Investigation of Molecular Size Effect on the Formation of Lignin Nanoparticles by Nanoprecipitation
Abstract
1. Introduction
2. Materials and Methods
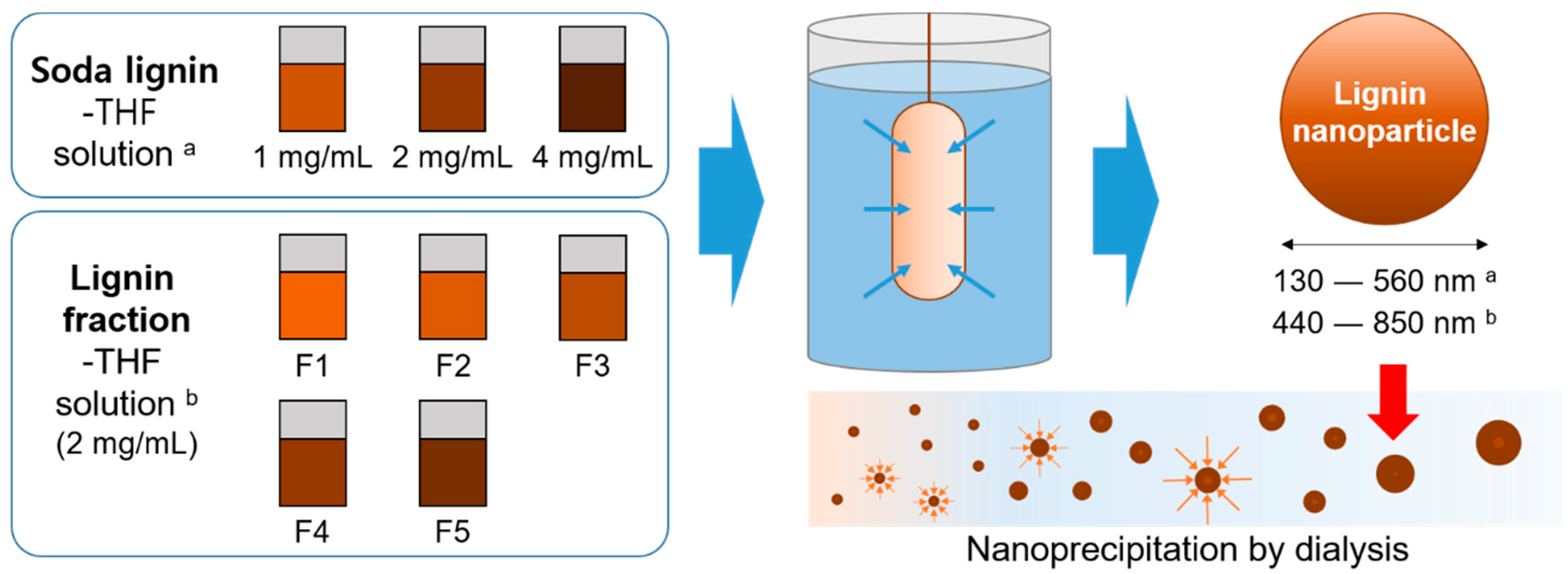
2.1. Lignin Fractionation
2.2. Lignin Nanoparticle Synthesis
2.3. Analytical Methods
3. Results and Discussion
3.1. Structural Features of Lignin Fractions
3.2. Effect of Lignin Concentration on Nanoparticle Synthesis
3.2.1. Particle Size and Shape
3.2.2. Surface Charge
3.3. Effect of Fractionation on Lignin Nanoparticle Synthesis
3.3.1. Particle Size and Shape
3.3.2. Colloidal Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gillet, S.; Aguedo, M.; Petitjean, L.; Morais, A.R.C.; da Costa Lopes, A.M.; Łukasik, R.M.; Anastas, P.T. Lignin transformations for high value applications: Towards targeted modifications using green chemistry. Green Chem. 2017, 19, 4200–4233. [Google Scholar] [CrossRef]
- Martínez, Á.T.; Speranza, M.; Ruiz-Dueñas, F.J.; Ferreira, P.; Camarero, S.; Guillén, F.; Martínez, M.J.; Gutiérrez Suárez, A.; Río Andrade, J.C.d. Biodegradation of lignocellulosics: Microbial, chemical, and enzymatic aspects of the fungal attack of lignin. Int. Microbiol. 2005, 8, 195–204. [Google Scholar]
- Fang, Z.; Smith, R.L., Jr. Production of Biofuels and Chemicals from Lignin; Springer: Berlin, Germany, 2016. [Google Scholar]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Gregorová, A.; Cibulková, Z.; Košíková, B.; Šimon, P. Stabilization effect of lignin in polypropylene and recycled polypropylene. Polym. Degrad. Stab. 2005, 89, 553–558. [Google Scholar] [CrossRef]
- Jiang, C.; He, H.; Jiang, H.; Ma, L.; Jia, D. Nano-lignin filled natural rubber composites: Preparation and characterization. Express Polym. Lett. 2013, 7, 480–493. [Google Scholar] [CrossRef]
- Sarkar, S.; Adhikari, B. Lignin-modified phenolic resin: Synthesis optimization. adhesive strength, and thermal stability. J. Adhes. Sci. Technol. 2000, 14, 1179–1193. [Google Scholar] [CrossRef]
- Park, J.; Hwang, H.; Kim, J.Y.; Choi, J.W. Applicability of lignin polymers for automobile brake pads as binder and filler materials and their performance characteristics. Environ Technol 2020, 41, 488–497. [Google Scholar] [CrossRef]
- Bianco, A.; Kostarelos, K.; Prato, M. Applications of carbon nanotubes in drug delivery. Curr. Opin. Chem. Biol. 2005, 9, 674–679. [Google Scholar] [CrossRef]
- Tortora, M.; Cavalieri, F.; Mosesso, P.; Ciaffardini, F.; Melone, F.; Crestini, C. Ultrasound Driven Assembly of Lignin into Microcapsules for Storage and Delivery of Hydrophobic Molecules. Biomacromolecules 2014, 15, 1634–1643. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.; Lintinen, K.; Kiriazis, A.; Hynninen, V.; Liu, Z.; Bauleth-Ramos, T.; Rahikkala, A.; Correia, A.; Kohout, T.; Sarmento, B.; et al. In vitro evaluation of biodegradable lignin-based nanoparticles for drug delivery and enhanced antiproliferation effect in cancer cells. Biomaterials 2017, 121, 97–108. [Google Scholar] [CrossRef]
