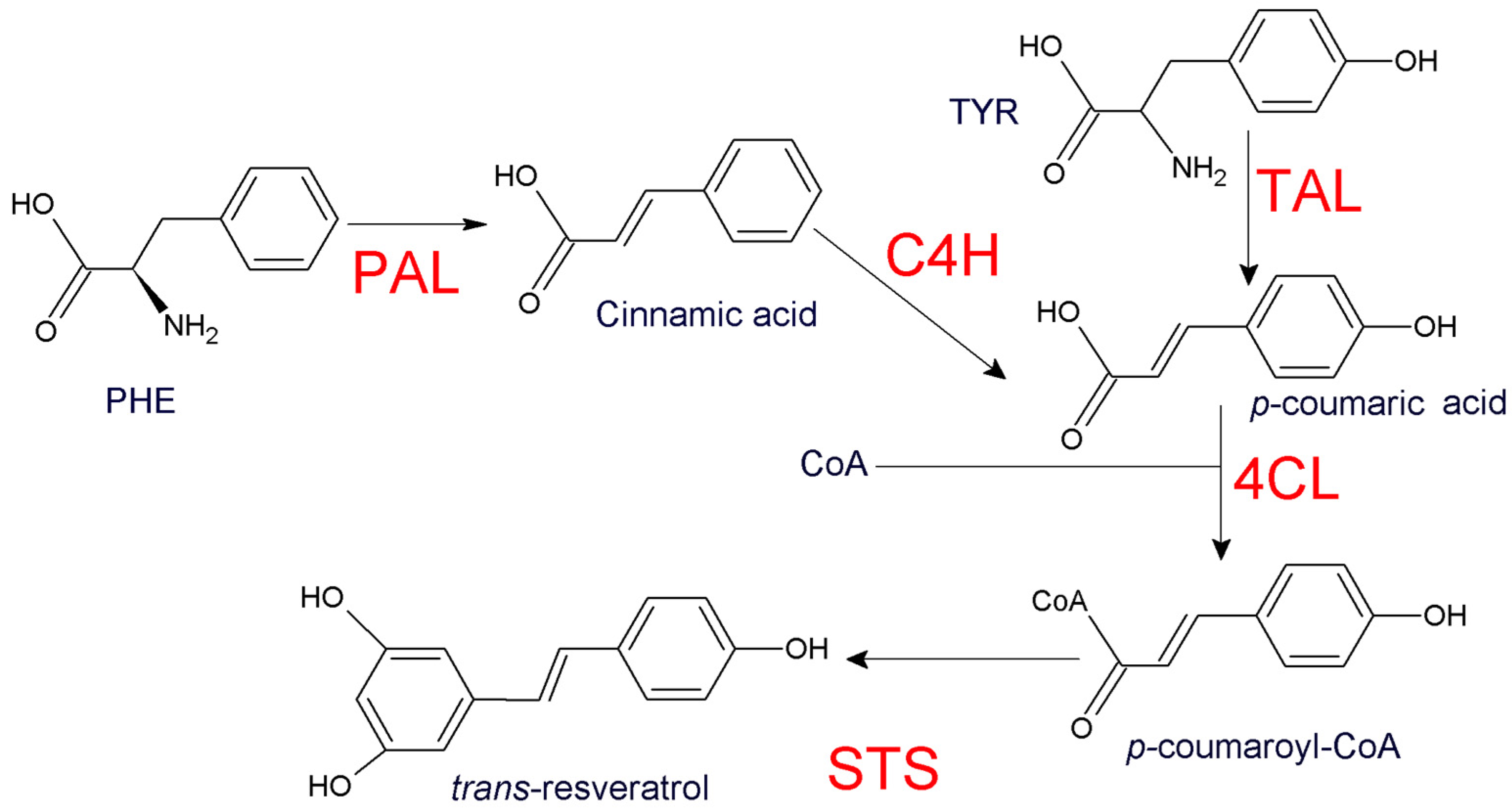
1. Introduction
2. Materials and Methods
2.1. Yeast Strain and DNA Sequences
2.2. Genetic Construction and Heterologous Expression
2.3. Yeast Viability and Effect of pH on p-Coumaric Acid Uptake
2.4. Batch Fermentation
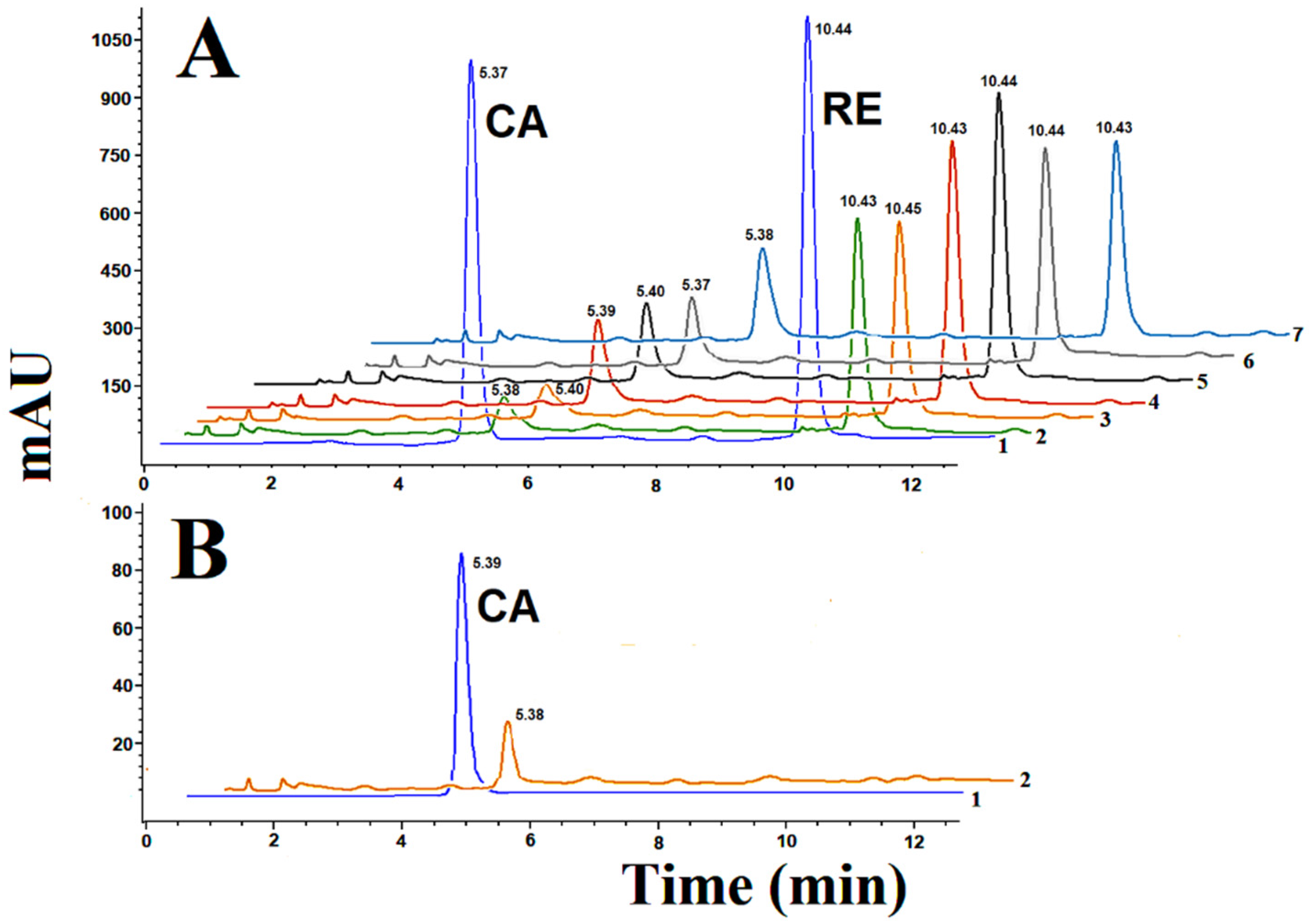
2.5. Extraction and Analysis of Resveratrol
2.6. Statistical Analysis
3. Results and Discussion
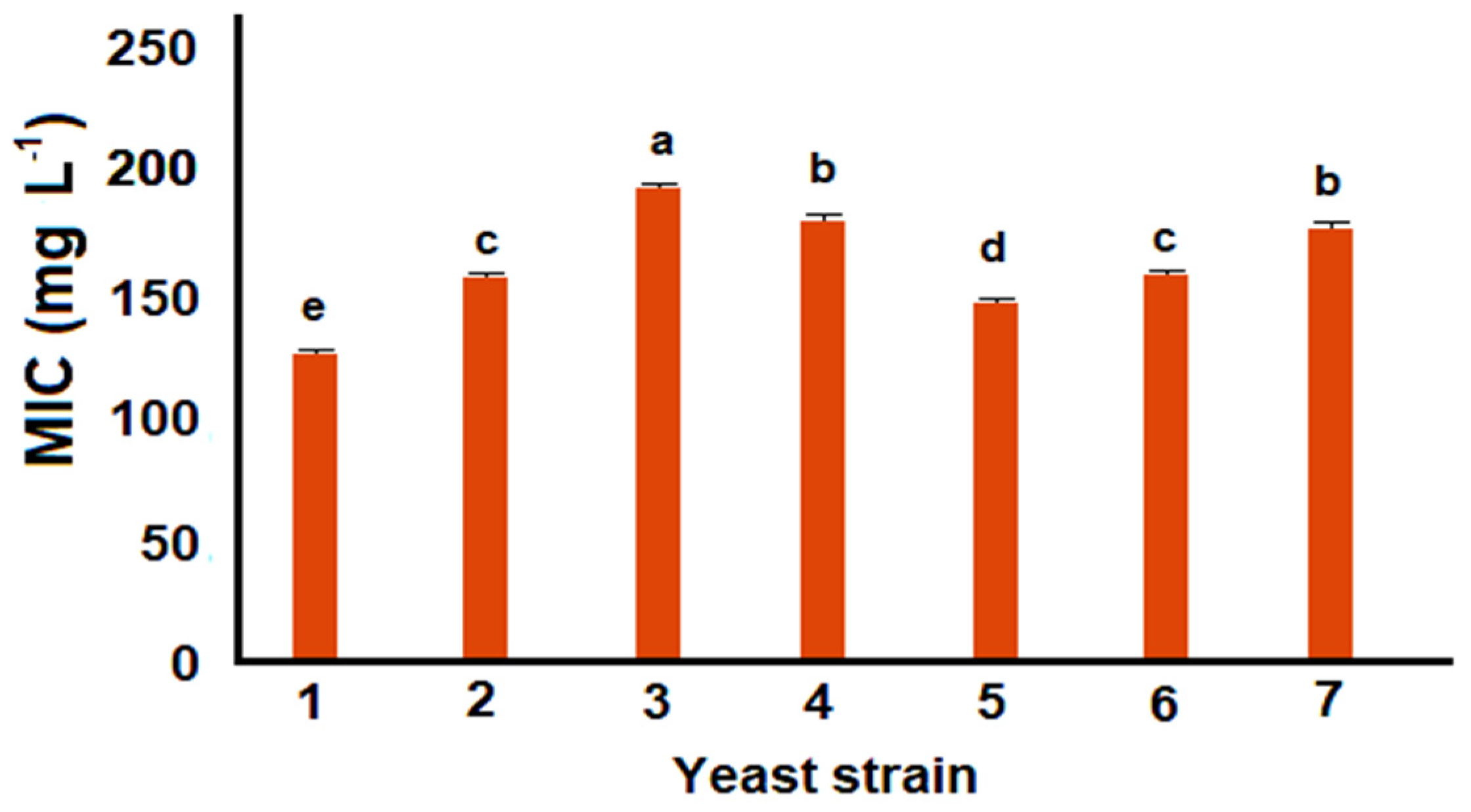
3.1. Tolerance of Yeast Strains to p-Coumaric Acid
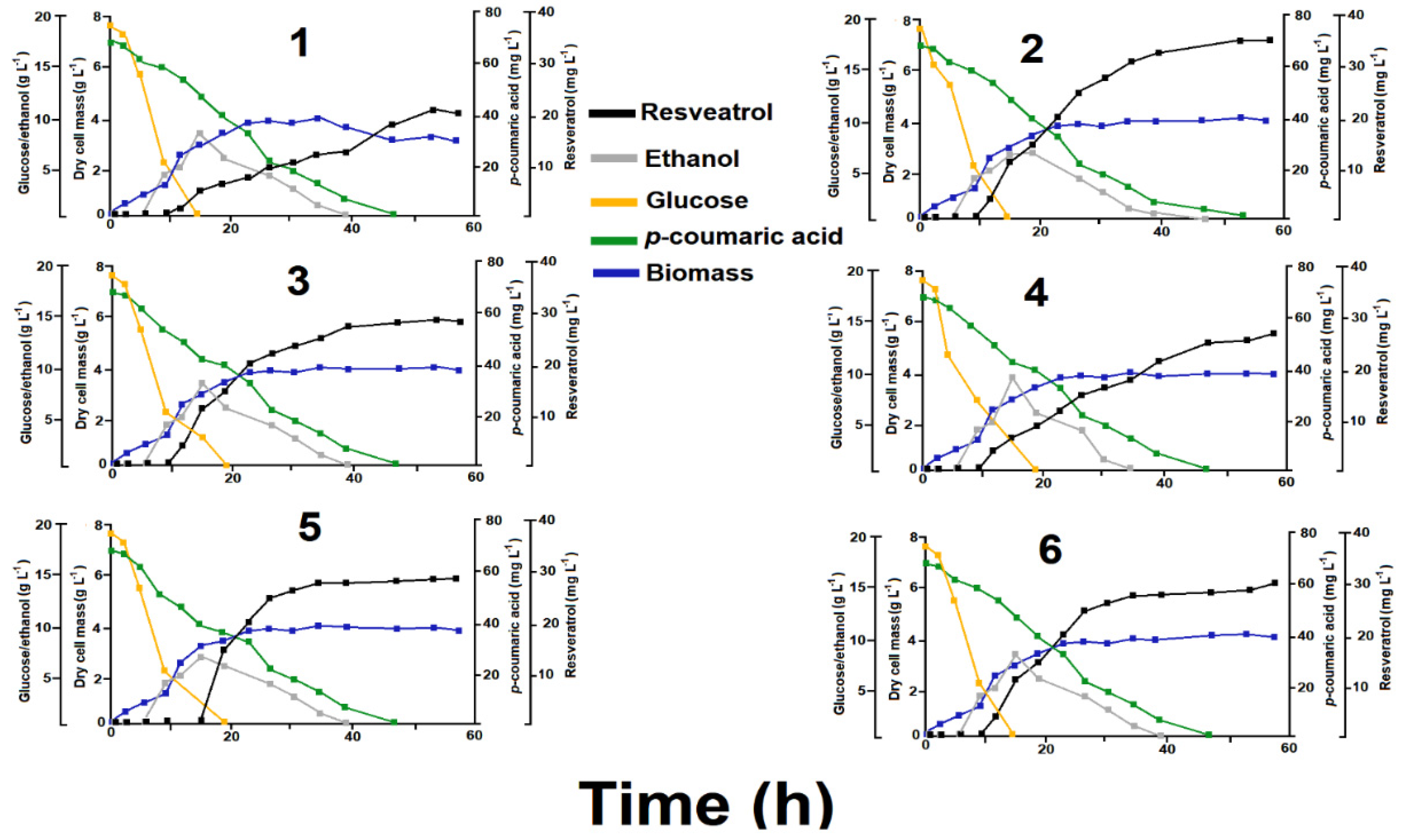
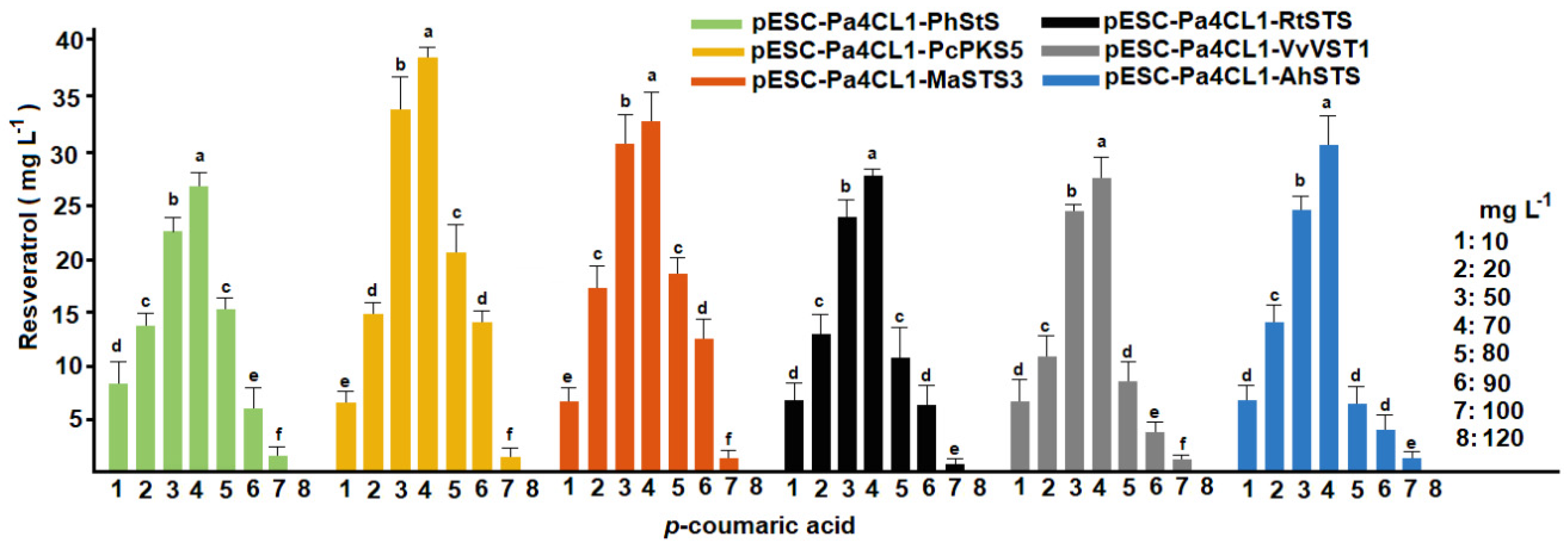
3.2. Conversion of p-Coumaric Acid into Resveratrol under Batch Fermentation Conditions
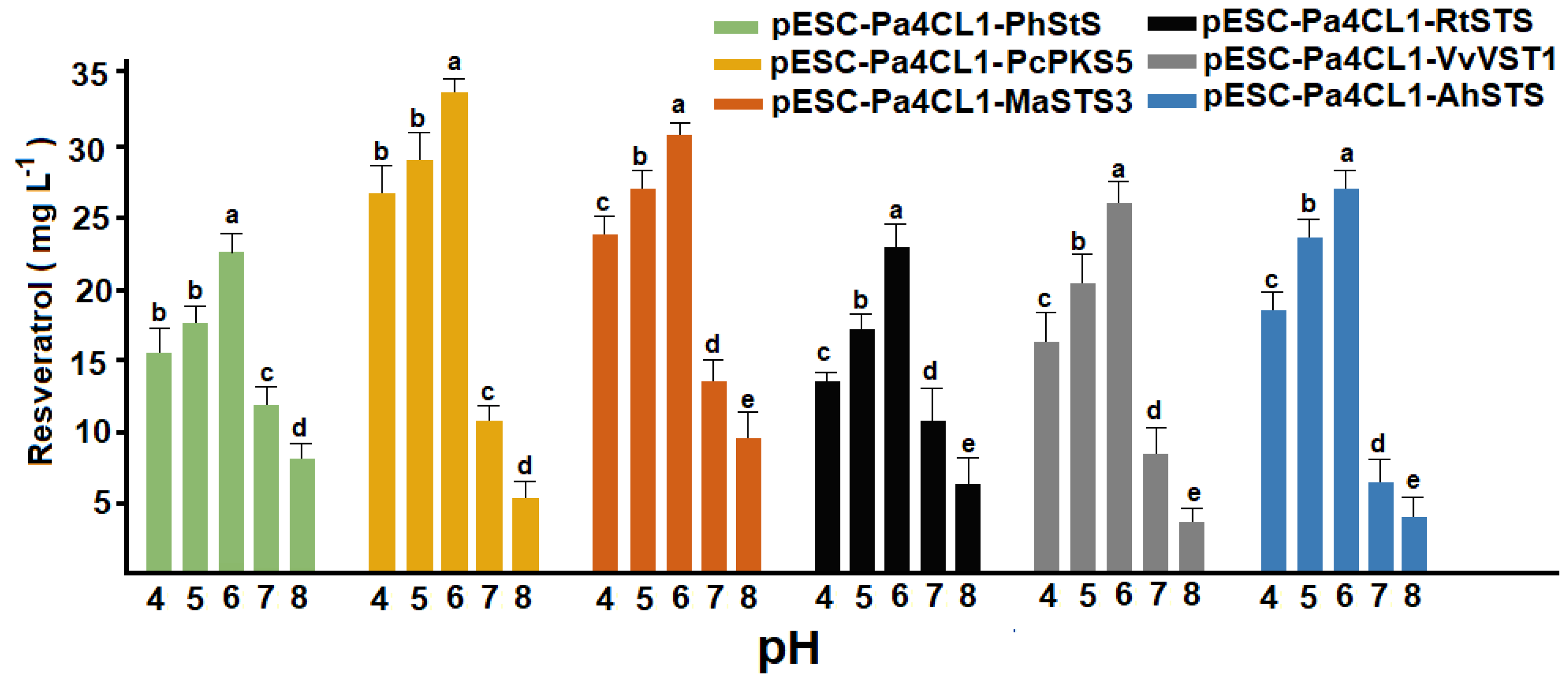
3.3. Effect of pH on p-Coumaric Acid Uptake
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Elshaer, M.; Chen, Y.; Wang, X.J.; Tang, X. Resveratrol: An overview of its anti-cancer mechanisms. Life Sci. 2018, 207, 340–349. [Google Scholar] [CrossRef] [PubMed]
- de la Lastra, C.A.; Villegas, I. Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and clinical implications. Biochem. Soc. Trans. 2007, 35, 1156–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhullar, K.S.; Hubbard, B.P. Lifespan and healthspan extension by resveratrol. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 1209–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samappito, S.; Page, J.E.; Schmidt, J.; De-Eknamkul, W.; Kutchan, T.M. Aromatic and pyrone polyketides synthesized by a stilbene synthase from Rheum tataricum. Phytochemistry 2003, 62, 313–323. [Google Scholar] [CrossRef]
- Pan, L.P.; Yu, S.Y.; Chen, C.J.; Li, H.; Wu, Y.L.; Li, H.H. Cloning a peanut resveratrol synthase gene and its expression in purple sweet potato. Plant Cell Rep. 2012, 31, 121–131. [Google Scholar] [CrossRef]
- Li, M.; Kildegaard, K.R.; Chen, Y.; Rodriguez, A.; Borodina, I.; Nielsen, J. De novo production of resveratrol from glucose or ethanol by engineered Saccharomyces cerevisiae. Metab. Eng. 2015, 32, 1–11. [Google Scholar] [CrossRef]
- Li, M.; Schneider, K.; Kristensen, M.; Borodina, I.; Nielsen, J. Engineering yeast for highlevel production of stilbenoid antioxidants. Sci. Rep. 2015, 6, 36827. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.G.; Fowler, Z.L.; Hueller, T.; Schaffer, S.; Koffas, M.A. High-yield resveratrol production in engineered Escherichia coli. Appl. Environ. Microbiol. 2015, 77, 3451–3460. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.; Zhao, S.; Li, Z.; Wang, Q.; Yao, F.; Yang, L.; Pan, J.; Liu, J. Evaluating the Effect of expressing a peanut resveratrol synthase gene in rice. PLoS ONE 2015, 10, e0136013. [Google Scholar] [CrossRef]
