Rigenera® Autologous Micrografts in Oral Regeneration: Clinical, Histological, and Radiographical Evaluations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population
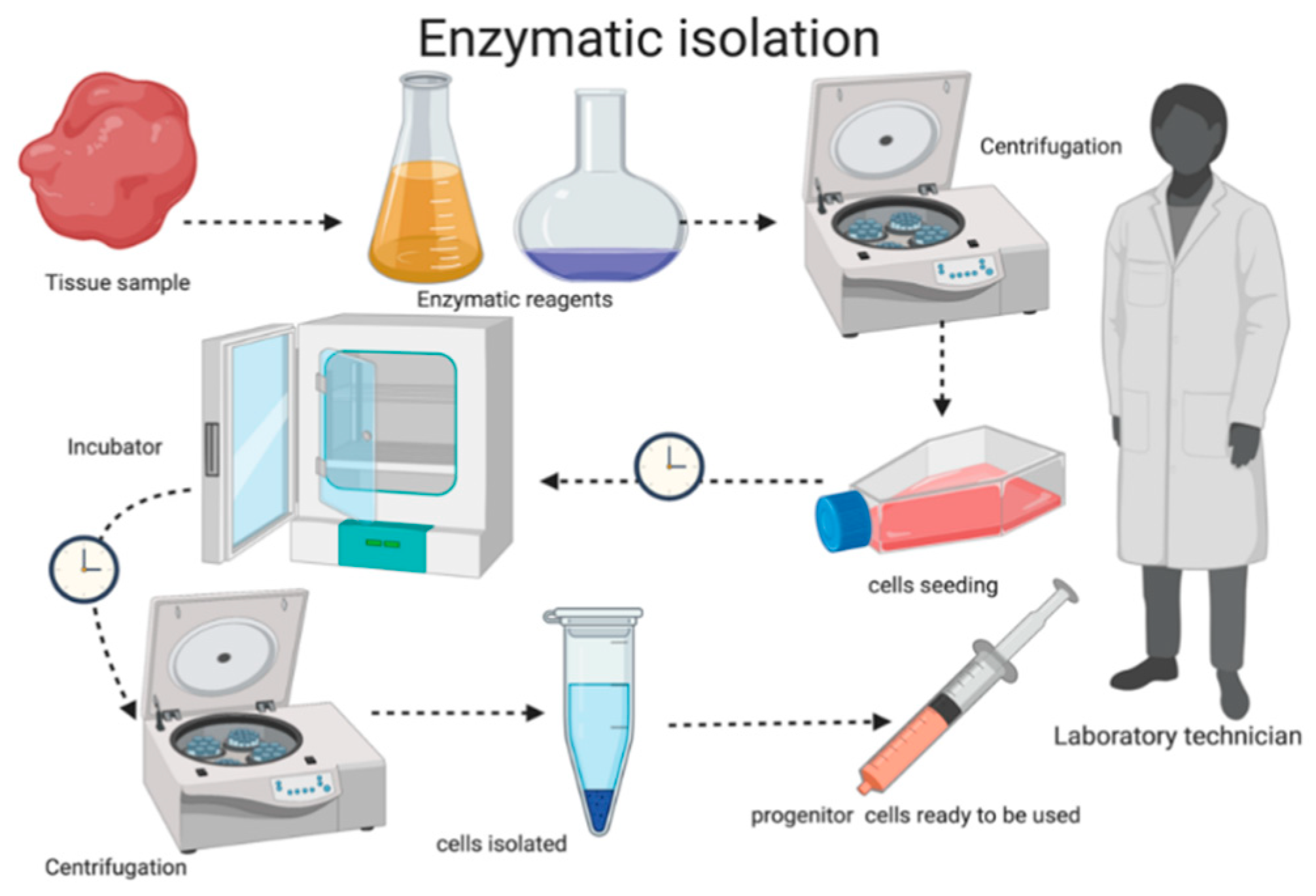
2.2. Autologous Micrograft Generation—Rigenera® Micrografting Technology
2.3. Surgery and Grafting Procedure
2.3.1. Sinus Lift
2.3.2. Periodontal Regeneration
2.3.3. Socket Preservation
2.4. Qualitative Analysis
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Grassia, V.; Nucci, L. New Materials in Oral Surgery. Materials 2020, 13, 1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Wu, W.; Qian, C.; Xiao, W.; Zhu, H.; Guo, J.; Meng, Z.; Zhu, J.; Ge, Z.; Cui, W. Advanced biomaterials for repairing and reconstruction of mandibular defects. Mater. Sci. Eng. C 2019, 103, 109858. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Mataliotakis, G.I.; Angoules, A.G.; Kanakaris, N.K.; Giannoudis, P.V. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: A systematic review. Injury 2011, 42, S3–S15. [Google Scholar] [CrossRef]
- Nkenke, E.; Weisbach, V.; Winckler, E.; Kessler, P.; Schultze-Mosgau, S.; Wiltfang, J.; Neukam, F.W. Morbidity of harvesting of bone grafts from the iliac crest for preprosthetic augmentation procedures: A prospective study. Int, J. Oral Maxillofac. Surg. 2004, 33, 157–163. [Google Scholar] [CrossRef]
- Sanz, M.; Dahlin, C.; Apatzidou, D.; Artzi, Z.; Bozic, D.; Calciolari, E.; De Bruyn, H.; Dommisch, H.; Donos, N.; Eickholz, P.; et al. Biomaterials and regenerative technologies used in bone regeneration in the craniomaxillofacial region: Consensus report of group 2 of the 15th European Workshop on Periodontology on Bone Regeneration. J. Clin. Periodontol. 2019, 46, 82–91. [Google Scholar]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: Which is the ideal biomaterial? J. Clin. Periodontol. 2019, 46, 92.S–102.S. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.M. Biomaterials for bone tissue engineering. Mater. Today 2008, 11, 18–25. [Google Scholar] [CrossRef]
- Chen, F.M.; Gao, L.N.; Tian, B.M.; Zhang, X.Y.; Zhang, Y.J.; Dong, G.Y.; Lu, H.; Chu, Q.; Xu, J.; Yu, Y.; et al. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: A randomized clinical trial. Stem Cell Res. Ther 2016, 7, 33. [Google Scholar] [CrossRef] [Green Version]
- Bajestan, M.N.; Rajan, A.; Edwards, S.P.; Aronovich, S.; Cevidanes, L.; Polymeri, A.; Travan, S.; Kaigler, D. Stem cell therapy for reconstruction of alveolar cleft and trauma defects in adults: A randomized controlled, clinical trial. Clin. Implant. Dent. Relat. Res. 2017, 19, 793–801. [Google Scholar] [CrossRef]
