Computer-Aided Surgical Simulation for Correcting Complex Limb Deformities in Children
Abstract
:1. Introduction
2. Materials and Method
2.1. Study Design and Case Series
2.2. From CT to 3D Digital Model
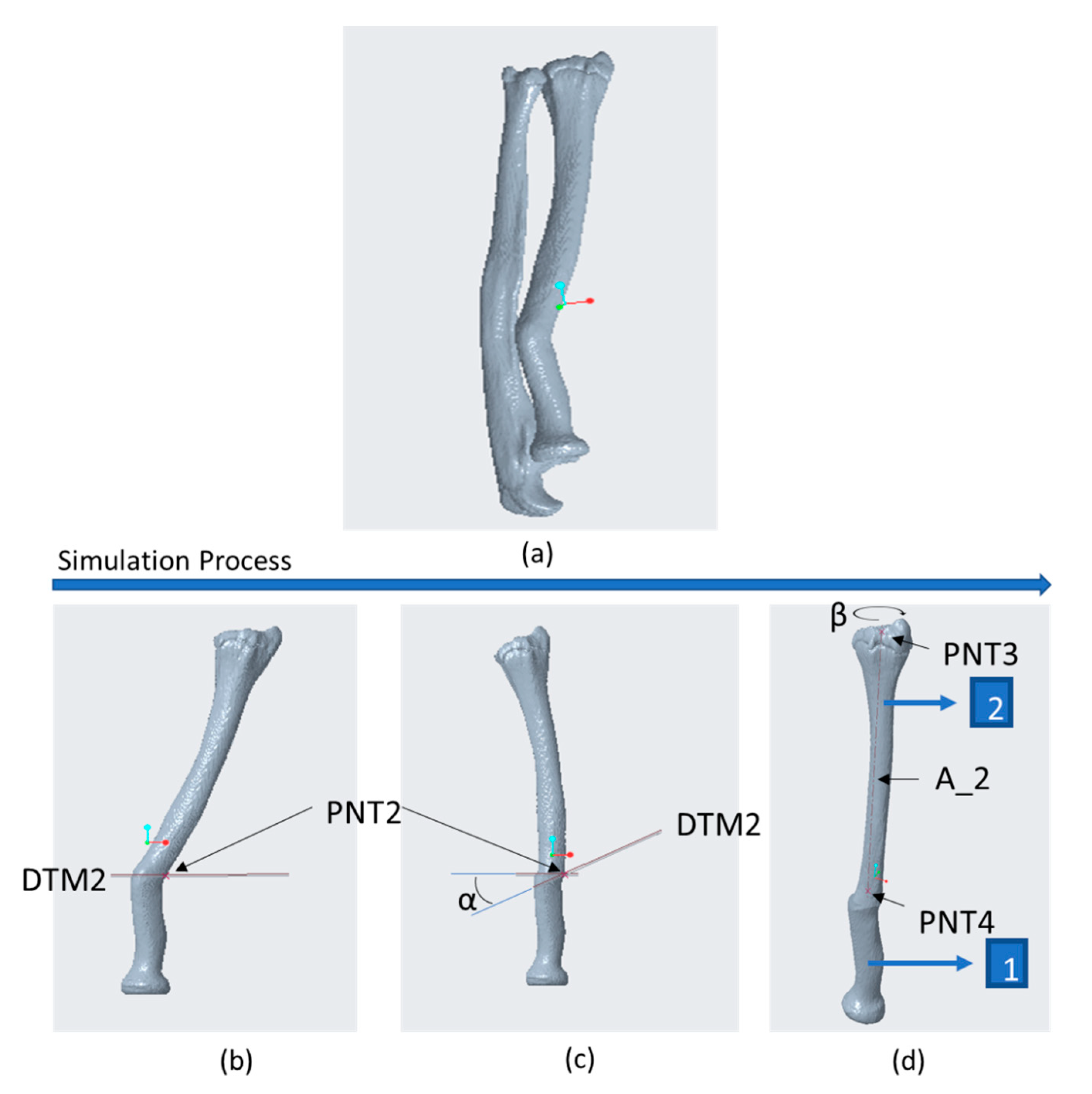
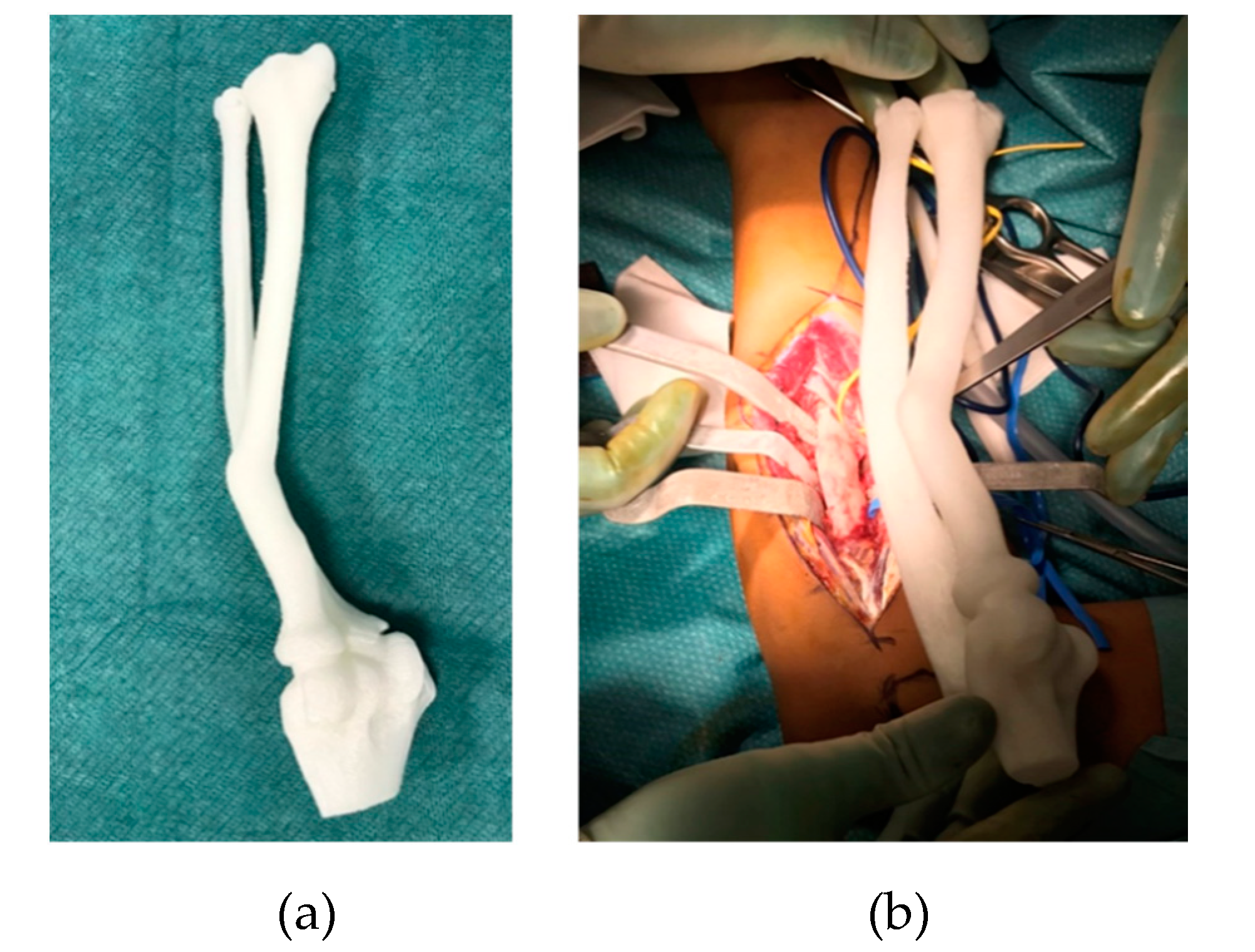
2.3. CASS
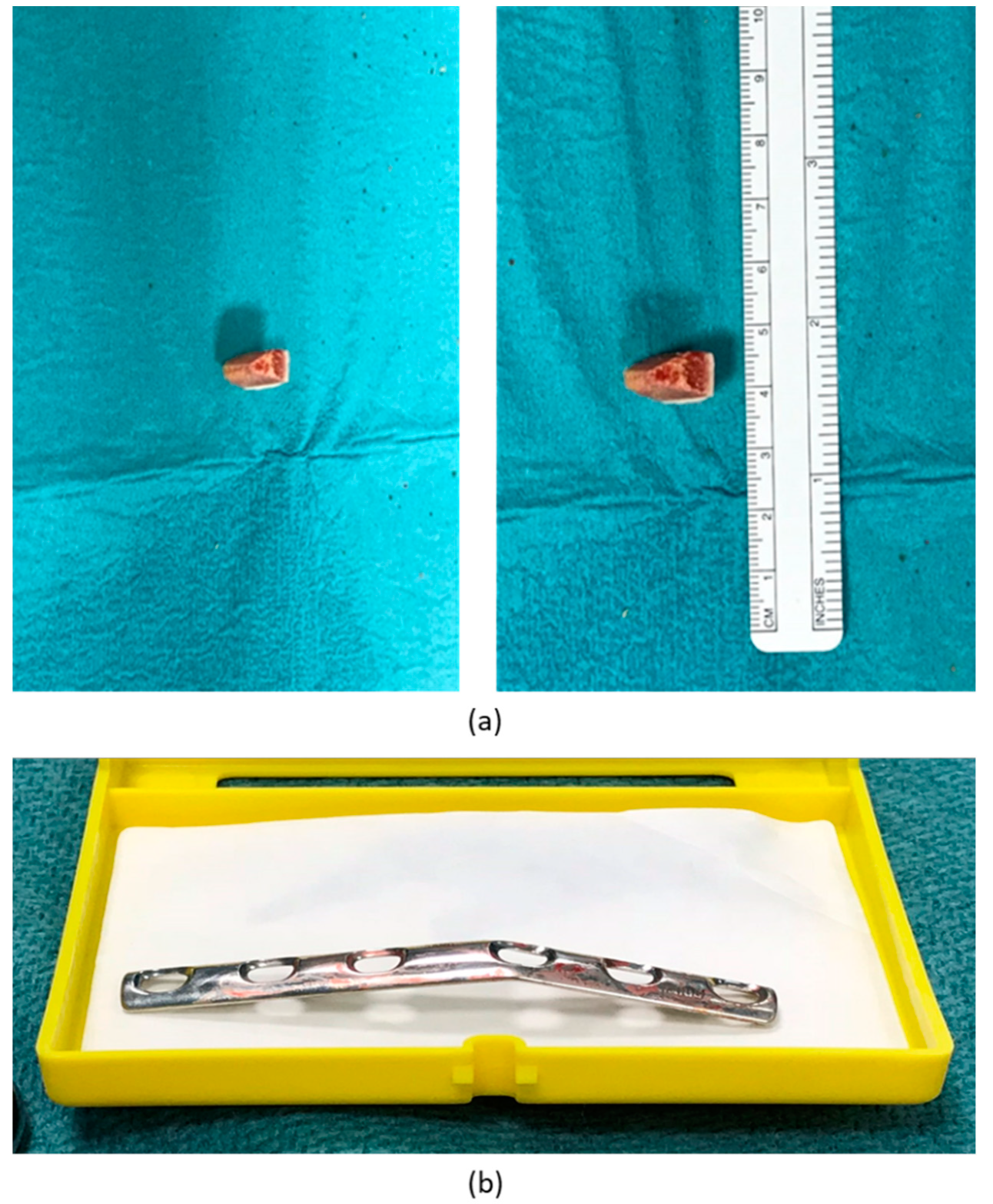
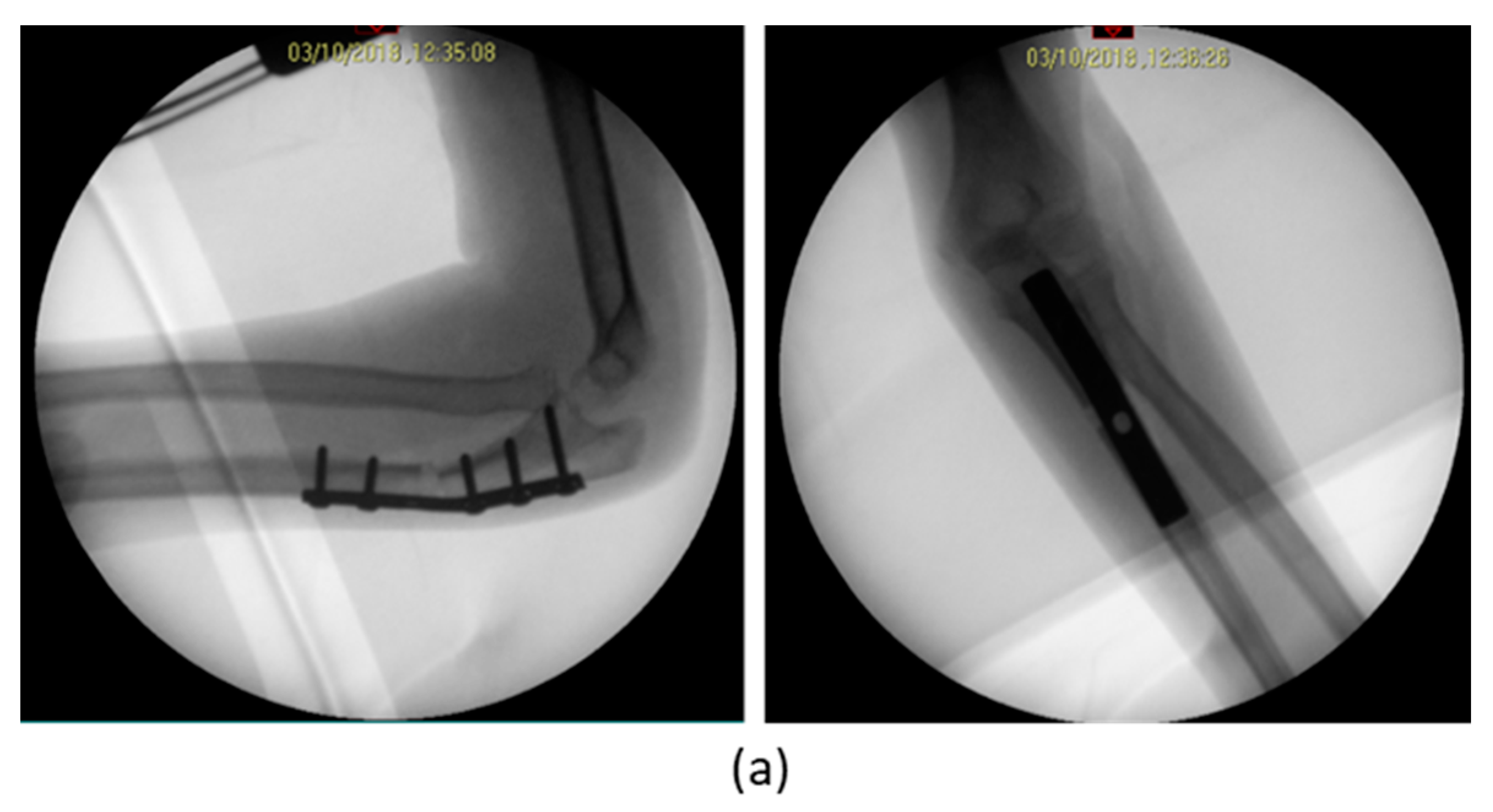
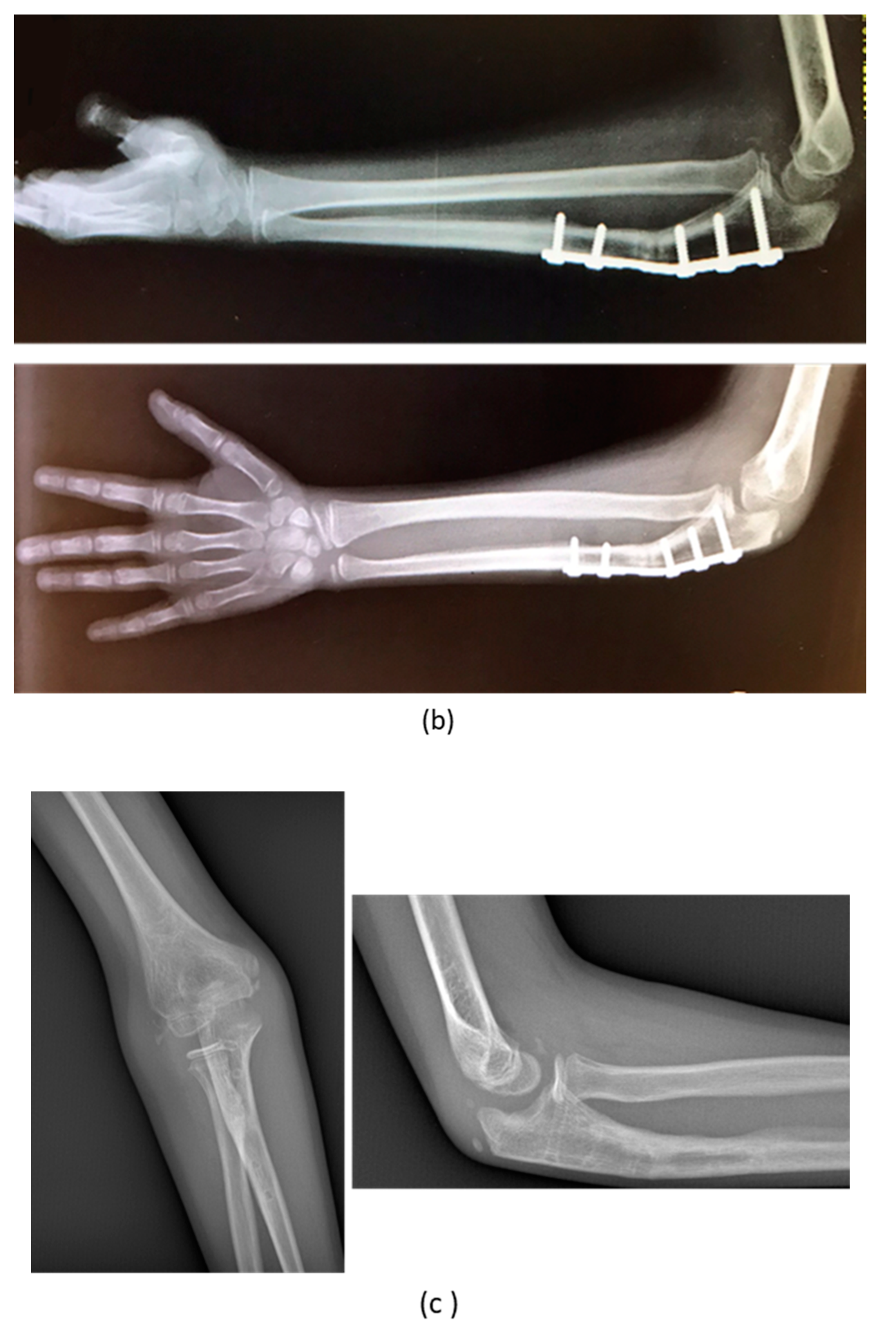
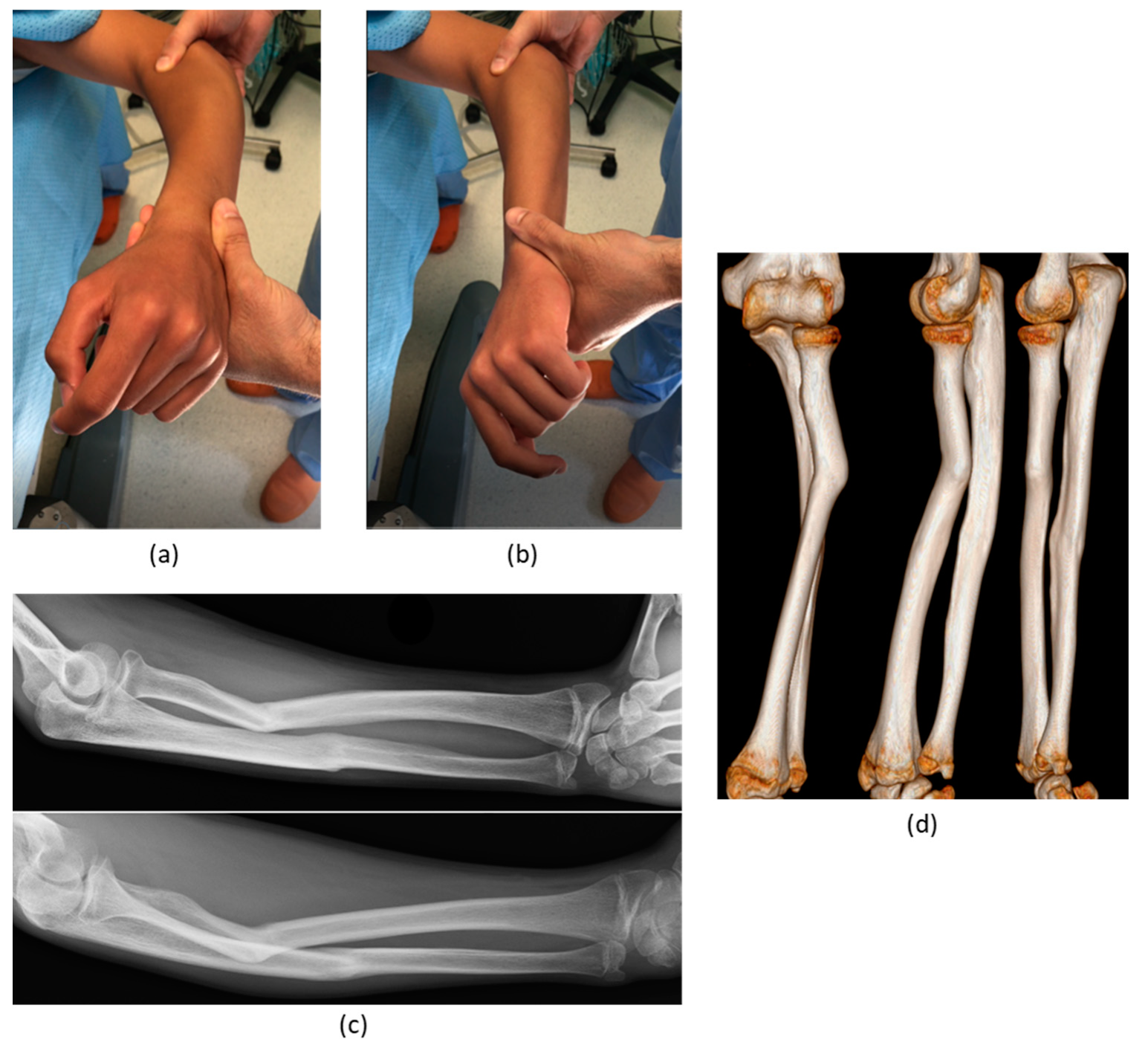
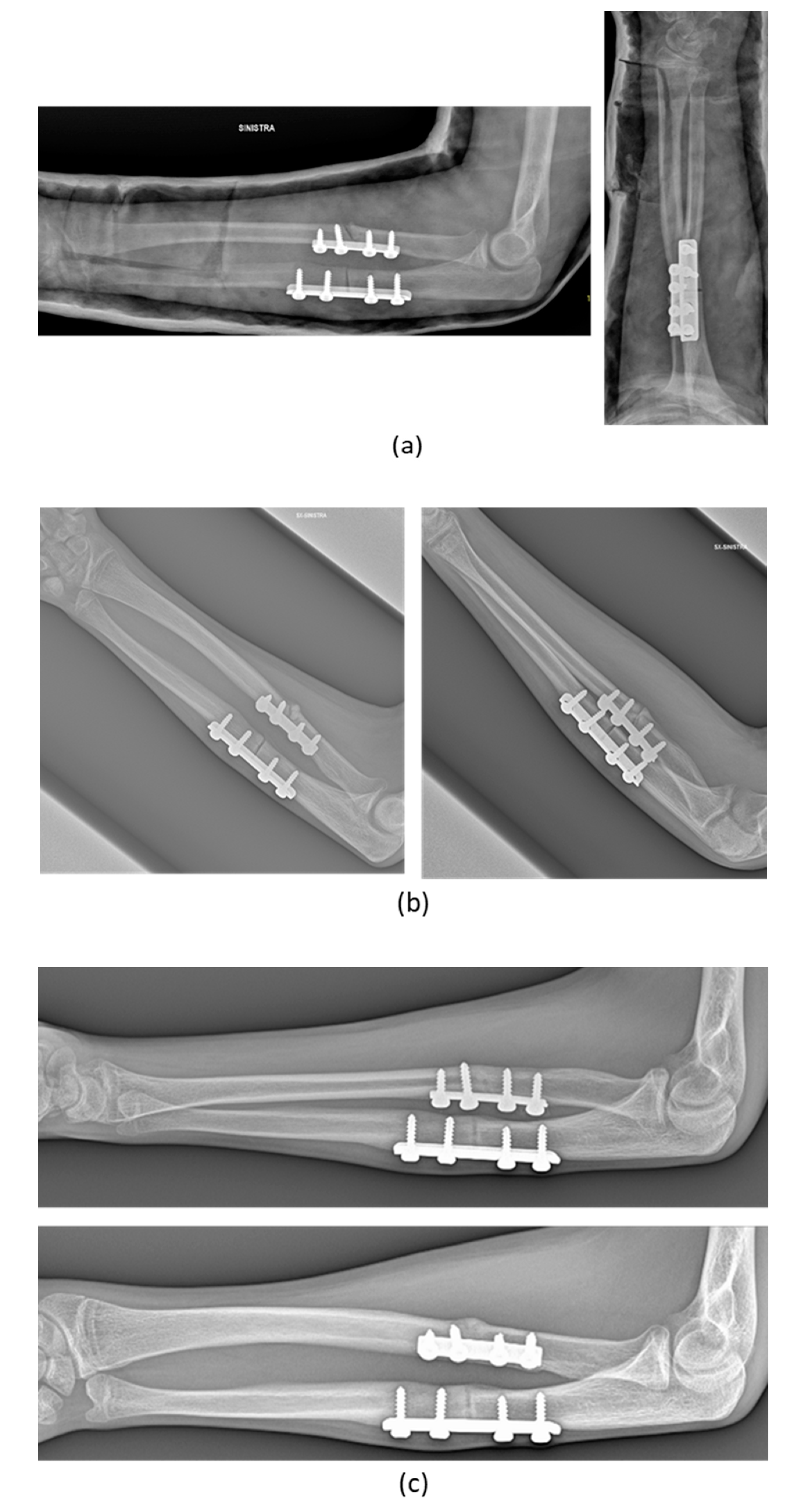
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bae, D.S. Simulation in Pediatric Orthopaedic Surgery. J. Pediatr. Orthop. 2015, 35, S26–S29. [Google Scholar] [CrossRef] [PubMed]