- Camacho-Zaragoza, J.M.; Hernández-Chávez, G.; Moreno-Avitia, F.M.; Ramírez-Iñiguez, R.; Martínez, A.; Gosset, G. Engineering of a microbial coculture of Escherichia coli strains for the biosynthesis of resveratrol. Microb. Cell Fact. 2016, 15, 163. [Google Scholar] [CrossRef] [Green Version]
- Jeandet, P.; Delaunois, B.; Aziz, A.; Donnez, D.; Vasserot, Y.; Cordelier, S.; Courot, E. Metabolic engineering of yeast and plants for the production of the biologically active hydroxystilbene, resveratrol. J. Biomed. Biotechnol. 2012, 579089. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hu, Y.; Wang, X.; Zhong, J.; Lin, Z. High Content of resveratrol in lettuce transformed with a stilbene synthase gene of Parthenocissus henryana. J. Agric. Food Chem. 2006, 54, 8082–8085. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.B.; Pandey, R.P.; Park, Y.; Sohng, J.K. Biotechnological advances in resveratrol production and its chemical diversity. Molecules 2019, 24, 2571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, S.Y.; Han, N.M.; Park, Y.C.; Kim, M.D.; Seo, J.H. Production of resveratrol from p-coumaric acid in recombinant Saccharomyces cerevisiae expressing 4-coumarate:coenzyme A ligase and stilbene synthase genes. Enzyme Microb. Technol. 2011, 48, 48–53. [Google Scholar] [CrossRef]
