A Maturity Estimation of Bell Pepper (Capsicum annuum L.) by Artificial Vision System for Quality Control
Abstract
:1. Introduction
2. Related Work
3. Materials and Methods
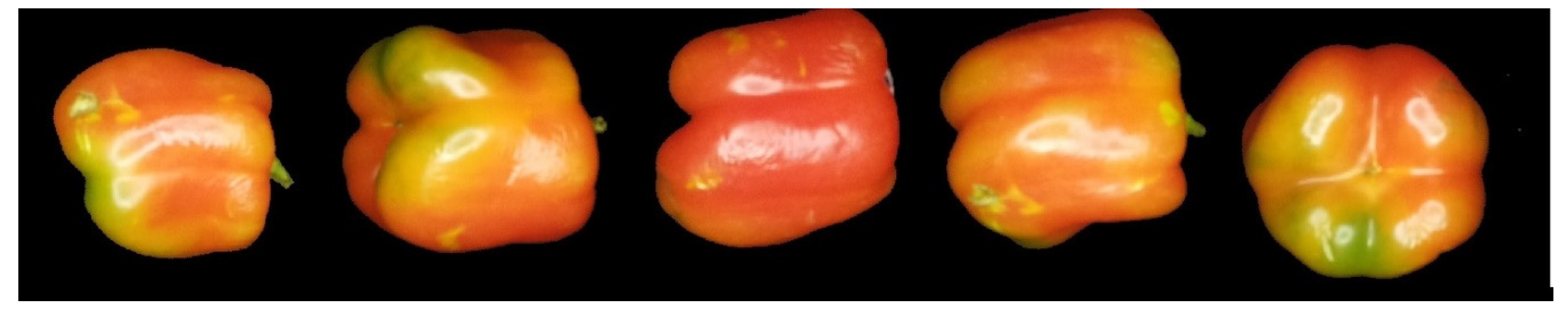
3.1. Samples
3.2. General Structure of Artificial Vision System
3.3. Image Acquisition
3.4. Automatic Sample Classification
3.5. Obtaining Regions of Interest
3.6. Maturity Status Estimator
3.6.1. Artificial Neural Network (ANN)
3.6.2. Fuzzy Logic
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Link to Images Database
References
- Tadesse, T.; Hewett, E.W.; Nichols, M.A.; Fisher, K.J. Changes in physicochemical attributes of sweet pepper cv. Domino during fruit growth and development. Sci. Hortic. 2002, 93, 91–103. [Google Scholar] [CrossRef]
- Frank, C.A.; Nelson, R.G.; Simonne, E.H.; Behe, B.K.; Simonne, A.H. Consumer preferences for color, price, and vitamin C content of bell peppers. HortScience 2001, 36, 795–800. [Google Scholar] [CrossRef] [Green Version]
- Luning, P.A.; van der Vuurst, D.V.R.; Yuksel, D.; Ebenhorst-Seller, T.; Wichers, H.J.; Roozen, J.P. Combined instrumental and sensory evalu- ation of flavor of fresh bell peppers (Capsicum annuum) harvested at three maturation stages. J. Agric. Food Chem. 1994, 42, 2855–2861. [Google Scholar] [CrossRef]
- Saltveit, M.E., Jr. Carbon dioxide, ethylene, and color development in ripening mature green bell peppers. J. Am. Soc. Hortic. Sci. 1977, 102, 523–525. [Google Scholar]
- Howard, L.R.; Hernandez-Brenes, C. An-tioxidant content and market quality of jalapeno pepper rings as affected by minimal processing and modified atmosphere packaging. J. Food Qual. 1998, 21, 317–327. [Google Scholar] [CrossRef]
- Howard, L.R.; Talcott, S.T.; Brenes, C.H.; Villalon, B. Changes in phytochemical and antioxidant activity of selected pepper cultivars (Capsicum species) as influenced by maturity. J. Agric. Food Chem. 2000, 48, 1713–1720. [Google Scholar] [CrossRef]
- Hornero-Mendez, D.; Minguez-Mosquera, M.I. Xanthophyll esterification accompanying carotenoid overaccumulation in chromoplast of Capsicumannuum ripening fruits is a constitutive process and useful for ripeness index. J. Agric. Food Chem. 2000, 48, 1617–1622. [Google Scholar] [CrossRef]
- Hornero-Mendez, D.; Minguez-Mosquera, M.I. Rapid spectrophotometric determination of red and yellow isochromic carotenoid fractions in paprika and red pepper oleoresins. J. Agric. Food Chem. 2001, 49, 3584–3588. [Google Scholar] [CrossRef]
- Markus, F.; Daood, H.G.; Kapitany, J.; Biacs, P.A. Change in the carotenoid and antioxidant content of spice red pepper (paprika) as a function of ripening and some technological factors. J. Agric. Food Chem. 1999, 47, 100–107. [Google Scholar] [CrossRef]
- Minguez-Mosquera, M.; Hornero-Mendez, D. Comparative study of the effect of paprika processing on the carotenoids in peppers (Capsi- cum annuum) of the Bola andAgridulce varieties. J. Agric. Food Chem. 1994, 42, 1555–1560. [Google Scholar] [CrossRef]
