Overview of the Cerebellar Function in Anticipatory Postural Adjustments and of the Compensatory Mechanisms Developing in Neural Dysfunctions
Abstract
1. Introduction
2. Overview of Cerebellar Functions
3. Overview of Anticipatory Postural Adjustments
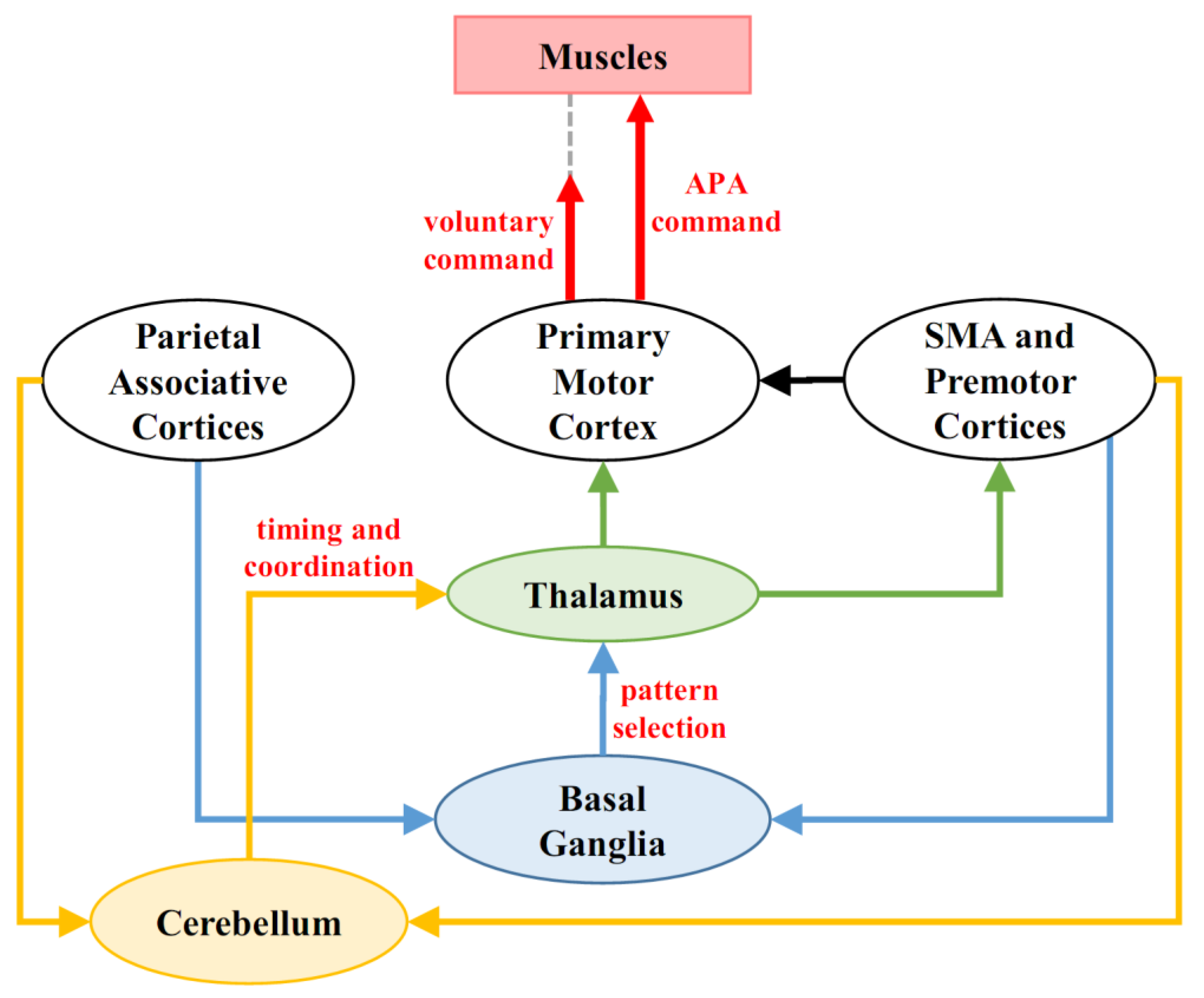
4. Cerebellum and Anticipatory Postural Control
5. Role of Cerebellum in the Ontogenesis of APA Control
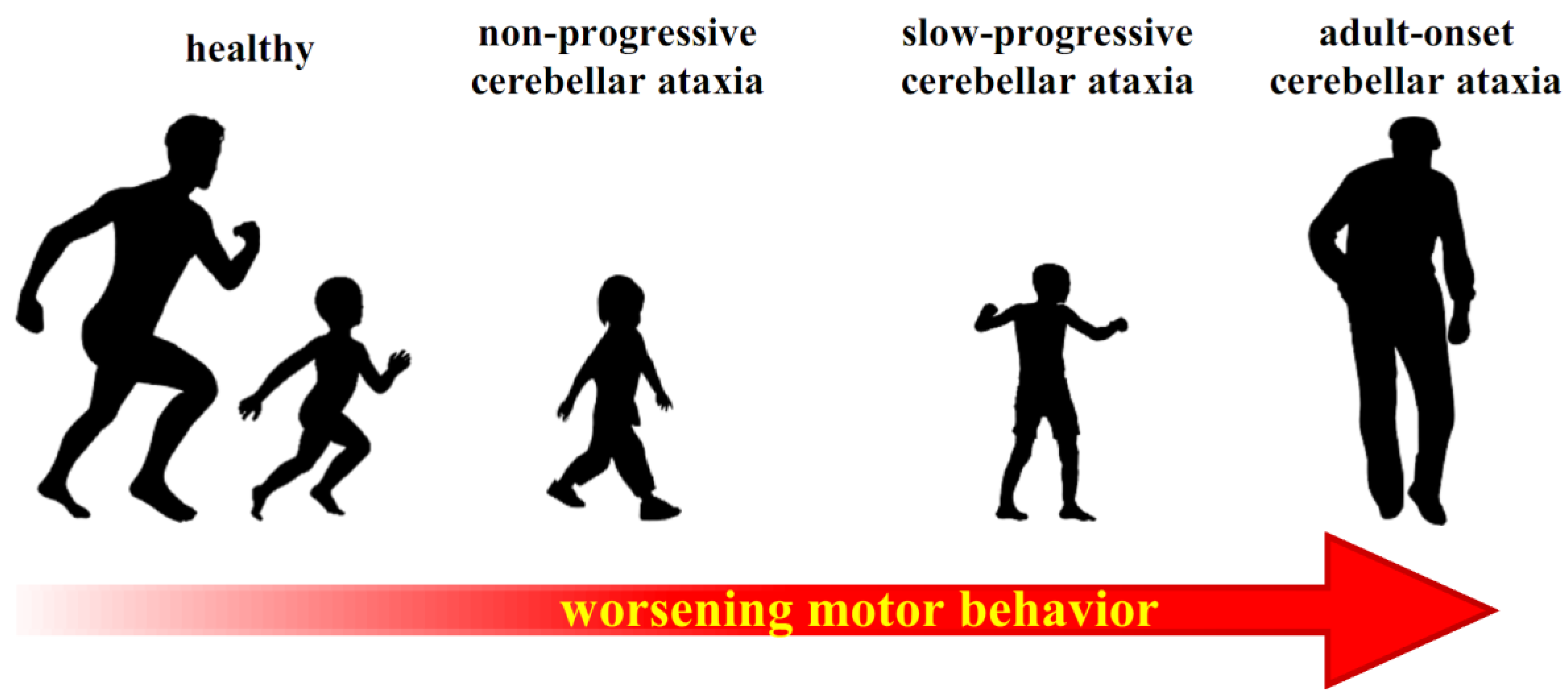
6. Compensatory Strategies in Cerebellar Dysfunctions
6.1. Neural Plasticity and Compensatory Mechanisms
6.2. A Possible Pathway for Compensating Cerebellar Dysfunction
7. Conclusions and Future Research
Author Contributions
Funding
Conflicts of Interest
References
- Moberget, T.; Karns, C.M.; Deouell, L.Y.; Lindgren, M.; Robert, T.; Ivry, R.B. Detecting Violations of Sensory Expectancies Following Cerebellar Degeneration: A Mismatch Negativity Study. Neuropsychologia 2009, 46, 2569–2579. [Google Scholar] [CrossRef]
- Mauk, M.D.; Medina, J.F.; Nores, W.L.; Ohyama, T. Cerebellar Function: Coordination, Learning or Timing? Curr. Biol. 2000, 10, 522–525. [Google Scholar] [CrossRef]
- Luciani, L. Il Cervelletto. Nuovi Studi Di Fisiologia Normale e Patologica. Philos. Rev. 1893, 2, 475–477. [Google Scholar]
- Albus, J.S. A Theory of Cerebellar Function. Math. Biosci. 1971, 10, 25–61. [Google Scholar] [CrossRef]
- Babinski, J. De l’asynergie Cérebelleuse. Rev. Neurol. 1899, 7, 806–816. [Google Scholar]
- Manto, M.; Bower, J.M.; Conforto, A.B.; Molinari, M.; Naito, E.; Nowak, D.A.; Oulad, N.; Taib, B. Consensus Paper: Roles of the Cerebellum in Motor Control—The Diversity of Ideas on Cerebellar Involvement in Movement. The Cerebellum 2012, 11, 457–487. [Google Scholar] [CrossRef]
- De Zeeuw, C.I.; Ten Brinke, M.M. Motor Learning and the Cerebellum. Cold Spring Harb. Perspect. Biol. 2015, 7. [Google Scholar] [CrossRef]
- D’Angelo, E.; Casali, S. Seeking a Unified Framework for Cerebellar Function and Dysfunction: From Circuit Operations to Cognition. Front. Neural Circuits 2012, 6, 116. [Google Scholar] [CrossRef]
- Ito, M. Control of Mental Activities by Internal Models in the Cerebellum. Nat. Rev. Neurosci. 2008, 9, 304–313. [Google Scholar] [CrossRef]
- Zagon, I.S.; McLaughlin, P.J.; Smith, S. Neural Populations in the Human Cerebellum: Estimations from Isolated Cell Nuclei. Brain Res. 1977, 127, 279–282. [Google Scholar] [CrossRef]
- Manto, M. Chapter 9—Cerebellar Motor Syndrome from Children to the Elderly. In The Cerebellum: From Embryology to Diagnostic Investigations; Elsevier: New York, NY, USA, 2018; Volume 154, pp. 151–166. [Google Scholar] [CrossRef]
- Ivry, R.B.; Spencer, R.M.; Zelaznik, H.N.; Diedrichsen, J. The Cerebellum and Event Timing. Ann. N. Y. Acad. Sci. 2002, 978, 302–317. [Google Scholar] [CrossRef] [PubMed]
- Bareš, M.; Apps, R.; Avanzino, L.; Breska, A.; D’Angelo, E.; Filip, P.; Gerwig, M.; Ivry, R.B.; Lawrenson, C.L.; Louis, E.D.; et al. Consensus Paper: Decoding the Contributions of the Cerebellum as a Time Machine. From Neurons to Clinical Applications. Cerebellum 2019, 18, 266–286. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.C.; Ivry, R.B. Spatial and Temporal Sequence Learning in Patients with Parkinson’s Disease or Spatial and Temporal Sequence Learning in Patients with Parkinson’s Disease or Cerebellar Lesions. J. Cogn. Neurosci. 2003, 15, 1232–1243. [Google Scholar] [CrossRef]