- Wang, C.; Zhi, S.; Liu, C.; Xu, F.; Zhao, A.; Wang, X.; Ren, Y.; Li, Z.; Yu, M. Characterization of stilbene synthase genes in mulberry (Morus atropurpurea) and metabolic engineering for the production of resveratrol in Escherichia coli. J. Agric. Food Chem. 2017, 65, 1659–1668. [Google Scholar] [CrossRef]
- Rose, M.D.; Winston, F.; Hieter, P. Methods in Yeast Genetics: A Laboratory Course Manual, 1th ed.; Cold Spring Harbor Press: New York, NY, USA, 1990. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtiter plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef]
- Lefebvre, D.; Gabriel, V.; Vayssier, Y.; Fontagné-Faucher, C. Simultaneous HPLC determination of sugars, organic acids and ethanol in sourdough process. LWT-Food Sci. Technol. 2002, 355, 407–414. [Google Scholar] [CrossRef]
- Glavnik, V.; Simonovska, B.; Albreht, A.; Vovk, I. TLC and HPLC screening of p-coumaric acid, trans-resveratrol, and pterostilbene in bacterial cultures, food supplements, and wine. J. Planar Chromat. 2012, 25, 251–258. [Google Scholar] [CrossRef]
- Watts, K.T.; Lee, P.C.; Schmidt-Dannert, C. Biosynthesis of plant-specific stilbene polyketides in metabolically engineered Escherichia coli. BMC Biotechnol. 2006, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Lou, Z.; Wang, H.; Rao, S.; Sun, J.; Ma, C.; Li, J. p-Coumaric acid kills bacteria through dual damage mechanisms. Food Control 2012, 25, 550–554. [Google Scholar] [CrossRef]
- Guo, Y.W.; Guo, H.L.; Li, X.; Huang, L.L.; Zhang, B.N.; Pang, X.B.; Liu, B.Y.; Ma, L.Q.; Wang, H. Two type III polyketide synthases from Polygonum cuspidatum: Gene structure, evolutionary route and metabolites. Plant Biotechnol. Rep. 2013, 7, 371–381. [Google Scholar] [CrossRef]
- Benvidi, A.; Dadras, A.; Abbasi, S.; Tezerjani, M.D.; Rezaeinasab, M.; Tabaraki, R.; Namazian, M. Experimental and computational study of the pK a of coumaric acid derivatives. J. Chin. Chem. Soc. 2019, 66, 589–593. [Google Scholar] [CrossRef]
- Han, M.; Yu, X. Enhanced expression of heterologous proteins in yeast cells via the modification of N-glycosylation sites. Bioengineered 2015, 6, 115–118. [Google Scholar] [CrossRef] [Green Version]
- Tropf, S.; Lanz, T.; Rensing, S.A.; Schröder, J.; Schröder, G. Evidence that stilbene synthases have developed from chalcone synthases several times in the course of evolution. J. Mol. Evol. 1994, 38, 610–618. [Google Scholar] [CrossRef]
- Gao, S.; Yu, H.N.; Xu, R.X.; Cheng, A.X.; Lou, H.X. Cloning and functional characterization of a 4-coumarate CoA ligase from liverwort Plagiochasma appendiculatum. Phytochemstry 2015, 111, 48–58. [Google Scholar] [CrossRef]