- Curl, A.L. The carotenoids of red bell peppers. J. Agric. Food Chem. 1962, 10, 504–509. [Google Scholar] [CrossRef]
- Molinari, A.F.; De Castro, L.R.; Antoniali, S.; Pornchaloempong, P.; Fox, A.J.; Sargent, S.A.; Lamb, E.M. The potential for bell pepper harvest before full color development. Proc. Fla. State Hort. Soc. 2000, 112, 143–146. [Google Scholar]
- Osuna-Garcia, J.A.; Wall, M.M.; Waddell, C.A. Endogenous levels of tocopherols and ascorbic acid during fruit ripening of new Mexi-can-type chile (Capsicum nnuum L.) cultivars. J. Agric. Food Chem. 1998, 46, 5093–5096. [Google Scholar] [CrossRef]
- Russo, V.M.; Howard, L.R. Carotenoids in pungent and nonpungent peppers at various developmental stages grown in the field and glasshouse. J. Sci. Food Agric. 2002, 82, 615–624. [Google Scholar] [CrossRef]
- Ryall, A.L.; Lipton, W.J. Handling transportation and storage of fruits and vegetables. In Vegetables and Melons, 2nd ed.; AVI Publ. Co.: Westport, CT, USA, 1979; p. 587. [Google Scholar]
- Yahia, E.M.; Contreras-Padilla, M.; Gonzalez-Aguilar, G. Ascorbic acid content in relation to ascorbic acid oxidase activity and polyamine content in tomato and bell pepper fruits during development, maturation and senescence. LWT 2001, 34, 452–457. [Google Scholar] [CrossRef] [Green Version]
- Matsufuji, H.; Nakamura, H.; Chino, M.; Takeda, M. Antioxidant activity of capsanthin and the fatty acid esters in paprika (Capsicum annuum). J. Agric. Food Chem. 1998, 46, 3468–3472. [Google Scholar] [CrossRef]
- Cantliffe, D.J.; Goodwin, P. Red color enhancement of pepper fruits by multiple applications of ethephon. J. Am. Soc. Hortic. Sci. 1975, 100, 157–161. [Google Scholar]
- Simonne, A.H.; Simonne, E.H.; Eitenmiller, R.R.; Mills, H.A.; Green, N.R. Ascorbic Acid and Provitamin A Contents in Unusually Colored Bell Peppers (Capsicum annuum L.). J. Food Compos. Anal. 1997, 10, 299–311. [Google Scholar] [CrossRef]
- Garcia-Mier, L.; Jimenez-Garcia, S.N.; Guevara-González, R.G.; Feregrino-Perez, A.A.; Contreras-medina, L.M.; Torres-Pacheco, I. Elicitor Mixtures Significantly Increase Bioactive Compounds, Antioxidant Activity, and Quality Parameters in Sweet Bell Pepper. J. Chem. 2015, 2015, 269296. [Google Scholar] [CrossRef] [Green Version]
- Chávez-Mendoza, C.; Sanchez, E.; Muñoz-Marquez, E.; Sida-Arreola, J.P.; Flores-Cordova, M.A. Bioactive Compounds and Antioxidant Activity in Different Grafted Varieties of Bell Pepper. Antioxidants 2015, 4, 427–446. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.Z.; Zhang, M.; Bhandari, B.; Guo, Z. Evaluation of the freshness of fresh-cut green bell pepper (Capsicum annuum var. grossum) using electronic nose. LWT 2018, 87, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Baenas, N.; Belović, M.; Ilic, N.; Moreno, D.A.; García-Viguera, C. Industrial use of pepper (Capsicum annum L.) derived products: Technological benefits and biological advantages. Food Chem. 2019, 274, 872–885. [Google Scholar] [CrossRef] [PubMed]