- Palesi, F.; De Rinaldis, A.; Castellazzi, G.; Calamante, F.; Muhlert, N.; Chard, D.; Tournier, J.D.; Magenes, G.; D’Angelo, E.; Wheeler-Kingshott, C.A.M.G. Contralateral Cortico-Ponto-Cerebellar Pathways Reconstruction in Humans in Vivo: Implications for Reciprocal Cerebro-Cerebellar Structural Connectivity in Motor and Non-Motor Areas. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Bostan, A.C.; Dum, R.P.; Strick, P.L. Cerebellar Networks with the Cerebral Cortex and Basal Ganglia. Trends Cogn. Sci. 2013, 241–254. [Google Scholar] [CrossRef]
- Blatt, G.J.; Oblak, A. Cerebellar Connections with Limbic Circuits: Anatomy and Functional Implications. Handb. Cerebellum Cerebellar Disord. 2013, 479–496. [Google Scholar] [CrossRef]
- Schmahmann, J.D.; Sherman, J.C. The Cerebellar Cognitive Affective Syndrome. Brain 1998, 121, 561–579. [Google Scholar] [CrossRef]
- Mochizuki, H.; Ugawa, Y. Cerebellar Ataxic Gait. Brain Nerve 2010, 62, 1203–1210. [Google Scholar]
- Schmahmann, J.D. Disorders of the Cerebellum: Ataxia, Dysmetria of Thought, and the Cerebellar Cognitive Affective Syndrome. J. Neuropsychiatry Clin. Neurosci. 2004, 16, 367–378. [Google Scholar] [CrossRef]
- Diener, H.-C.; Dichgans, J.; Guschlbauer, B.; Bacher, M.; Rapp, H.; Klockgether, T. The Coordination of Posture and Voluntary Movement in Patients with Cerebellar Dysfunction. Mov. Disord. 1992, 7, 14–22. [Google Scholar] [CrossRef]
- Diener, H.C.; Dichgans, J.; Bacher, M.; Gompf, B. Quantification of Postural Sway in Normals and Patients with Cerebellar Diseases. Electroencephalogr. Clin. Neurophysiol. 1984, 57, 134–142. [Google Scholar] [CrossRef]
- Asaka, T.; Wang, Y. Feedforward Postural Muscle Modes and Multi-Mode Coordination in Mild Cerebellar Ataxia. Exp. Brain Res. 2011, 210, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Earhart, G.M.; Bastian, A.J. Selection and Coordination of Human Locomotor Forms Following Cerebellar Damage. J. Neurophysiol. 2001, 85, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Morton, S.M.; Bastian, A.J. Cerebellar Control of Balance and Locomotion. Neuroscientist 2004, 10, 247–259. [Google Scholar] [CrossRef]
- Manto, M. Mechanisms of Human Cerebellar Dysmetria: Experimental Evidence and Current Conceptual Bases. J. Neuroeng. Rehabil. 2009, 18, 1–18. [Google Scholar] [CrossRef]
- Martino, G.; Ivanenko, Y.P.; Serrao, M.; Ranavolo, A.; D’Avella, A.; Draicchio, F.; Conte, C.; Casali, C.; Lacquaniti, F. Locomotor Patterns in Cerebellar Ataxia. J. Neurophysiol. 2014, 112, 2810–2821. [Google Scholar] [CrossRef]
- Bastian, A.J. Learning to Predict the Future: The Cerebellum Adapts Feedforward Movement Control. Curr. Opin. Neurobiol. 2006, 645–649. [Google Scholar] [CrossRef]
- Morton, S.M.; Bastian, A.M.Y.J. Mechanisms of Cerebellar Gait Ataxia. Cerebellum 2007, 18, 79–86. [Google Scholar] [CrossRef]
- Bouisset, S.; Do, M. Posture, Dynamic Stability and Voluntary Movement. Posture, Stabilité, Dynamique et Mouvement Volontaire. Neurophysiol Clin. 2008, 38, 345–362. [Google Scholar] [CrossRef]
- Bouisset, S.; Zattara, M. A Sequence of Postural Movements Precedes Voluntary Movement. Neurosci. Lett. 1981, 22, 263–270. [Google Scholar] [CrossRef]
- Massion, J. Movement, Posture and Equilibrium: Interaction and Coordination. Prog. Neurobiol. 1992, 38, 35–56. [Google Scholar] [CrossRef]