- Gateno, J.; Xia, J.J.; Teichgraeber, J.F.; Christensen, A.M.; Lemoine, J.J.; Liebschner, M.A.K.; Gliddon, M.J.; Briggs, M.E. Clinical Feasibility of Computer-Aided Surgical Simulation (CASS) in the Treatment of Complex Cranio-Maxillofacial Deformities. J. Oral Maxillofac. Surg. 2007, 65, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.J.; Zhou, Y.X.; Chen, L.B.; Wang, J.W.; Kan, W.S.; Ma, D.Z. Simulated surgeries of acetabular reconstruction for high-dislocated hips. Chin. J. Exp. Surg. 2016, 33, 820–822. [Google Scholar]
- Bao, H.D.; Yan, P.; Zhu, F.; Qiu, Y.; Zhu, Z.Z.; Wang, B.; Qian, B.P.; Yu, Y. Technology of pre-operative digitalized simulation of osteotomy in degenerative spinal deformity with sagittal malalignment. Chin. J. Orthop. 2016, 36, 321–328. [Google Scholar]
- Zhang, Y.Z.; Lu, S.; Chen, B.; Zhao, J.M.; Liu, R.; Pei, G.X. Application of computer-aided design osteotomy template for treatment of cubitus varus deformity in teenagers: A pilot study. J. Shoulder Elb. Surg. 2011, 20, 51–56. [Google Scholar] [CrossRef]
- Jiang, H.; Li, M.; Wu, Y. Application of computer simulation in the treatment of traumatic cubitus varus deformity in children. Medicine (Baltimore) 2019, 98, e13882. [Google Scholar] [CrossRef]
