Multi-mode Microscopic Hyperspectral Imager for the Sensing of Biological Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. System Setup and Calibration
2.2. Epi-illumination and Trans-illumination
3. Results
3.1. Zebrafish Detection on Transmission Mode
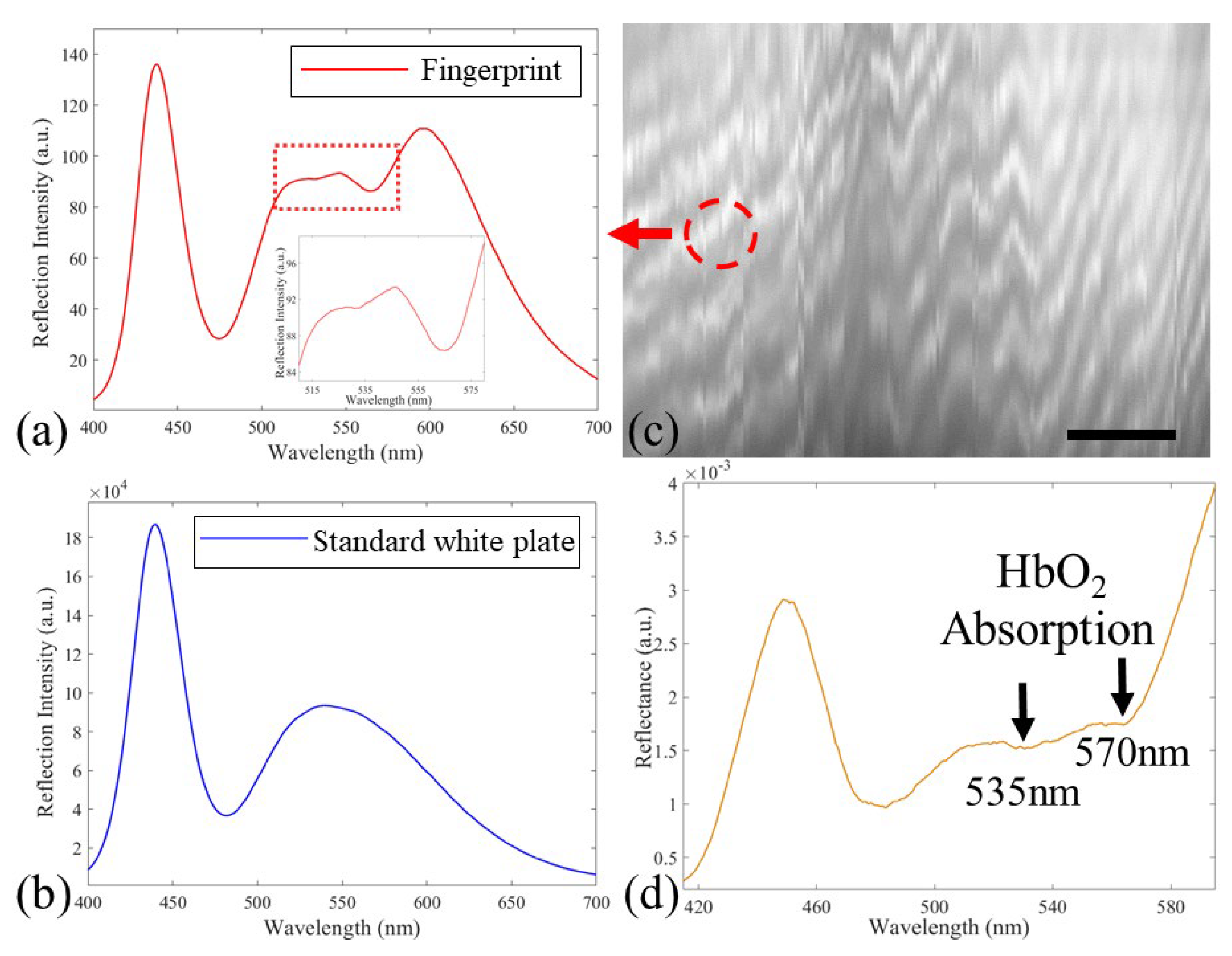
3.2. Human Fingerprint Detection in Reflection Mode
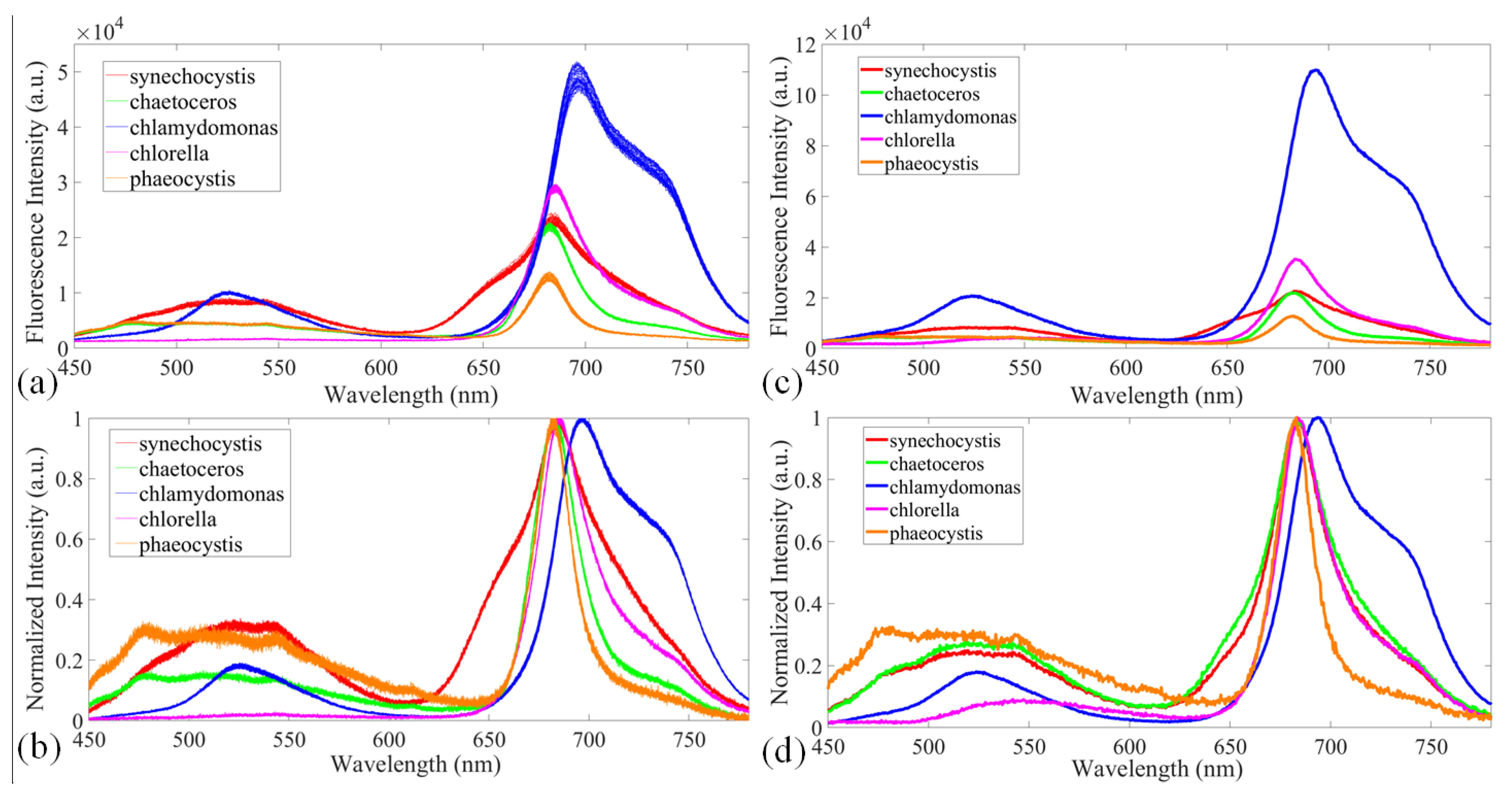
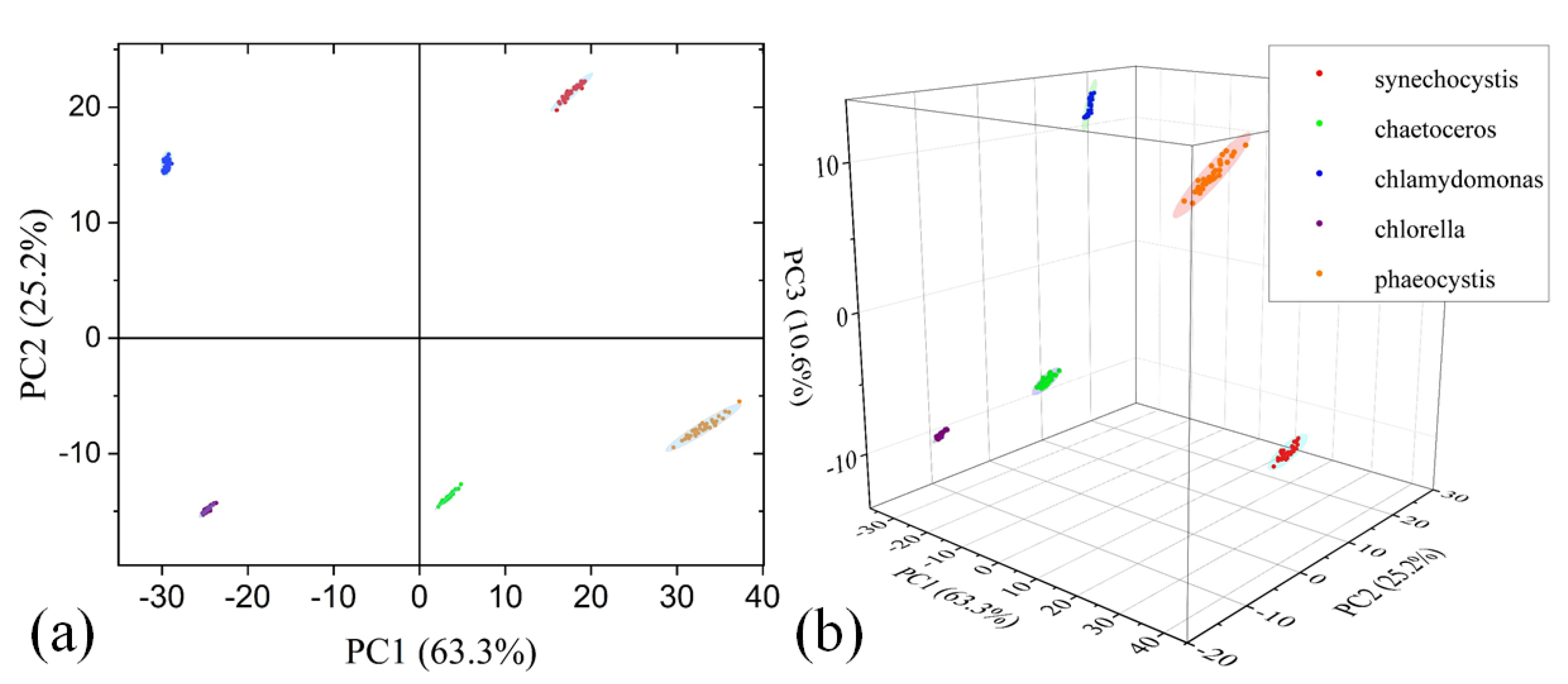
3.3. Disaster-causing Microalgae Classification on Fluorescence Mode
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, G.; Kulinski, P.; Hubert, P.; Deguine, A.; Petitprez, D.; Crumeyrolle, S.; Fertein, E.; Deboudt, K.; Flament, P.; Sigrist, M.W.; et al. Filter-Free Light Absorption Measurement of Volcanic Ashes and Ambient Particulate Matter Using Multi-Wavelength Photoacoustic Spectroscopy. Prog. Electromagn. Res. 2019, 166, 59–74. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, M.; Okamoto, C.; Shinoda, K.; Komagata, H.; Iwamoto, C.; Ohuchida, K.; Hashizume, M.; Shimizu, A.; Kobayashi, N. Detection of pancreatic tumor cell nuclei via a hyperspectral analysis of pathological slides based on stain spectra. Biomed. Opt. Express 2019, 10, 4568–4588. [Google Scholar] [CrossRef]
- Cai, F.; Chen, J.; Xie, X.; Xie, J. The design and implementation of portable rotational scanning imaging spectrometer. Opt. Commun. 2020, 459, 125016. [Google Scholar] [CrossRef]