- Caronni, A.; Cavallari, P. Anticipatory Postural Adjustments Stabilise the Whole Upper-Limb Prior to a Gentle Index Finger Tap. Exp. Brain Res. 2009, 194, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Bruttini, C.; Esposti, R.; Bolzoni, F.; Cavallari, P. Higher Precision in Pointing Movements of the Preferred vs. Non-Preferred Hand Is Associated with an Earlier Occurrence of Anticipatory Postural Adjustments. Front. Hum. Neurosci. 2016, 10, 365. [Google Scholar] [CrossRef] [PubMed]
- Belen’kii, V.Y.; Gurfinkel’, V.S.; Pal’tsev, Y.I. Elements of Control of Voluntary Movements. Biophys. Oxf. 1967, 12, 154–161. [Google Scholar]
- Bouisset, S.; Zattara, M. Biomechanical Study of the Programming of Anticipatory Postural Adjustments Associated with Voluntary Movement. J. Biomech. 1987, 20, 735–742. [Google Scholar] [CrossRef]
- Friedli, W.G.; Hallett, M.; Simon, S.R. Postural Adjustments Associated with Rapid Voluntary Arm Movements. I. Electromyographic Data. J. Neurol. Neurosurg. Psychiatry 1984, 47, 611–622. [Google Scholar] [CrossRef]
- Honeine, J.; Schieppati, M.; Crisafulli, O.; Do, M. The Neuro-Mechanical Processes That Underlie Goal-Directed Medio-Lateral APA during Gait Initiation. Front. Hum. Neurosci. 2016, 10, 1–17. [Google Scholar] [CrossRef]
- Crenna, P.; Frigo, C. A Motor Programme for the Initiation of Forward-oriented Movements in Humans. J. Physiol. 1991, 437, 635–653. [Google Scholar] [CrossRef]
- Aruin, A.S.; Latash, M.L. The Role of Motor Action in Anticipatory Postural Adjustments Studied with Self-Induced and Externally Triggered Perturbations. Exp. Brain Res. 1995, 106, 291–300. [Google Scholar] [CrossRef]
- Yiou, E.; Caderby, T. Balance Control during Gait Initiation: State-of-the-Art and Research Perspectives. World J. Orthop. 2017, 8, 815–828. [Google Scholar] [CrossRef]
- Crenna, P.; Carpinella, I.; Rabuffetti, M.; Rizzone, M.; Lopiano, L.; Lanotte, M.; Ferrarin, M. Impact of Subthalamic Nucleus Stimulation on the Initiation of Gait in Parkinson’s Disease. Exp. Brain Res. 2006, 172, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Cavallari, P.; Bolzoni, F.; Bruttini, C.; Esposti, R. The Organization and Control of Intra-Limb Anticipatory Postural Adjustments and Their Role in Movement Performance. Front. Hum. Neurosci. 2016, 10, 525. [Google Scholar] [CrossRef] [PubMed]
- Almeida, G.L.; Hong, D.A.; Corcos, D.; Gottlieb, G.L. Organizing Principles for Voluntary Movement: Extending Single-Joint Rules. J. Neurophysiol. 1995, 74, 1374–1381. [Google Scholar] [CrossRef] [PubMed]
- Gribble, P.L.; Ostry, D.J. Compensation for Interaction Torques during Single- and Multijoint Limb Movement. J. Neurophysiol. 1999, 82, 2310–2326. [Google Scholar] [CrossRef]
- Hopf, H.C.; Hufschmidt, H.J. Coordination of Adjoining Muscles in Simple Voluntary Movements. Dtsch. Z. Nervenheilkd. 1963, 185, 191–202. [Google Scholar]
- Aoki, F. Activity Patterns of Upper Arm Muscles in Relation to Direction of Rapid Wrist Movement in Man. Exp. Brain Res. 1991, 83, 679–682. [Google Scholar] [CrossRef]
- Bruttini, C.; Esposti, R.; Bolzoni, F.; Cavallari, P. Ischemic Block of the Forearm Abolishes Finger Movements but Not Their Associated Anticipatory Postural Adjustments. Exp. Brain Res. 2014, 232, 1739–1750. [Google Scholar] [CrossRef]
- Esposti, R.; Bruttini, C.; Bolzoni, F.; Cavallari, P. Intended Rather than Actual Movement Velocity Determines the Latency of Anticipatory Postural Adjustments. Exp. Brain Res. 2015, 233, 397–403. [Google Scholar] [CrossRef]