- Barbier, L.; Ramos, E.; Mendiola, J.; Rodriguez, O.; Santamaria, G.; Santamaria, J.; Arteagoitia, I. Autologous dental pulp mesenchymal stem cells for inferior third molar post-extraction socket healing: A split-mouth randomised clinical trial. Med. Oral Patol. Oral Cir. Bucal. 2018, 23, 469–477. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, B.P.; Soleas, I.M.; Ferko, N.C.; Cameron, C.G.; Hinoul, P. Prolonged Operative Duration Increases Risk of Surgical Site Infections: A Systematic Review. Surg. Infect. 2017, 18, 722–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trovato, L.; Monti, M.; Del Fante, C.; Cervio, M.; Lampinen, M.; Ambrosio, L.; Redi, C.A.; Perotti, C.; Kankuri, E.; Ambrosio, G.; et al. A New Medical Device Rigeneracons Allows to Obtain Viable Micro-Grafts From Mechanical Disaggregation of Human Tissues. J. Cell Physiol. 2015, 2 30, 2299–2303. [Google Scholar] [CrossRef]
- Monti, M.; Graziano, A.; Rizzo, S.; Perotti, C.; Del Fante, C.; d’Aquino, R.; Redi, C.A.; Rodriguez, Y.; Baena, R. In Vitro and In Vivo Differentiation of Progenitor Stem Cells Obtained After Mechanical Digestion of Human Dental Pulp. J. Cell Physiol. 2017, 232, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Giaccone, M.; Brunetti, M.; Camandona, M.; Trovato, L.; Graziano, A. A New Medical Device, Based on Rigenera Protocol, in the Management of Complex Wounds. J. Stem Cells Res. Rev. Rep. 2014, 1, 3–5. [Google Scholar]
- Ceccarelli, G.; Graziano, A.; Benedetti, L.; Imbriani, M.; Romano, F.; Ferrarotti, F.; Aimetti, M.; Cusella de Angelis, G.M. Osteogenic Potential of Human Oral-Periosteal Cells (PCs) Isolated From Different Oral Origin: An In Vitro Study. J. Cell Physiol. 2016, 231, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Baena, R.R.; D’Aquino, R.; Graziano, A.; Trovato, L.; Aloise, A.C.; Ceccarelli, G.; Cusella, G.; Pelegrine, A.A.; Lupi, S.M. Autologous periosteum-derived micrografts and PLGA/HA enhance the bone formation in sinus lift augmentation. Front. Cell Dev. Biol. 2017, 1–7. [Google Scholar]
- Brunelli, G.; Motroni, A.; Graziano, A.; D’Aquino, R.; Zollino, I.; Carinci, F. Sinus lift tissue engineering using autologous pulp micro-grafts: A case report of bone density evaluation. J. Indian Soc. Periodontol. 2013, 17, 644–647. [Google Scholar]
- Lupi, S.M.; Rodriguez y Baena, A.; Todaro, C.; Ceccarelli, G.; Rodriguez y Baena, R. Maxillary sinus lift using autologous periosteal micrografts: A new regenerative approach and a case report of a 3-year follow-up. Case Rep. Dent. 2018, 2018, 3023096. [Google Scholar]
- Ferrarotti, F.; Romano, F.; Gamba, M.N.; Quirico, A.; Giraudi, M.; Audagna, M.; Aimetti, M. Human intrabony defect regeneration with micrografts containing dental pulp stem cells: A randomized controlled clinical trial. J. Clin. Periodontol. 2018, 45, 841–850. [Google Scholar] [CrossRef]