- Takeyasu, Y.; Oka, K.; Miyake, J.; Kataoka, T.; Moritomo, H.; Murase, T. Preoperative, computer simulation-based, three-dimensional corrective osteotomy for cubitus varus deformity with use of a custom-designed surgical device. J. Bone Jt. Surg. Ser. A 2013, 95, e173. [Google Scholar] [CrossRef]
- Frizziero, L.; Santi, G.M.; Liverani, A.; Giuseppetti, V.; Trisolino, G.; Maredi, E.; Stilli, S. Paediatric Orthopaedic Surgery with 3D Printing: Improvements and Cost Reduction. Symmetry 2019, 11, 1317. [Google Scholar] [CrossRef] [Green Version]
- Osti, F.; Santi, G.; Neri, M.; Liverani, A.; Frizziero, L.; Stilli, S.; Maredi, E.; Zarantonello, P.; Gallone, G.; Stallone, S.; et al. CT Conversion Workflow for Intraoperative Usage of Bony Models: From DICOM Data to 3D Printed Models. Appl. Sci. 2019, 9, 708. [Google Scholar] [CrossRef] [Green Version]
- InVesalius. Available online: https://invesalius.github.io/ (accessed on 18 April 2019).
- MeshLab. Available online: https://www.meshlab.net/ (accessed on 20 April 2019).
- Available online: http://www.meshmixer.com/ (accessed on 20 April 2019).
- blender.org—Home of the Blender Project—Free and Open 3D Creation Software. Available online: https://www.blender.org/ (accessed on 9 May 2019).
- Software di Modellazione 3D Creo Parametric | PTC. Available online: https://www.ptc.com/it/products/creo/parametric (accessed on 21 February 2019).