- Hall, L.M.; Brauer, S.; Horak, F.; Hodges, P.W. Adaptive Changes in Anticipatory Postural Adjustments with Novel and Familiar Postural Supports. J. Neurophysiol. 2010, 103, 968–976. [Google Scholar] [CrossRef]
- Caronni, A.; Bolzoni, F.; Esposti, R.; Bruttini, C.; Cavallari, P. Accuracy of Pointing Movements Relies upon a Specific Tuning between Anticipatory Postural Adjustments and Prime Mover Activation. Acta Physiol. 2013. [Google Scholar] [CrossRef]
- Petersen, T.H.; Rosenberg, K.; Petersen, N.C.; Nielsen, J.B. Cortical Involvement in Anticipatory Postural Reactions in Man. Exp. Brain Res. 2009, 193, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Palmer, E.; Cafarelli, E.; Ashby, P. The Processing of Human Ballistic Movements Explored by Stimulation over the Cortex. J. Physiol. 1994, 481, 509–520. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Massion, J.; Ioffe, M.; Fran, C.S.; Gantcheva, R. Acquisition of Anticipatory Postural Adjustments in a Bimanual Load-Lifting Task: Normal and Pathological Aspects. Exp. Brain Res. 1999, 128, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Bolzoni, F.; Bruttini, C.; Esposti, R.; Castellani, C.; Cavallari, P. Transcranial Direct Current Stimulation of SMA Modulates Anticipatory Postural Adjustments without Affecting the Primary Movement. Behav. Brain Res. 2015, 291, 407–413. [Google Scholar] [CrossRef]
- Schmitz, C.; Jenmalm, P.; Westling, G.; Ehrsson, H.H.; Forssberg, H. 8.5 Anticipatory Postural Adjustments in a Bimanualload-Lifting Task: Central Aspects. Gait Posture 2005, 21. [Google Scholar] [CrossRef]
- Schepens, B.; Drew, T. Independent and Convergent Signals From the Pontomedullary Reticular Formation Contribute to the Control of Posture and Movement During Reaching in the Cat. J. Neurophysiol. 2004, 92, 2217–2238. [Google Scholar] [CrossRef]
- Bolzoni, F.; Esposti, R.; Marchese, S.M.; Pozzi, N.G.; Ramirez-pasos, U.E.; Isaias, I.U.; Cavallari, P. Disrupt of Intra-Limb APA Pattern in Parkinsonian Patients Performing Index-Finger Flexion. Front. Physiol. 2018, 9, 1–8. [Google Scholar] [CrossRef]
- Viallet, F.; Massion, J.; Massarino, R.; Khalil, R. Performance of a Bimanual Load-Lifting Task by Parkinsonian Patients. J. Neurol. Neurosurg. Psychiatry 1987, 50, 1274–1283. [Google Scholar] [CrossRef]
- Diedrichsen, J.; Verstynen, T.; Lehman, S.L.; Ivry, R.B. Cerebellar Involvement in Anticipating the Consequences of Self-Produced Actions During Bimanual Movements. J Neurophysiol 2005, 93, 801–812. [Google Scholar] [CrossRef]
- Hugon, M.; Massion, J.; Wiesendanger, M. Anticipatory Postural Changes Induced by Active Unloading and Comparison with Passive Unloading in Man. Pfliigers Arch. 1982, 393, 292–296. [Google Scholar] [CrossRef]
- Isaias, I.U.; Dipaola, M.; Michi, M.; Marzegan, A.; Volkmann, J.; Roidi, M.L.R.; Frigo, C.A.; Cavallari, P. Gait Initiation in Children with Rett Syndrome. PLoS ONE 2014, 9, e92736. [Google Scholar] [CrossRef] [PubMed]
- Dipaola, M.; Frigo, C.A.; Cavallari, P.; Iasias, I.U. Alterations of Load Transfer Mechanism during Gait Initiation in Parkinson’s Disease. In Proceedings of the E-Health and Bioengineering Conference, EHB 2017, Sinaia, Romania, 22–24 June 2017; pp. 579–582. [Google Scholar] [CrossRef]
- Timmann, D.; Horak, F.B. Perturbed Step Initiation in Cerebellar Subjects: 2. Modification of Anticipatory Postural Adjustments. Exp. Brain Res. 2001, 141, 110–120. [Google Scholar] [CrossRef]