- Aimetti, M.; Ferrarotti, F.; Gamba, M.N.; Giraudi, M.; Romano, F. Regenerative Treatment of Periodontal Intrabony Defects Using Autologous Dental Pulp Stem Cells: A 1-Year Follow-Up Case Series. Int. J. Periodontics Restorative Dent. 2018, 38, 5–58. [Google Scholar] [CrossRef]
- Graziano, A.; Carinci, F.; Scolaro, S.; D’Aquino, R. Periodontal tissue generation using autologous dental ligament micro-grafts: Case report with 6 months follow-up. Ann. Oral Maxillofac. Surg. 2013, 1, 20. [Google Scholar] [CrossRef]
- d’Aquino, R.; De Rosa, A.; Lanza, V.; Tirino, V.; Laino, L.; Graziano, A.; Desiderio, V.; Laino, G.; Papaccio, G. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur. Cell Mater. 2009, 18, 75–83. [Google Scholar] [CrossRef] [PubMed]
- d’Aquino, R.; Trovato, L.; Graziano, A.; Ceccarelli, G.; Cusella de Angelis, G.; Marangini, A.; Nisio, A.; Galli, M.; Pasi, M.; Finotti, M.; et al. Periosteum-derived micro-grafts for tissue regeneration of human maxillary bone. J. Transl. Sci. 2016, 2, 125–129. [Google Scholar] [CrossRef] [Green Version]
- Lang, N.P.; Joss, A.; Orsanic, T.; Gusberti, F.A.; Siegrist, B.E. Bleeding on probing. A predictor for the progression of periodontal disease? J. Clin. Periodontol. 1986, 13, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Bartold, P.M. Periodontal health. J. Clin. Periodontol. 2018, 45, S9–S16. [Google Scholar] [CrossRef] [Green Version]
- Isola, G.; Polizzi, A.; Iorio-Siciliano, V.; Alibrandi, A.; Ramaglia, L.; Leonardi, R. Effectiveness of a nutraceutical agent in the non-surgical periodontal therapy: A randomized, controlled clinical trial. Clin. Oral Investig. 2020. [Google Scholar] [CrossRef]
- Lo Giudice, A.; Quinzi, V.; Ronsivalle, V.; Farronato, M.; Nicotra, C.; Indelicato, F.; Isola, G. Evaluation of Imaging Software Accuracy for 3-Dimensional Analysis of the Mandibular Condyle. A Comparative Study Using a Surface-to-Surface Matching Technique. Int. J. Environ. Res. Public Health 2020, 17, E4789. [Google Scholar] [CrossRef]
- Shanbhag, S.; Suliman, S.; Pandis, N.; Stavropoulos, A.; Sanz, M.; Mustafa, K. Cell therapy for orofacial bone regeneration: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 162–182. [Google Scholar] [CrossRef] [Green Version]
- Svolacchia, F.; De Francesco, F.; Trovato, L.; Graziano, A.; Ferraro, G.A. An innovative regenerative treatment of scars with dermal micrografts. J. Cosmet. Derm.. 2016, 15, 245–253. [Google Scholar] [CrossRef]
- Ferretti, C. Periosteum derived stem cells for regenerative medicine proposals: Boosting current knowledge. World J. Stem Cells 2014, 6, 266. [Google Scholar] [CrossRef]