- Wan, P.; Toudeshki, A.; Tan, H.; Ehsani, R. A methodology for fresh tomato maturity detection using computer vision. Comput. Electron. Agric. 2018, 146, 43–50. [Google Scholar] [CrossRef]
- Zhang, B.; Gu, B.; Tian, G.; Zhou, J.; Huang, J.; Xiong, Y. Challenges and solutions of optical-based nondestructive quality inspection for robotic fruit and vegetable grading systems: A technical review. Trends Food Sci. Technol. 2018, 81, 213–231. [Google Scholar] [CrossRef]
- Li, C.; Cao, Q.; Guo, F. A method for color classification of fruits based on machine vision. WSEAS Trans. Syst. 2009, 8, 312–321. [Google Scholar]
- Cavallo, D.P.; Cefola, M.; Pace, B.; Logrieco, A.F.; Attolico, G. Non-destructive automatic quality evaluation of fresh-cut iceberg lettuce through packaging material. J. Food Eng. 2018, 223, 46–52. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, L.R.; Chen, K. Prediction of firmness parameters of tomatoes by portable visible and near-infrared spectroscopy. J. Food Eng. 2018, 222, 185–1984. [Google Scholar] [CrossRef]
- Guo, W.; Li, W.; Yang, B.; Zhu, Z.Z.; Liu, D.; Zhu, X. A novel noninvasive and cost-effective handheld detector on soluble solids content of fruits. J. Food Eng. 2019, 257, 1–9. [Google Scholar] [CrossRef]
- Munera, S.; Besada, C.; Aleixos, N.; Talens, P.; Salvador, A.; Sun, D.W.; Cubero, S.; Blasco, J. Non-destructive assessment of the internal quality of intact persimmon using colour and VIS/NIR hyperspectral imaging. LWT 2017, 77, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Estrada, B.; Bernal, M.A.; Diaz, J.; Pomar, F.; Merino, F. Fruit development in Capsicum annuum: Changes in capsaicin, lignin, free phenolics, and peroxidase patterns. J. Agric. Food Chem. 2000, 48, 6234–6239. [Google Scholar] [CrossRef]
- Goel, N.; Sehgal, P. Fuzzy classification of pre-harvest tomatoes for ripeness estimation—An approach based on automatic rule learning using decision tree. Appl. Soft Comput. J. 2015, 36, 45–56. [Google Scholar] [CrossRef]
- Villaseñor-Aguilar, M.J.; Botello-Álvarez, J.E.; Pérez-Pinal, F.J.; Cano-Lara, M.; León-Galván, M.F.; Bravo-Sánchez, M.G.; Barranco-Gutierrez, A.I. Fuzzy classification of the maturity of the tomato using a vision system. J. Sensors 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Constante, P.; Gordon, A.; Chang, O.; Pruna, E.; Acuna, F.; Escobar, I. Artificial Vision Techniques to Optimize Strawberrys Industrial Classification. IEEE Lat. Am. Trans. 2016, 14, 2576–2581. [Google Scholar] [CrossRef]
- Fashi, M.; Naderloo, L.; Javadikia, H. The relationship between the appearance of pomegranate fruit and color and size of arils based on image processing. Postharvest Biol. Technol. 2019, 154, 52–57. [Google Scholar] [CrossRef]
- Osterloh, A.; Ebert, G.; Held, W.-H. Lagerung von Obst Und Südfrüchten; Verlag Ulmer: Stuttgart, Germany, 1996; p. 253. [Google Scholar]