| Oligonuclotide Pairs (5->3′) | PCR Conditions | Restriction Sites Added | Target |
|---|---|---|---|
| GGCCGGATCCCGATTTGGGCGCGAATCC GGCCGAATTCTGTTTTATATTTGTTGTAA | 3 min at 94 °C initial denaturation, 35 cycles of 30 s at 94 °C, 30 at 55 °C and 1 min at 72 °C | BamHI EcoRI | Amplification and insertion of PGK promoter into pESC-TRP vector |
| GGCCGGATCCAGTTTATCATTATCAATACTCG AGCCTGGGCCCTCTAGAATCCGTCGAAACTAAG | 3 min at 94 °C initial denaturation, 35 cycles of 30 s at 94 °C, 30 at 56 °C and 1 min 20 s at 72 °C | BamHI ApaI | Amplification and insertion of GPD promoter into the pESC-TRP vector |
| CGGGGCCCATGGGTTATGAGAAATCAG CGCTCGAGTCACAGCTTAGAGCTGGG | 3 min at 94 °C initial denaturation, 30 cycles of 30 s at 94 °C, 30 at 57 °C and 1 min 30 s at 72 °C | ApaI XhoI | Amplification and insertion of Pa4CL1 from Plagiochasma appendiculatum into the pESC-TRP-PGPD-PPGK |
| CTAGGACTAGTATGGCTAGTGTGGAGGAAT GGAAAGATCTTTAATTAGTAACCATAG | 3 min at 94 °C initial denaturation, 30 cycles of 30 s at 94 °C, 30 at 58 °C and 1 min 20 s at 72 °C | SpeI BglII | Amplification and insertion of PhStS from Parthenocissus henryana into the pESC-TRP-PGPD-PPGK |