- Viganò, M.; Tessaro, I.; Trovato, L.; Colombini, A.; Scala, M.; Magi, A.; Toto, A.; Peretti, G.; de Girolamo, L. Rationale and pre-clinical evidences for the use of autologous cartilage micrografts in cartilage repair. J. Orthop. Surg. Res. 2018, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nummi, A.; Nieminen, T.; Pätilä, T.; Lampinen, M.; Lehtinen, M.L.; Kivistö, S.; Holmström, M.; Wilkman, E.; Teittinen, K.; Laine, M.; et al. Epicardial delivery of autologous atrial appendage micrografts during coronary artery bypass surgery-safety and feasibility study. Pilot Feasibility Stud. 2017, 3, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trubiani, O.; Marconi, G.D.; Pierdomenico, S.D.; Piattelli, A.; Diomede, F.; Pizzicannella, J. Human Oral Stem Cells, Biomaterials and Extracellular Vesicles: A Promising Tool in Bone Tissue Repair. Int. J. Mol. Sci. 2019, 20, 4987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakahara, H.; Bruder, S.P.; Haynesworth, S.E.; Holecek, J.J.; Baber, M.A.; Goldberg, V.M.; Caplan, A.I. Bone and cartilage formation in diffusion chambers by subcultured cells derived from the periosteum. Bone 1990, 11, 181–188. [Google Scholar] [CrossRef]
- Tong, C.K.; Vellasamy, S.; Chong Tan, B.; Abdullah, M.; Vidyadaran, S.; Fong Seow, H.; Ramasamy, R. Generation of mesenchymal stem cell from human umbilical cord tissue using a combination enzymatic and mechanical disassociation method. Cell Biol. Int. 2011, 35, 221–226. [Google Scholar] [CrossRef]
- Abdel Meguid, E.; Ke, Y.; Ji, J.; El-Hashash, A.H.K. Stem cells applications in bone and tooth repair and regeneration: New insights, tools, and hopes. J. Cell Physiol. 2018, 233, 1825–1835. [Google Scholar] [CrossRef]
- Isola, G.; Giudice, A.L.; Polizzi, A.; Alibrandi, A.; Patini, R.; Ferlito, S. Periodontitis and Tooth Loss Have Negative Systemic Impact on Circulating Progenitor Cell Levels: A Clinical Study. Genes 2019, 10, 1022. [Google Scholar] [CrossRef] [Green Version]
- Mao, J.J.; Robey, P.G.; Prockop, D.J. Stem cells in the face: Tooth regeneration and beyond. Cell Stem Cell. 2012, 11, 291–301. [Google Scholar] [CrossRef] [Green Version]
- Sanz, A.R.; Carrión, F.S.; Chaparro, A.P. Mesenchymal stem cells from the oral cavity and their potential value in tissue engineering. Periodontol. 2000 2015, 67, 251–267. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Santonocito, S.; Alibrandi, A.; Ferlito, S. Expression of Salivary and Serum Malondialdehyde and Lipid Profile of Patients with Periodontitis and Coronary Heart Disease. Int. J. Mol. Sci. 2019, 20, 6061. [Google Scholar] [CrossRef] [Green Version]


| Authors (Year) | Study Design | Effective Population (n. of Patients) | n. of Chirurgical Sites | Study Group(s) | Control Group | Mean Age (Years) | n. Male | n. Female | Type of InterventIon |
|---|---|---|---|---|---|---|---|---|---|