- Richard, A.; Van Hamme, A.; Drevelle, X.; Golmard, J.; Meunier, S.; Welter, M. Contribution of the Supplementary Motor Area and the Cerebellum to the Anticipatory Postural Adjustments and Execution Phases of Human Gait Initiation. Neuroscience 2017. [Google Scholar] [CrossRef] [PubMed]
- Di Lazzaro, V.; Pilato, F.; Saturno, E.; Oliviero, A.; Dileone, M.; Mazzone, P.; Insola, A.; Tonali, P.A.; Ranieri, F.; Huang, Y.Z.; et al. Theta-Burst Repetitive Transcranial Magnetic Stimulation Suppresses Specific Excitatory Circuits in the Human Motor Cortex. J. Physiol. 2005, 3, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.H.; Hefter, H.; Mertens, M.; Freund, H. Disturbances in Human Arm Movement Trajectory Due to Mild Cerebellar Dysfunction. J. Neurol. Neurosurg. Psychiatry 1990, 53, 306–313. [Google Scholar] [CrossRef]
- Manto, M.; Godaux, E.; Jacquy, J. Cerebellar Hypermetria Is Larger When the Inertial Load Is Artificially Increased. Ann. Neurol. 1994, 35, 45–52. [Google Scholar] [CrossRef]
- Müller, F.; Dichgans, J. Impairments of Precision Grip in Two Patients with Acute Unilateral Cerebellar Lesions: A Simple Parametric Test for Clinical Use. Neuropsychologia 1994. [Google Scholar] [CrossRef]
- Babin-Ratté, S.; Sirigu, A.; Gilles, M.; Wing, A. Impaired Anticipatory Finger Grip-Force Adjustments in a Case of Cerebellar Degeneration. Exp. Brain Res. 1999. [Google Scholar] [CrossRef]
- Bruttini, C.; Esposti, R.; Bolzoni, F.; Vanotti, A.; Mariotti, C.; Cavallari, P. Temporal Disruption of Upper-Limb Anticipatory Postural Adjustments in Cerebellar Ataxic Patients. Exp. Brain Res. 2015, 233, 197–203. [Google Scholar] [CrossRef]
- Yamaura, H.; Hirai, H.; Yanagihara, D. Postural Dysfunction in a Transgenic Mouse Model of Spinocerebellar Ataxia Type 3. Neuroscience 2013. [Google Scholar] [CrossRef]
- Wang, S.S.; Kloth, A.D.; Badura, A. The Cerebellum, Sensitive Periods, and Autism. Neuron 2014, 83, 518–532. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, C.J. The Cerebellum and Neurodevelopmental Disorders. Cerebellum 2017, 15, 34–37. [Google Scholar] [CrossRef] [PubMed]
- De Graaf-Peters, V.B.; Bakker, H.; Van Eykern, L.A.; Otten, B.; Hadders-Algra, M. Postural Adjustments and Reaching in 4- and 6-Month-Old Infants: An EMG and Kinematical Study. Exp. Brain Res. 2007, 181, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Hedberg, A.; Carlberg, E.B.; Forssberg, H.; Hadders-Algra, M. Development of Postural Adjustments in Sitting Position during the First Half Year of Life. Dev. Med. Child Neurol. 2005, 47, 312–320. [Google Scholar] [CrossRef]
- Van der Fits, I.B.; Klip, A.W.; van Eykern, L.A.; Hadders-Algra, M. Postural Adjustments during Spontaneous and Goal-Directed Arm Movements in the First Half Year of Life. Behav. Brain Res. 1999, 106, 75–90. [Google Scholar] [CrossRef]
- Barela, J.A.; Jeka, J.J.; Clark, J.E. The Use of Somatosensory Information during the Aquisition of Independent Upright Stance. Infant. Behav. Dev. 1999, 22, 87–102. [Google Scholar] [CrossRef]
- Van der Fits, I.B.; Otten, E.; Klip, A.W.; Van Eykern, L.A.; Hadders-Algra, M. The Development of Postural Adjustments during Reaching in 6- to 18-Month-Old Infants. Evidence for Two Transitions. Exp. Brain Res. 1999, 126, 517–528. [Google Scholar] [CrossRef]
- Witherington, D.C.; Von Hofsten, C.; Rosander, K.; Robinette, A.; Woollacott, M.H.; Bertenthal, B.I. The Development of Anticipatory Postural Adjustments in Infancy. Infancy 2002, 3, 495–517. [Google Scholar] [CrossRef]