| CTAGGACTAGTATGGCGGCTTCCACAGAGT GGAAAGATCTTTAAATGATTGGAACAGAAC | 3 min at 94 °C initial denaturation, 30 cycles of 30 s at 94 °C, 30 at 58 °C and 1 min 20 s at 72 °C | SpeI BglII | Amplification and insertion of PcPKS5 from Polygonum cuspidatum into the pESC-TRP-PGPD-PPGK |
| CTAGGACTAGTATGGCACCGAACAATGTATC GGAAAGATCTCTATGCAACGATAGGTACAG | 3 min at 94 °C initial denaturation, 30 cycles of 30 s at 94 °C, 30 at 58 °C and 1 min 20 s at 72 °C | SpeI BglII | Amplification and insertion of MaSTS3 Morus alba var. atropurpurea into the pESC-TRP-PGPD-PPGK |
| CTAGGACTAGTATGGCCCCAGAGGAGTCTC GGAAAGATCTTCAAGTGATTAGTGGTACGC | 3 min at 94 °C initial denaturation, 30 cycles of 30 s at 94 °C, 30 at 58 °C and 1 min 20 s at 72 °C | SpeI BglII | Amplification and insertion of RtSTS from Rheum tataricum into the pESC-TRP-PGPD-PPGK |
| CTAGGACTAGTATGGCCAGCGTTGAAGAG GGAAAGATCTTTAATTAGTAACCGTCGGTA | 3 min at 94 °C initial denaturation, 30 cycles of 30 s at 94 °C, 30 at 58 °C and 1 min 20 s at 72 °C | SpeI BglII | Amplification and insertion of VvVST1n from Vitis vinifera into the pESC-TRP-PGPD-PPGK |
| CTAGGACTAGATGGTTTCAGTGTCTGGC GGAAAGATCTTTATATCGCCATACTCCTAAG | 3 min at 94 °C initial denaturation, 30 cycles of 30 s at 94 °C, 30 at 58 °C and 1 min 20 s at 72 °C | SpeI BglII | Amplification and insertion of AhSTS from Arachis hypogaea into the pESC-TRP-PGPD-PPGK |