| Baena et al. 2017 [16] | Retrospective study | 24 | 24 | Micrografts+hydroxyapatite | Deprotenized bovine bone;hydroxyapatite alone | 45–64 | 12 | 12 | Sinus lift |
| Brunelli et al. 2013 [17] | Case report | 1 | 1 | Micrografts+collagen sponge | - | 45 | 1 | - | Sinus lift |
| Lupi et al. 2018 [18] | Case report | 1 | 1 | Micrografts+hydroxyapatite | - | 52 | - | 1 | Sinus lift |
| Ferrarotti et al. 2018 [19] | Randomized clinical trials | 29 | 29 | Micrografts+collagen sponge | Collagen sponge | 39–69 | 13 | 14 | Periodontal regeneration |
| Aimetti et al. 2018 [20] | Case series | 11 | 11 | Micrografts+collagen sponge | - | 43–59 | 6 | 5 | Periodontal regeneration |
| Graziano et al. 2013 [21] | Case report | 1 | 2 | Micrografts+collagen sponge | Collagen sponge | 32 | - | 1 | Periodontal regeneration |
| d’Aquino et al. 2016 [22] | Multicentric study | 35 | 35 | Micrografts+collagen sponge | Collagen sponge | 25–64 | 14 | 21 | Socket preservation |
| d’Aquino et al. 2009 [23] | Case series | 7 | 14 | Micrografts+collagen sponge | Collagen sponge | 24–40 | 1 | Socket preservation |
| Sinus Lift | |||||||
| Authors (Year) | Type of Evaluation | Follow-up | Histological Parameters | Radiographic Parameters | Clinical Parameters | Type of Tissue | Site |
| Baena et al. 2017 [16] | Periapical RX and Histological. | 4 Months | Vital mineralized tissue(VTM); nonmineralized tissue (NMT); nonvital mineralized tissue (NVMT). | Grey scale (Mineralization). | - | Periosteal sample | Inner layer of the flap |
| Brunelli et al. 2013 [17] | CT scans and Periapical RX. | 4 Months | - | Hounsfield unit scale and grey scale (Mineralization). | - | Pulp tissue | Third molar |
| Lupi et al. 2018 [18] | CT scans and Histological. | 4 Months/3 Years | Calcified matrix and organic matrix | Hounsfield unit scale (Mineralization). | - | Persiosteal sample | Inner layer of the flap |
| Periodontal Regeneration | |||||||
| Authors (Year) | Type of Evaluation | Follow-up | Histological Parameters | Radiographic Parameters | Clinical Parameters | Type of Tissue | Site |
| Ferrarotti et al. 2018 [19] | Clinical and peripical RX. | 6 months/12 months | - | Intrabony defct depth(IBD). | PI;BoP;PD;CAL; REC. | Pulp tissue | Third molar |
| Aimetti et al. 2018 [20] | Clinical and peripical RX. | 1 year | - | Intrabony defct depth(IBD). | PI;BoP;PD;CAL; REC. | Pulp tissue | Third molar |
| Graziano et al. 2013 [21] | Clinical and CT scans. | 6 months | - | Hounsfield unit scale (Mineralization). | PI;BoP;PD;CAL; REC. | Periodontal ligament | Third molar |
| Socket Preservation | |||||||
| Authors (Year) | Type of Evaluation | Follow-up | Histological Parameters | Radiographic Parameters | Clinical Parameters | Type of Tissue | Site |