- Nielsen, J.H.; Pedersen, C.; Kiorboe, T.; Nikolajsen, T.; Brydegaard, M.; Rodrigo, P.J. Investigation of autofluorescence in zooplankton for use in classification of larval salmon lice. Appl. Opt. 2019, 58, 7022–7027. [Google Scholar] [CrossRef]
- Tan, S.; Tsang, L. Efficient Broadband Evaluations of Lattice Green's Functions via Imaginary Wavenumber Components Extractions. Prog. Electromagn. Res. 2019, 164, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Mollaei, M.; Kurdjumov, S.; Hurshkainen, A.; Simovski, C. Decoupling of Two Closely Located Dipoles by a Single Passive Scatterer for Ultra-High Field MRI. Prog. Electromagn. Res. 2019, 164, 155–166. [Google Scholar] [CrossRef] [Green Version]
- Cai, F.; Wang, T.; Wu, J.; Zhang, X. Handheld four-dimensional optical sensor. Optik 2020, 203. [Google Scholar] [CrossRef]
- Lu, G.; Fei, B. Medical hyperspectral imaging: A review. J. Biomed. Opt. 2014, 19, 10901. [Google Scholar] [CrossRef]
- Baek, I.; Kim, M.; Cho, B.-K.; Mo, C.; Barnaby, J.; McClung, A.; Oh, M. Selection of Optimal Hyperspectral Wavebands for Detection of Discolored, Diseased Rice Seeds. Appl. Sci. 2019, 9, 1027. [Google Scholar] [CrossRef] [Green Version]
- Fournier, G.; Ardouin, J.-P.; Levesque, M. Modeling Sea Bottom Hyperspectral Reflectance. Appl. Sci. 2018, 8, 2680. [Google Scholar] [CrossRef] [Green Version]
- Schie, I.W.; Alber, L.; Gryshuk, A.L.; Chan, J.W. Investigating drug induced changes in single, living lymphocytes based on Raman micro-spectroscopy. Analyst 2014, 139, 2726–2733. [Google Scholar] [CrossRef]