- Hadders-Algra, M. Development of Postural Control during the First 18 Months of Life. Neural Plast. 2005, 12, 99–108. [Google Scholar] [CrossRef]
- Rubinstein, M.; Denays, R.; Ham, H.R.; Piepsz, A.; VanPachterbeke, T.; Haumont, D.; Noël, P. Functional Imaging of Brain Maturation in Humans Using Iodine-123 Iodoamphetamine and SPECT. J. Nucl. Med. 1989, 30, 1982–1985. [Google Scholar]
- Chugani, H.T. A Critical Period of Brain Development: Studies of Cerebral Glucose Utilization with PET. Prev. Med. Baltim. 1998, 27, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Jover, M.; Schmitz, C.; Bosdure, E.; Chabrol, B.; Assaiante, C. Anticipatory Postural Adjustments in a Bimanual Load-Lifting Task in Children with Duchenne Muscular Dystrophy. Neurosci. Lett. 2006, 403, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Hay, L.; Redon, C. Feedforward versus Feedback Control in Children and Adults Subjected to a Postural Disturbance. Exp. Brain Res. 1999, 125, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Haas, G.; Diener, H.C.; Rapp, H.; Dichgans, J. Development of Feedback and Feedforward Control of Upright Stance. Dev. Med. Child Neurol. 1989, 31, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Riach, C.L.; Hayes, K.C. Anticipatory Postural Control in Children. J. Mot. Behav. 1990, 22, 250–266. [Google Scholar] [CrossRef]
- Ledebt, A.; Bril, B.; Brenière, Y. The Build-up of Anticipatory Behaviour: An Analysis of the Development of Gait Initiation in Children. Exp. Brain Res. 1998, 120, 9–17. [Google Scholar] [CrossRef]
- Malouin, F.; Richards, C.L. Preparatory Adjustments during Gait Initiation in 4–6-Year-Old Children. Gait Posture 2000, 11, 239–253. [Google Scholar] [CrossRef]
- Schmitz, C.; Martin, N.; Assaiante, C. Building Anticipatory Postural Adjustment during Childhood: A Kinematic and Electromyographic Analysis of Unloading in Children from 4 to 8 Years of Age. Exp. Brain Res. 2002, 142, 354–364. [Google Scholar] [CrossRef]
- Cignetti, F.; Vaugoyeau, M.; Decker, L.M.; Grosbras, M.-H.; Girard, N.; Chaix, Y.; Péran, P.; Assaiante, C. Brain Network Connectivity Associated with Anticipatory Postural Control in Children and Adults. Cortex 2018, 108, 210–221. [Google Scholar] [CrossRef]
- Farinelli, V.; Palmisano, C.; Marchese, S.M.; Strano, C.M.M.; Arrigo, S.D.; Pantaleoni, C.; Ardissone, A.; Nardocci, N.; Esposti, R.; Cavallari, P. Postural Control in Children with Cerebellar Ataxia. Appl. Sci. 2020, 10, 1606. [Google Scholar] [CrossRef]
- Ivry, R.B.; Keele, S.W. Timing Functions of the Cerebellum. J. Cogn. Neurosci. 1989, 1, 136–152. [Google Scholar] [CrossRef] [PubMed]
- Cerri, G.; Esposti, R.; Locatelli, M.; Cavallari, P. Coupling of Hand and Foot Voluntary Oscillations in Patients Suffering Cerebellar Ataxia: Different Effect of Lateral or Medial Lesions on Coordination. Progress Brain Res. 2005, 227–241. [Google Scholar] [CrossRef]
- D’Angelo, E. Rebuilding Cerebellar Network Computations from Cellular Neurophysiology. Front. Cell. Neurosci. 2010. [Google Scholar] [CrossRef] [PubMed]
- Titomanlio, L.; Romaniello, R.; Borgatti, R. Cerebellar Agenesis. Handb. Cerebellum Cerebellar Disord. 2005, 1855–1872. [Google Scholar] [CrossRef]
- Lu, C.; Huffmaster, S.L.A.; Harvey, J.C.; MacKinnon, C.D. Anticipatory Postural Adjustment Patterns during Gait Initiation across the Adult Lifespan. Gait Posture 2017, 57, 182–187. [Google Scholar] [CrossRef]