- Di Gennaro, G.; Trisolino, G. Chronic Radial Head Dislocation Caused by Focal Fibrocartilaginous Dysplasia (FFCD) of the Ulna: A Case Report and Review of the Literature. EC Paediatr. 2018, 7, 312–314. [Google Scholar]
- Miyake, J.; Murase, T.; Oka, K.; Moritomo, H.; Sugamoto, K.; Yoshikawa, H. Computer-assisted corrective osteotomy for malunited diaphyseal forearm fractures. J. Bone Jt. Surg. Ser. A 2012, 94, e150. [Google Scholar] [CrossRef]
- Miyake, J.; Oka, K.; Kataoka, T.; Moritomo, H.; Sugamoto, K.; Murase, T. 3-dimensional deformity analysis of malunited forearm diaphyseal fractures. J. Hand Surg. Am. 2013, 38, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Colaris, J.W.; Oei, S.; Reijman, M.; Holscher, H.; Allema, J.H.; Verhaar, J.A.N. Three-dimensional imaging of children with severe limitation of pronation/supination after a both-bone forearm fracture. Arch. Orthop. Trauma Surg. 2014, 134, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.; Oka, K.; Miyamura, S.; Shigi, A.; Tanaka, H.; Sugamoto, K.; Yoshikawa, H.; Murase, T. Three-Dimensional In Vivo Analysis of Malunited Distal Radius Fractures With Restricted Forearm Rotation. J. Orthop. Res. 2019, 37, 1881–1891. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, L.F.P.; Barbieri, C.H.; Mazzer, N.; Zatiti, S.C.A.; Bellucci, Â.D.; Nogueira-Barbosa, M.H. Intraobserver and interobserver reliability of radial torsion angle measurements by a new and alternative method with computed tomography. Clinics 2010, 65, 1093–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bockhorn, L.; Gardner, S.S.; Dong, D.; Karmonik, C.; Elias, S.; Gwathmey, F.W.; Harris, J.D. Application of three-dimensional printing for pre-operative planning in hip preservation surgery. J. Hip Preserv. Surg. 2019, 6, 164–169. [Google Scholar] [CrossRef] [Green Version]