- Perrin, S.; Li, H.; Leong-Hoi, A.; Lecler, S.; Montgomery, P. Illumination conditions in microsphere-assisted microscopy. J. Microsc. 2019, 274, 69–75. [Google Scholar] [CrossRef]
- Craig, J.; Sansonetti, M.; Joseph, R. Wavelengths of spectral lines in mercury pencil lamps. Appl. Opt. 1996, 35, 1. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.; Gemperline, P.J.; Walker, D. Wavelength calibration method for a CCD detector and multichannel fiber-optic probes. Appl. Spectrosc. 1995, 49, 1841–1845. [Google Scholar] [CrossRef]
- Cai, F.; Wang, Y.; Gao, M.; He, S. The design and implementation of a low-cost multispectral endoscopy through galvo scanning of a fiber bundle. Opt. Commun. 2018, 428, 1–6. [Google Scholar] [CrossRef]
- Leblond, F.; Davis, S.C.; Valdes, P.A.; Pogue, B.W. Pre-clinical whole-body fluorescence imaging: Review of instruments, methods and applications. J. Photochem. Photobiol. B 2010, 98, 77–94. [Google Scholar] [CrossRef] [Green Version]
- Damestani, Y.; Galan-Hoffman, D.E.; Ortiz, D.; Cabrales, P.; Aguilar, G. Inflammatory response to implantation of transparent nanocrystalline yttria-stabilized zirconia using a dorsal window chamber model. Nanomedicine 2016, 12, 1757–1763. [Google Scholar] [CrossRef]
- Cai, F.; Gao, M.; Li, J.; Lu, W.; Wu, C. Compact Dual-Channel (Hyperspectral and Video) Endoscopy. Front. Phys. 2020, 8. [Google Scholar] [CrossRef]