| d’Aquino et al. 2009 [22] | Clinical, peripical RX, Histological and Immunofluorescence. | 3 months | Calcified matrix, organic matrix and expression of osteonectin and osteocalcin. | Grey scale (Mineralization). | Horizontal and vertical dimension of the sokcet; PD;CAL. | Pulp tissue | Third molar |
| d’Aquino et al. 2016 [23] | Clinical, Histological and periapical RX. | 1 to 4 months | Calcified matrix and organic matrix | Grey scale (Mineralization). | Horizontal and vertical dimension of the sokcet | Persiosteal sample | Inner layer of the flap |
| Sinus Lift | ||
| Baena et al. 2017 [16] | Micro-grafts group | Bone substitutes |
| Histological | VMT = 58.5 ± 2.5%; NMT = 41.4 ± 5.6%; NVMT = N/A | Hidroxiapatite alone VMT = 20.2 ± 3.1%; NMT = 5.5 ± 1.6%; NVMT = N/A; Bovine bone alone VMT = 48 ± 2.5%; NMT = 20.5 ± 3.1%; NVMT = 31.5 ± 2.3% |
| Radiographical | High mineralization | High mineralization |
| Periodontal regeneration | ||
| Ferrarotti et al. 2018 [19] | Micro-grafts group | Collagen sponge group |
| Clinical | PD = 8.3 ± 1.2 mm; CAL = 10.0 ± 1.6 mm; | PD = 7.9 ± 1.3 mm; CAL = 9.4 ± 1.5 mm; |
| Radiographical | IBD = 6.4 ± 1.4 mm | IBD = 5.6 ± 1.0 mm |
| Graziano et al. 2013 [21] | Micro-grafts group | Collagen sponge group |
| Clinical | PD reduction from 12 to 3 mm | PD reduction from 11 to 7 mm |
| Radiographical | High mineralization | Low mineralization |
| Socket preservation | ||
| d’Aquino et al. 2009 [22] | Micro-grafts group | Collagen sponge group |
| Clinical | CAL = 6.2±2.3 mm | CAL = 4.4±1.2 mm |
| Histological | lamellar architecture | immature Bone |
| Radiographical | High mineralization | Low mineralization |
| d’Aquino et al. 2016 [23] | Micro-grafts group | Collagen sponge group |
| Clinical | Horizontal reduction = 25% Vertical reduction = 0.60% | Horizontal reduction = 30% Vertical reduction = 1.5% |
| Histological | calcified matrix | organic matrix |
| Radiographical | High mineralization | Low mineralization |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mummolo, S.; Mancini, L.; Quinzi, V.; D’Aquino, R.; Marzo, G.; Marchetti, E. Rigenera® Autologous Micrografts in Oral Regeneration: Clinical, Histological, and Radiographical Evaluations. Appl. Sci. 2020, 10, 5084. https://doi.org/10.3390/app10155084
Mummolo S, Mancini L, Quinzi V, D’Aquino R, Marzo G, Marchetti E. Rigenera® Autologous Micrografts in Oral Regeneration: Clinical, Histological, and Radiographical Evaluations. Applied Sciences. 2020; 10(15):5084. https://doi.org/10.3390/app10155084
Chicago/Turabian StyleMummolo, Stefano, Leonardo Mancini, Vincenzo Quinzi, Riccardo D’Aquino, Giuseppe Marzo, and Enrico Marchetti. 2020. "Rigenera® Autologous Micrografts in Oral Regeneration: Clinical, Histological, and Radiographical Evaluations" Applied Sciences 10, no. 15: 5084. https://doi.org/10.3390/app10155084