- Perica, E.; Sun, Z. Patient-specific three-dimensional printing for pre-surgical planning in hepatocellular carcinoma treatment. Quant. Imaging Med. Surg. 2017, 7, 668–677. [Google Scholar] [CrossRef] [Green Version]
- Shen, S.; Wang, P.Z.; Li, X.Y.; Han, X.; Tan, H.L. Pre-operative simulation using a three-dimensional printing model for surgical treatment of old and complex tibial plateau fractures. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.H.; Liao, H.; Tan, X.Y.; Xing, W.R.; Zhou, Q.; Zheng, Y.S.; Cao, H.Y.; Zeng, C.J. Surgical treatment for both-column acetabular fractures using pre-operative virtual simulation and three-dimensional printing techniques. Chin. Med. J. (Engl.) 2020, 133, 395–401. [Google Scholar] [CrossRef]
- Hung, C.C.; Li, Y.T.; Chou, Y.C.; Chen, J.E.; Wu, C.C.; Shen, H.C.; Yeh, T. Te Conventional plate fixation method versus pre-operative virtual simulation and three-dimensional printing-assisted contoured plate fixation method in the treatment of anterior pelvic ring fracture. Int. Orthop. 2019, 43, 425–431. [Google Scholar] [CrossRef]
- Grassi, F.R.; Grassi, R.; Vivarelli, L.; Dallari, D.; Govoni, M.; Nardi, G.M.; Kalemaj, Z.; Ballini, A. Design techniques to optimize the scaffold performance: Freeze-dried bone custom-made allografts for maxillary alveolar horizontal ridge augmentation. Materials 2020, 13, 1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalski, A.; Stopa, M.; Miśkowiak, B. Use of multimedia technology in the doctor- patient relationship for obtaining patient informed consent. Med. Sci. Monit. 2016, 22, 3994–3999. [Google Scholar] [CrossRef] [PubMed] [Green Version]

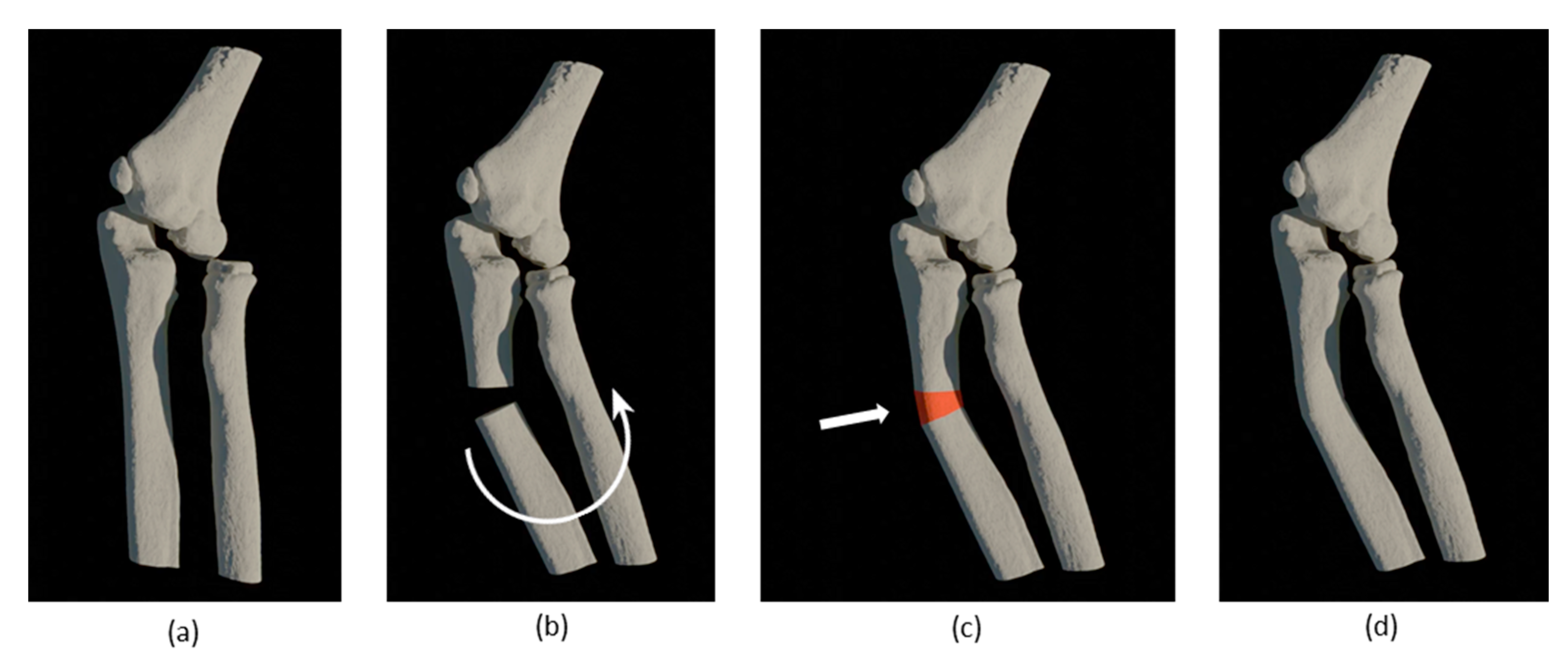
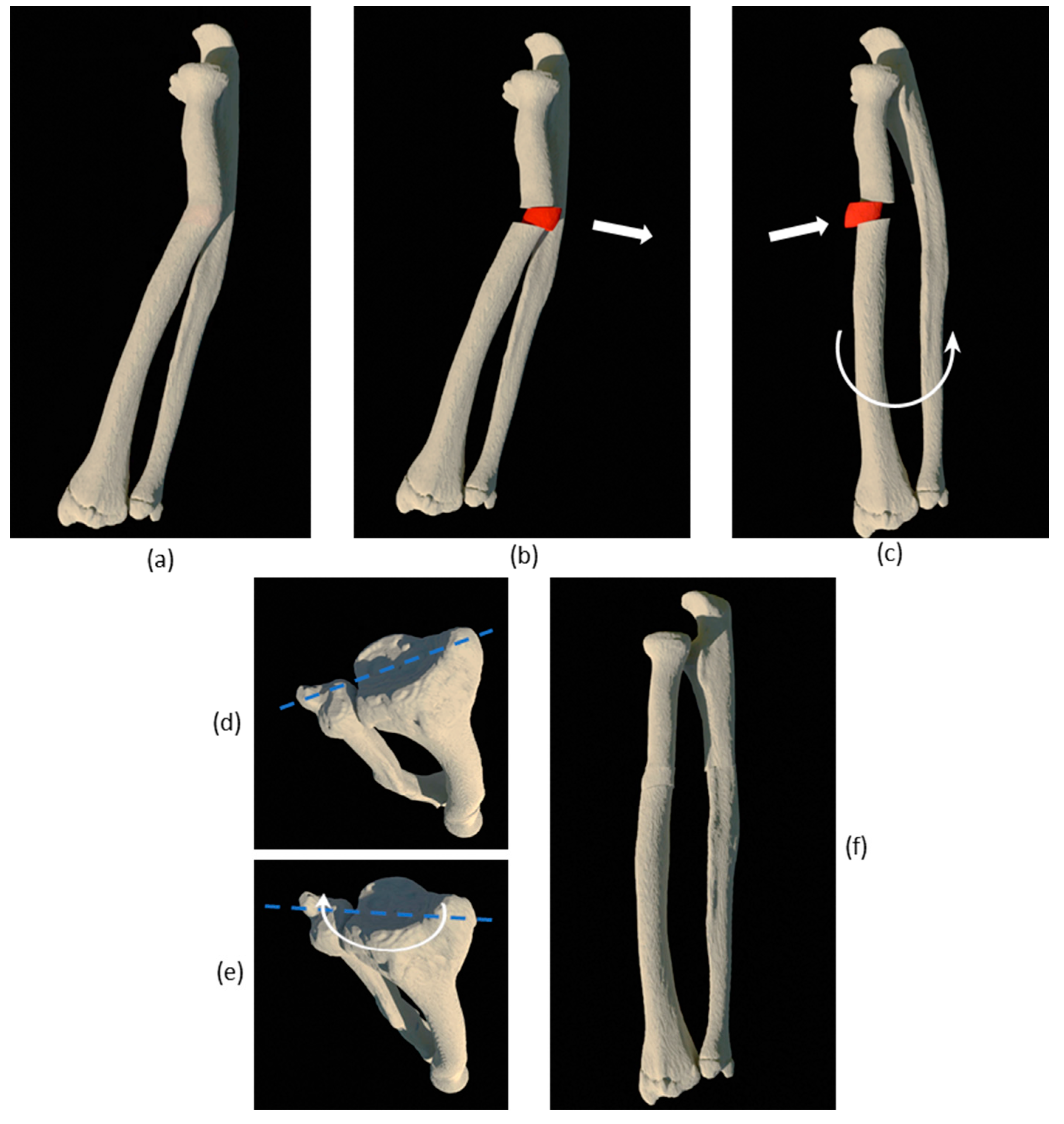
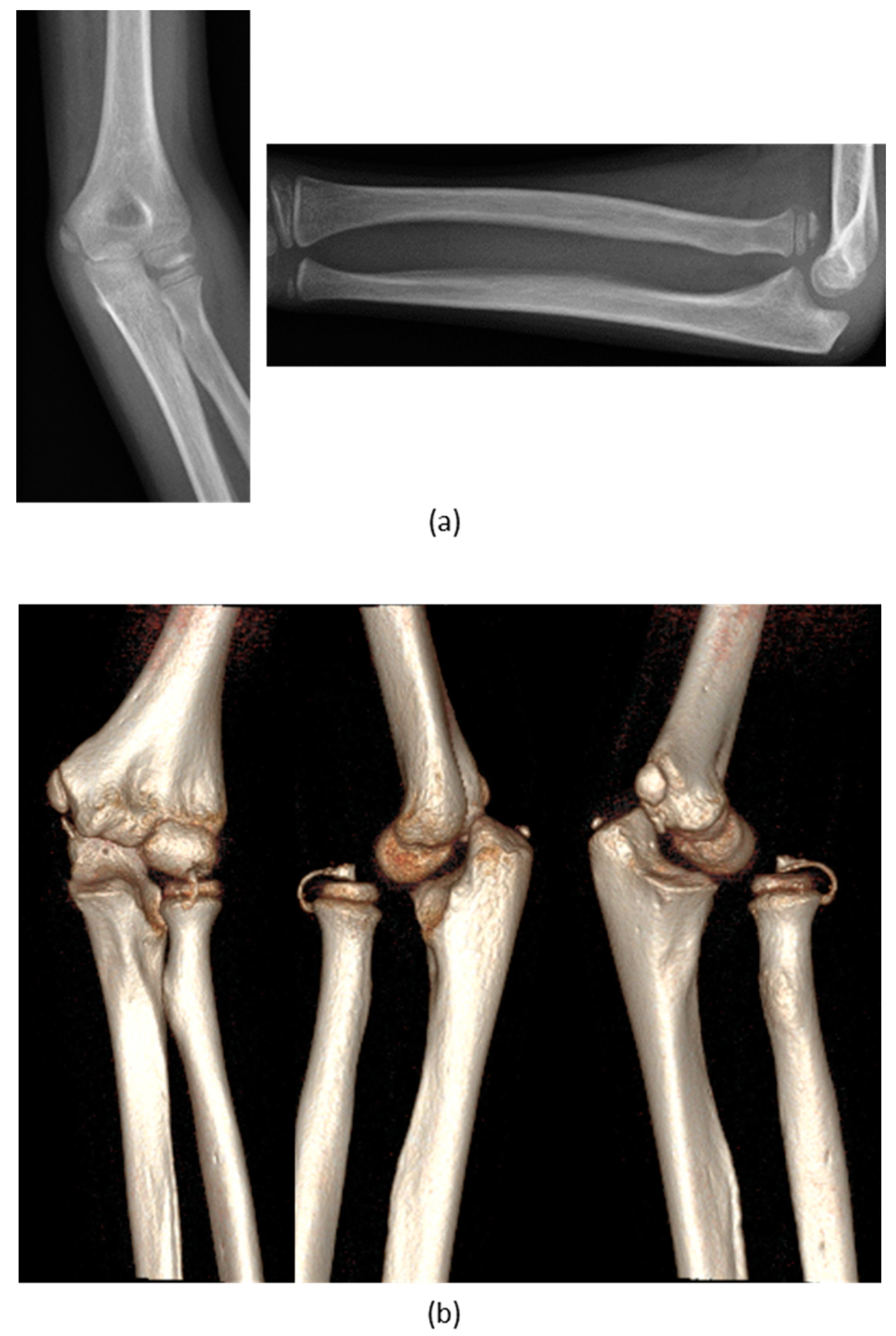
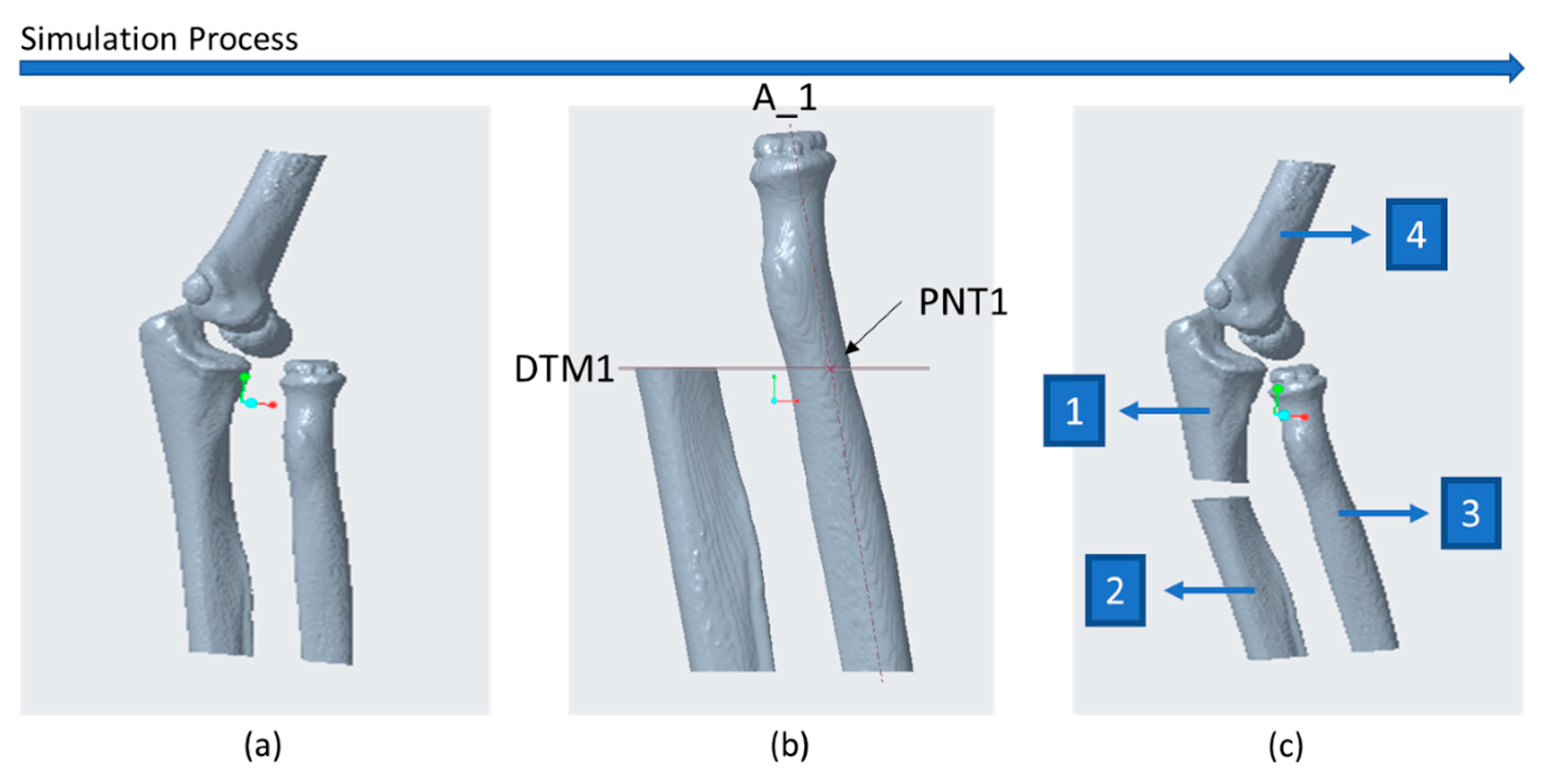
| No. | Sex and Age (years) | Diagnose | Type of Deformity | Type of Intervention | Complications | Radiographic Outcome |
|---|---|---|---|---|---|---|