- Zhu, Y.; Yin, X.; Jia, X.; Hu, J. Latent Fingerprint Segmentation Based on Convolutional Neural Networks. In Proceedings of the IEEE Workshop on Information Forensics and Security (WIFS), Rennes, France, 4–7 December 2017; IEEE: Piscataway, NJ, USA, 2017; ISBN 9781509067701. [Google Scholar] [CrossRef]
- Militello, C.; Rundo, L.; Minafra, L.; Cammarata, F.P.; Calvaruso, M.; Conti, V.; Russo, G. MF2C3: Multi-Feature Fuzzy Clustering to Enhance Cell Colony Detection in Automated Clonogenic Assay Evaluation. Symmetry 2020, 12, 773. [Google Scholar] [CrossRef]
- Ruiz-Santaquiteria, J.; Bueno, G.; Deniz, O.; Vallez, N.; Cristobal, G. Semantic versus instance segmentation in microscopic algae detection. Eng. Appl. Artif. Intell. 2020, 87, 103271. [Google Scholar] [CrossRef]
- Edwards, P.; Zhang, C.; Zhang, B.; Hong, X.; Nagarajan, V.K.; Yu, B.; Liu, Z. Smartphone based optical spectrometer for diffusive reflectance spectroscopic measurement of hemoglobin. Sci. Rep. 2017, 7, 12224. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, M.A.S.; Campbell, M.; Afify, A.M.; Huang, E.C.; Chan, J.W. Hyperspectral Raman microscopy can accurately differentiate single cells of different human thyroid nodules. Biomed. Opt. Express 2019, 10, 4411–4421. [Google Scholar] [CrossRef] [PubMed]
- Bevacqua, T.; Bellizzi, G.; Isernia, T.; Crocco, L. A Method for Effective Permittivity and Conductivity Mapping of Biological Scenarios via Segmented Contrast Source Inversion. Prog. Electromagn. Res. 2019, 164, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Kho, E.; Dashtbozorg, B.; de Boer, L.L.; Van de Vijver, K.K.; Sterenborg, H.; Ruers, T.J.M. Broadband hyperspectral imaging for breast tumor detection using spectral and spatial information. Biomed. Opt. Express 2019, 10, 4496–4515. [Google Scholar] [CrossRef] [Green Version]