- Kennard, M.A. Age and Other Factors in Motor Recovery from Precentral Lesions in Monkeys. Am. J. Physiol. Content 1936, 115, 138–146. [Google Scholar] [CrossRef]
- Kennard, M.A. Cortical Reorganization of Motor Function: Studies on Series of Monkeys of Various Ages from Infancy to Maturity. Arch. Neurol. Psychiatry 1942, 48, 227–240. [Google Scholar] [CrossRef]
- Tong, L.; Spear, P.D.; Kalil, R.E. Effects of Corpus Callosum Section on Functional Compensation in the Posteromedial Lateral Suprasylvian Visual Area after Early Visual Cortex Damage in Cats. J. Comp. Neurol. 1987, 256, 128–136. [Google Scholar] [CrossRef]
- Guido, W.; Spear, P.D.; Tong, L. How Complete Is Physiological Compensation in Extrastriate Cortex after Visual Cortex Damage in Kittens? Exp. Brain Res. 1992, 91, 455–466. [Google Scholar] [CrossRef]
- Guido, W.; Spear, P.D.; Tong, L. Functional Compensation in the Lateral Suprasylvian Visual Area Following Bilateral Visual Cortex Damage in Kittens. Exp. Brain Res. 1990, 83, 219–224. [Google Scholar] [CrossRef]
- Spear, P.D. Plasticity Following Neonatal Visual Cortex Damage in Cats. Can. J. Physiol. Pharmacol. 1995, 73, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Bridge, H.; Thomas, O.; Jbabdi, S.; Cowey, A. Changes in Connectivity after Visual Cortical Brain Damage Underlie Altered Visual Function. Brain 2008, 131, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Buckner, R.; Corbetta, M.; Schatz, J.; Raichle, M.; Petersen, S. Preserved Speech Abilities and Compensation Following Prefrontal Damage. Proc. Natl. Acad. Sci. USA 1996, 93, 1249–1253. [Google Scholar] [CrossRef]
- Léonard, B.; de Partz, M.-P.; Grandin, C.; Pillon, A. Domain-Specific Reorganization of Semantic Processing after Extensive Damage to the Left Temporal Lobe. Neuroimage 2009, 45, 572–586. [Google Scholar] [CrossRef] [PubMed]
- Hagoort, P.; Wassenaar, M.; Brown, C. Real-Time Semantic Compensation in Patients with Agrammatic Comprehension: Electrophysiological Evidence for Multiple-Route Plasticity. Proc. Natl. Acad. Sci. USA 2003, 100, 4340–4345. [Google Scholar] [CrossRef]
- Obayashi, S. Frontal Dynamic Activity as a Predictor of Cognitive Dysfunction after Pontine Ischemia. NeuroRehabilitation 2019, 44, 251–261. [Google Scholar] [CrossRef]
- Fregni, F.; Pascual-Leone, A. Hand Motor Recovery After Stroke: Tuning the Orchestra to Improve Hand Motor Function. Cogn. Behav. Neurol. 2006, 19, 21–33. [Google Scholar] [CrossRef]
- Murata, Y.; Higo, N.; Hayashi, T.; Nishimura, Y.; Sugiyama, Y.; Oishi, T.; Tsukada, H.; Isa, T.; Onoe, H. Temporal Plasticity Involved in Recovery from Manual Dexterity Deficit after Motor Cortex Lesion in Macaque Monkeys. J. Neurosci. 2015, 35, 84–95. [Google Scholar] [CrossRef]
- Yamamoto, X.T.; Hayashi, X.T.; Murata, X.Y.; Ose, T.; Higo, X.N. Premotor Cortical-Cerebellar Reorganization in a Macaque Model of Primary Motor Cortical Lesion and Recovery. J. Neurosci. 2019, 39, 8484–8496. [Google Scholar] [CrossRef]
- Umeda, T.; Isa, T. Differential Contributions of Rostral and Caudal Frontal Forelimb Areas to Compensatory Process after Neonatal Hemidecortication in Rats. Eur. J. Neurosci. 2011, 34, 1453–1460. [Google Scholar] [CrossRef]
- Graveline, C.J.; Mikulis, D.J.; Crawley, A.P.; Hwang, P.A. Regionalized Sensorimotor Plasticity after Hemispherectomy FMRI Evaluation. Pediatr. Neurol. 1998, 19, 337–342. [Google Scholar] [CrossRef]