- Wills, R.B.H.; McGlasson, B.; Graham, D.; Joyce, D. Postharvest: An Introduction to the Physiology and Handling of Fruit, Vegetables and Ornamentals; CAB International: New York, NY, USA, 1998; p. 262. [Google Scholar]
- Kays, S. Postharvest Biology; Exon Press: Athens, GA, USA, 2004; p. 568. [Google Scholar]
- El-Mesery, H.S.; Mao, H.; Abomohra, A.E.-F. Applications of Non-destructive Technologies for Agricultural and Food Products Quality Inspection. Sensors 2019, 19, 846. [Google Scholar] [CrossRef] [Green Version]
- Harel, B.; Parmet, Y.; Edan, Y. Maturity classification of sweet peppers using image datasets acquired in different times. Comput. Ind. 2020, 121, 103274. [Google Scholar] [CrossRef]
- Elhariri, E.; El-Bendary, N.; Hussein, A.M.M.; Hassanien, A.E.; Badr, A. Bell Pepper Ripeness Classification based on Support Vector Machine. In Proceedings of the 2014 International Conference on Engineering and Technology (ICET), Cairo, Egypt, 19–20 April 2014. [Google Scholar]
- Shamili, M. The estimation of mango fruit total soluble solids using image processing technique. Sci. Hortic. 2019, 249, 383–389. [Google Scholar] [CrossRef]
- Li, X.; Wei, Y.; Xu, J.; Feng, X.; Wu, F.; Zhou, R.; Jin, J.; Xu, K.; Yu, X.; He, Y. SSC and pH for sweet assessment and maturity classification of harvested cherry fruit based on NIR hyperspectral imaging technology. Postharvest Biol. Technol. 2018, 143, 112–118. [Google Scholar] [CrossRef]
- Teerachaichayut, S.; Huong, T.H. Non-destructive prediction of total soluble solids, titratable acidity and maturity index of limes by near infrared hyperspectral imaging. Postharvest Biol. Technol. 2017, 133, 20–25. [Google Scholar] [CrossRef]
- Arias, R.; Lee, T.-C.; Logendra, L.; Janes, H. Correlation of Lycopene Measured by HPLC with the L*, a*, b* Color Readings of a Hydroponic Tomato and the Relationship of Maturity with Color and Lycopene Content. J. Agric. Food Chem. 2000, 48, 1697–1702. [Google Scholar] [CrossRef]
- Leiva-Valenzuela, G.A.; Lu, R.; Aguilera, J.M. Prediction of firmness and soluble solids content of blueberries using hyperspectral reflectance imaging. J. Food Eng. 2013, 115, 91–98. [Google Scholar] [CrossRef]
- Rahman, A.; Kandpal, L.M.; Lohumi, S.; Kim, M.S.; Lee, H.; Mo, C.; Cho, B.-K. Nondestructive Estimation of Moisture Content, pH and Soluble Solid Contents in Intact Tomatoes Using Hyperspectral Imaging. Appl. Sci. 2017, 7, 109. [Google Scholar] [CrossRef]

















| Green Mask | |||||
| H min | H max | S min | S max | V min | V max |
| 47 | 118 | 0 | 255 | 0 | 255 |
| Yellow Mask | |||||
| H min | H max | S min | S max | V min | V max |
| 32 | 46 | 0 | 255 | 0 | 255 |
| Orange Mask | |||||
| H min | H max | S min | S max | V min | V max |
| 15 | 32 | 0 | 255 | 0 | 255 |
| Red Mask | |||||
| L min | L min | L min | L min | L min | L min |
| 241 | 155 | 11 | 247 | 0 | 255 |
| Inputs | Number of Neurons in the Hidden Layer | Output | Epochs | Accuracy | |
|---|---|---|---|---|---|