- Sigernes, F.; Syrjasuo, M.; Storvold, R.; Fortuna, J.; Grotte, M.E.; Johansen, T.A. Do it yourself hyperspectral imager for handheld to airborne operations. Opt. Express 2018, 26, 6021–6035. [Google Scholar] [CrossRef]
- Simon, J.C.; Lucas, S.A.; Staninec, M.; Tom, H.; Chan, K.H.; Darling, C.L.; Cozin, M.J.; Lee, R.C.; Fried, D. Near-IR transillumination and reflectance imaging at 1300 nm and 1500–1700 nm for in vivo caries detection. Lasers. Surg. Med. 2016, 48, 828–836. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Forsberg, E.; Guo, Y.; Cai, F.; He, S. Light-Sheet Microscopy for Surface Topography Measurements and Quantitative Analysis. Sensors 2020, 20, 2842. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Jiang, Y.; He, S. Multi-mode Microscopic Hyperspectral Imager for the Sensing of Biological Samples. Appl. Sci. 2020, 10, 4876. https://doi.org/10.3390/app10144876
Xu Z, Jiang Y, He S. Multi-mode Microscopic Hyperspectral Imager for the Sensing of Biological Samples. Applied Sciences. 2020; 10(14):4876. https://doi.org/10.3390/app10144876
Chicago/Turabian StyleXu, Zhanpeng, Yiming Jiang, and Sailing He. 2020. "Multi-mode Microscopic Hyperspectral Imager for the Sensing of Biological Samples" Applied Sciences 10, no. 14: 4876. https://doi.org/10.3390/app10144876