| Strain | 50 mg L−1 | 70 mg L−1 |
|---|---|---|
| pESC-Pa4CL1-PhStS | 23.7 ± 2.6 b | 27.1 ± 3.1 c |
| pESC-Pa4CL1- PcPKS5 | 34.5 ± 4.1 a | 39.9 ± 2.5 a |
| pESC-Pa4CL1-MaSTS3 | 31.3 ± 4.7 a | 34.4 ± 3.8 b |
| pESC-Pa4CL1-RtSTS | 24.2 ± 3.1 b | 28.5 ± 2.4 c |
| pESC-Pa4CL1-VvVST1 | 25.4 ± 1.8 b | 27.6 ± 3.7 c |
| pESC-Pa4CL1-AhSTS | 24.7 ± 2.9 b | 30.9 ± 4.3 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villa-Ruano, N.; Rivera, A.; Rubio-Rosas, E.; Landeta-Cortés, G.; Varela-Caselis, J.L.; Romero-Arenas, O. Comparative Activity of Six Recombinant Stilbene Synthases in Yeast for Resveratrol Production. Appl. Sci. 2020, 10, 4847. https://doi.org/10.3390/app10144847
Villa-Ruano N, Rivera A, Rubio-Rosas E, Landeta-Cortés G, Varela-Caselis JL, Romero-Arenas O. Comparative Activity of Six Recombinant Stilbene Synthases in Yeast for Resveratrol Production. Applied Sciences. 2020; 10(14):4847. https://doi.org/10.3390/app10144847
Chicago/Turabian StyleVilla-Ruano, Nemesio, Antonio Rivera, Efraín Rubio-Rosas, Gerardo Landeta-Cortés, Jenaro Leocadio Varela-Caselis, and Omar Romero-Arenas. 2020. "Comparative Activity of Six Recombinant Stilbene Synthases in Yeast for Resveratrol Production" Applied Sciences 10, no. 14: 4847. https://doi.org/10.3390/app10144847