- Martinez Rivas, C.J.; Tarhini, M.; Badri, W.; Miladi, K.; Greige-Gerges, H.; Nazari, Q.A.; Galindo Rodriguez, S.A.; Roman, R.A.; Fessi, H.; Elaissari, A. Nanoprecipitation process: From encapsulation to drug delivery. Int. J. Pharm. 2017, 532, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, J.Y.; Youn, H.J.; Choi, J.W. Fractionation of lignin macromolecules by sequential organic solvents systems and their characterization for further valuable applications. Int J Biol Macromol 2018, 106, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Park, S.Y.; Lee, J.H.; Choi, I.-G.; Choi, J.W. Sequential solvent fractionation of lignin for selective production of monoaromatics by Ru catalyzed ethanolysis. Rsc. Adv. 2017, 7, 53117–53125. [Google Scholar] [CrossRef]
- Lievonen, M.; Valle-Delgado, J.J.; Mattinen, M.-L.; Hult, E.-L.; Lintinen, K.; Kostiainen, M.A.; Paananen, A.; Szilvay, G.R.; Setälä, H.; Österberg, M. A simple process for lignin nanoparticle preparation. Green Chem. 2016, 18, 1416–1422. [Google Scholar] [CrossRef]
- Sun, R.; Tomkinson, J.; Jones, G.L. Fractional characterization of ash-AQ lignin by successive extraction with organic solvents from oil palm EFB fibre. Polym. Degrad. Stab. 2000, 68, 111–119. [Google Scholar] [CrossRef]
- Kawamura, H.; Hayashi, T.; Inoue, Y.; Chujo, R. Molecular weight and tacticity fractionations in successive extraction of highly isotactic polypropylene with n-alkane solvents. Macromolecule 1989, 22, 2181–2186. [Google Scholar] [CrossRef]
- Gref, R.; Domb, A.; Quellec, P.; Blunk, T.; Müller, R.; Verbavatz, J.-M.; Langer, R. The controlled intravenous delivery of drugs using PEG-coated sterically stabilized nanospheres. Adv. Drug Deliv. Rev. 1995, 16, 215–233. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hedeman, H.; Muir, I.; Illum, L.; Davis, S.S. An investigation of the filtration capacity and the fate of large filtered sterically-stabilized microspheres in rat spleen. Biochim. Et Biophys. Acta (Bba)-Gen. Subj. 1993, 1157, 233–240. [Google Scholar] [CrossRef]
- Devine, D.V.; Wong, K.; Serrano, K.; Chonn, A.; Cullis, P.R. Liposome—Complement interactions in rat serum: Implications for liposome survival studies. Biochim. Et Biophys. Acta (Bba) Biomembr. 1994, 1191, 43–51. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size. shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Toy, R.; Peiris, P.M.; Ghaghada, K.B.; Karathanasis, E. Shaping cancer nanomedicine: The effect of particle shape on the in vivo journey of nanoparticles. Nanomedicine 2014, 9, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Miladi, K.; Sfar, S.; Fessi, H.; Elaissari, A. Nanoprecipitation process: From particle preparation to in vivo applications. In Polymer Nanoparticles for Nanomedicines; Springer: Berlin, Germany, 2016; pp. 17–53. [Google Scholar]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Morris, K.F.; Cutak, B.J.; Dixon, A.M.; Larive, C.K. Analysis of diffusion coefficient distributions in humic and fulvic acids by means of diffusion ordered NMR spectroscopy. Anal. Chem. 1999, 71, 5315–5321. [Google Scholar] [CrossRef] [PubMed]


| Samples | Yield (wt %) | Molecular Weight | Phenolic OH (mmol/g) | Methoxyl (mmol/g) | ||
|---|---|---|---|---|---|---|
| Mw | Mn | PDI | ||||
| SL | - | 3130 | 1030 | 3.0 | 1.8 | 3.4 |
| F1 | 30.1 | 1190 | 660 | 1.8 | 2.6 | 3.9 |
| F2 | 25.5 | 2550 | 1200 | 2.1 | 2.2 | 3.9 |
| F3 | 24.7 | 3680 | 1630 | 2.3 | 1.7 | 3.5 |
| F4 | 2.0 | 5310 | 2110 | 2.5 | 1.6 | 3.4 |
| F5 | 11.2 | 14,650 | 4700 | 3.1 | 0.8 | 3.1 |
| INS | 6.5 | - | - | - | - | 1.3 |
| Samples | Amount of Solute (%) | Molecular Weight | ||
|---|---|---|---|---|
| Mw | Mn | PDI | ||
| SL | - | 3130 | 1030 | 3.0 |
| 1 mg/mL | 92.0 | 1810 | 820 | 2.2 |
| 2 mg/mL | 87.7 | 2160 | 880 | 2.5 |
| 4 mg/mL | 86.5 | 2490 | 980 | 2.5 |
| 2 mg/mL F1 | 100.0 | 1150 | 850 | 1.3 |
| 2 mg/mL F2 | 100.0 | 2250 | 1530 | 1.5 |
| 2 mg/mL F3 | 93.0 | 3390 | 2170 | 1.6 |
| 2 mg/mL F4 | 94.2 | 5900 | 3630 | 1.6 |
| 2 mg/mL F5 | 45.5 | 6000 | 2290 | 2.6 |
| 2 mg/mL INS | - | - | - | - |
| Samples | Size from Image (nm) | Peak Size (nm) | PD c |
|---|---|---|---|
| NP-C1 a | 404 | 128.4 | 0.3 |
| NP-C2 | 468 | 466.7 | 1.0 |
| NP-C4 | 590 | 559.7 | 1.0 |
| NP-F1 b | 474 | 444.8 | 0.8 |
| NP-F2 | 508 | 592.9 | 1.0 |
| NP-F3 | 1024 | 850.3 | 1.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.H.; Park, S.Y.; Choi, I.-G.; Choi, J.W. Investigation of Molecular Size Effect on the Formation of Lignin Nanoparticles by Nanoprecipitation. Appl. Sci. 2020, 10, 4910. https://doi.org/10.3390/app10144910
Lee JH, Park SY, Choi I-G, Choi JW. Investigation of Molecular Size Effect on the Formation of Lignin Nanoparticles by Nanoprecipitation. Applied Sciences. 2020; 10(14):4910. https://doi.org/10.3390/app10144910
Chicago/Turabian StyleLee, Jae Hoon, Shin Young Park, In-Gyu Choi, and Joon Weon Choi. 2020. "Investigation of Molecular Size Effect on the Formation of Lignin Nanoparticles by Nanoprecipitation" Applied Sciences 10, no. 14: 4910. https://doi.org/10.3390/app10144910
APA StyleLee, J. H., Park, S. Y., Choi, I.-G., & Choi, J. W. (2020). Investigation of Molecular Size Effect on the Formation of Lignin Nanoparticles by Nanoprecipitation. Applied Sciences, 10(14), 4910. https://doi.org/10.3390/app10144910