- Sebastianelli, L.; Versace, V.; Taylor, A.; Brigo, F.; Nothdurfter, W.; Saltuari, L.; Trinka, E.; Nardone, R. Functional Reorganization after Hemispherectomy in Humans and Animal Models: What Can We Learn about the Brain’s Resilience to Extensive Unilateral Lesions? Brain Res. Bull. 2017, 131, 156–167. [Google Scholar] [CrossRef]
- Herbet, G.; Duffau, H. Revisiting the Functional Anatomy of the Human Brain: Toward a Meta-Networking Theory of Cerebral Functions. Physiol. Rev. 2020, 100, 1181–1228. [Google Scholar] [CrossRef]
- Bostan, A.C.; Dum, R.P.; Strick, P.L. The Basal Ganglia Communicate with the Cerebellum. Proc. Natl. Acad. Sci. USA 2010, 107, 8452–8456. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, E.; Tremblay, L.; Féger, J.; Carras, P.L.; Strick, P.L. The Cerebellum Communicates with the Basal Ganglia. Nat. Neurosci. 2005, 8, 1491–1493. [Google Scholar] [CrossRef] [PubMed]
- Vitale, F.; Mattei, C.; Capozzo, A.; Pietrantoni, I.; Mazzone, P.; Scarnati, E. Cholinergic Excitation from the Pedunculopontine Tegmental Nucleus to the Dentate Nucleus in the Rat. Neuroscience 2016, 317, 12–22. [Google Scholar] [CrossRef]
- Mori, F.; Okada, K.; Nomura, T.; Kobayashi, Y. The Pedunculopontine Tegmental Nucleus as a Motor and Cognitive Interface between the Cerebellum and Basal Ganglia. Front. Neurosci. 2016, 10, 1–8. [Google Scholar] [CrossRef]
- Bostan, A.C.; Strick, P.L. The Basal Ganglia and the Cerebellum: Nodes in an Integrated Network. Nat. Rev. Neurosci. 2018, 19, 338–350. [Google Scholar] [CrossRef]
- Wu, T.; Hallett, M. The Cerebellum in Parkinson’s Disease. Brain 2013, 136, 696–709. [Google Scholar] [CrossRef]
- Yu, H.; Sternad, D.; Corcos, D.M.; Vaillancourt, D.E. Role of Hyperactive Cerebellum and Motor Cortex in Parkinson’s Disease. Neuroimage 2007, 35, 222–233. [Google Scholar] [CrossRef]
- Simioni, A.C.; Dagher, A.; Fellows, L.K. Compensatory Striatal-Cerebellar Connectivity in Mild-Moderate Parkinson’s Disease. NeuroImage Clin. 2016, 10, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Martinu, K.; Monchi, O. Cortico-Basal Ganglia and Cortico-Cerebellar Circuits in Parkinson’s Disease: Pathophysiology or Compensation? Behav. Neurosci. 2013, 127, 222–236. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchese, S.M.; Farinelli, V.; Bolzoni, F.; Esposti, R.; Cavallari, P. Overview of the Cerebellar Function in Anticipatory Postural Adjustments and of the Compensatory Mechanisms Developing in Neural Dysfunctions. Appl. Sci. 2020, 10, 5088. https://doi.org/10.3390/app10155088
Marchese SM, Farinelli V, Bolzoni F, Esposti R, Cavallari P. Overview of the Cerebellar Function in Anticipatory Postural Adjustments and of the Compensatory Mechanisms Developing in Neural Dysfunctions. Applied Sciences. 2020; 10(15):5088. https://doi.org/10.3390/app10155088
Chicago/Turabian StyleMarchese, Silvia Maria, Veronica Farinelli, Francesco Bolzoni, Roberto Esposti, and Paolo Cavallari. 2020. "Overview of the Cerebellar Function in Anticipatory Postural Adjustments and of the Compensatory Mechanisms Developing in Neural Dysfunctions" Applied Sciences 10, no. 15: 5088. https://doi.org/10.3390/app10155088
APA StyleMarchese, S. M., Farinelli, V., Bolzoni, F., Esposti, R., & Cavallari, P. (2020). Overview of the Cerebellar Function in Anticipatory Postural Adjustments and of the Compensatory Mechanisms Developing in Neural Dysfunctions. Applied Sciences, 10(15), 5088. https://doi.org/10.3390/app10155088