| Model 1 | 4 | 4 | 4 | 10 | 92% |
| Model 2 | 4 | 5 | 4 | 10 | 98% |
| Model 3 | 4 | 8 | 4 | 10 | 100% |
| Model 4 | 4 | 10 | 4 | 10 | 100% |
| Model 5 | 4 | 15 | 4 | 10 | 100% |
| Inputs | Number of Neurons in the Hidden Layer | Output | Epochs | Mean Squared Error (MSE) | Pearson Correlation Coefficient (R) | |
|---|---|---|---|---|---|---|
| Model 6 | 4 | 4 | 1 | 10 | 0.5483 | 0.68659 |
| Model 7 | 4 | 5 | 1 | 10 | 0.5013 | 0.50130 |
| Model 8 | 4 | 8 | 1 | 10 | 0.5016 | 0.73176 |
| Model 9 | 4 | 10 | 1 | 10 | 0.4676 | 0.75910 |
| Model 10 | 4 | 15 | 1 | 10 | 0.3888 | 0.79543 |
| Number of GROI Membership Functions | Number of YROI Membership Functions | Number of OROI Membership Functions | Number of RROI Membership Functions | Training Error RMSE 1 × 10−6 | |
|---|---|---|---|---|---|
| Model 11 | 2 | 2 | 2 | 2 | 930.46 |
| Model 12 | 3 | 2 | 2 | 3 | 479.86 |
| Model 13 | 2 | 3 | 3 | 2 | 19.466 |
| Model 14 | 3 | 3 | 3 | 3 | 9.8679 |
| Model 15 | 4 | 4 | 4 | 4 | 2.0339 |
| Number of GROI Membership Functions | Number of YROI Membership Functions | Number of OROI Membership Functions | Number of RROI Membership Functions | Mean Squared Error (MSE) | Root Mean Squared Error (RMSE) | Pearson Correlation Coefficient (R) | |
|---|---|---|---|---|---|---|---|
| Model 16 | 3 | 3 | 3 | 3 | 5.923 | 2.433 | 0.499 |
| Model 17 | 4 | 3 | 3 | 4 | 0.891 | 0.944 | 0.696 |
| Model 18 | 3 | 4 | 4 | 3 | 1.645 | 1.282 | 0.424 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villaseñor-Aguilar, M.-J.; Bravo-Sánchez, M.-G.; Padilla-Medina, J.-A.; Vázquez-Vera, J.L.; Guevara-González, R.-G.; García-Rodríguez, F.-J.; Barranco-Gutiérrez, A.-I. A Maturity Estimation of Bell Pepper (Capsicum annuum L.) by Artificial Vision System for Quality Control. Appl. Sci. 2020, 10, 5097. https://doi.org/10.3390/app10155097
Villaseñor-Aguilar M-J, Bravo-Sánchez M-G, Padilla-Medina J-A, Vázquez-Vera JL, Guevara-González R-G, García-Rodríguez F-J, Barranco-Gutiérrez A-I. A Maturity Estimation of Bell Pepper (Capsicum annuum L.) by Artificial Vision System for Quality Control. Applied Sciences. 2020; 10(15):5097. https://doi.org/10.3390/app10155097
Chicago/Turabian StyleVillaseñor-Aguilar, Marcos-Jesús, Micael-Gerardo Bravo-Sánchez, José-Alfredo Padilla-Medina, Jorge Luis Vázquez-Vera, Ramón-Gerardo Guevara-González, Francisco-Javier García-Rodríguez, and Alejandro-Israel Barranco-Gutiérrez. 2020. "A Maturity Estimation of Bell Pepper (Capsicum annuum L.) by Artificial Vision System for Quality Control" Applied Sciences 10, no. 15: 5097. https://doi.org/10.3390/app10155097
APA StyleVillaseñor-Aguilar, M.-J., Bravo-Sánchez, M.-G., Padilla-Medina, J.-A., Vázquez-Vera, J. L., Guevara-González, R.-G., García-Rodríguez, F.-J., & Barranco-Gutiérrez, A.-I. (2020). A Maturity Estimation of Bell Pepper (Capsicum annuum L.) by Artificial Vision System for Quality Control. Applied Sciences, 10(15), 5097. https://doi.org/10.3390/app10155097