| 1 | F, 9 | Missed Monteggia Lesion | Post-traumatic | Proximal ulnar osteotomy stabilized with a 6-holes plate | None | Corrected |
| 2 | M, 13 | Focal fibrocartilagineous dysplasia | Congenital | Bifocal osteotomy stabilized with external fixator | Heterotopic bone formations | Fair (residual dislocation of the radial head with shortening of the ulna) |
| 3 | M, 15 | Post-traumatic forearm malunion deformity | Post-traumatic | Bifocal osteotomy stabilized with 1/3 tubular plate | None | Corrected |
| 4 | M, 7 | Bilateral genu varum in Blount disease | Congenital | Double tibial osteotomy | None | Good (partial varus recurrence) |
| 5 | F, 6 | Coxa vara in Progressive Pseudorheumatoid Dysplasia | Congenital | Valgus derotational osteotomy | None | Corrected |
| Ref. | PROCEDURES | CT Reconstruction | 3D Optimization | Preparation for 3D Printing | 3D Simulation | Total Cost |
|---|---|---|---|---|---|---|
| OUR PROCEDURE | Invesalius + meshlab/meshmixer + blender + PTC creo | YES (free) | YES (free) | YES (free) | YES | Only PTC Creo for 3D simulation |
| Bockhorn et al., 2019 [21] | ImageJ + Paraview + MeshMixer | YES (free) | LIMITED (free) | LIMITED (free) | NO | NO |
| Perica et al., 2017 [22] | Analyze 12.0 + Geomagic Wrap | YES | YES | YES | NO | Analyze 12.0, Geomagic Wrap |
| Shen et al., 2020 [23] Huang et al., 2020 [24] | Materialise Mimics | YES | YES | YES | NO | Materialise Mimics |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frizziero, L.; Santi, G.M.; Liverani, A.; Napolitano, F.; Papaleo, P.; Maredi, E.; Gennaro, G.L.D.; Zarantonello, P.; Stallone, S.; Stilli, S.; et al. Computer-Aided Surgical Simulation for Correcting Complex Limb Deformities in Children. Appl. Sci. 2020, 10, 5181. https://doi.org/10.3390/app10155181
Frizziero L, Santi GM, Liverani A, Napolitano F, Papaleo P, Maredi E, Gennaro GLD, Zarantonello P, Stallone S, Stilli S, et al. Computer-Aided Surgical Simulation for Correcting Complex Limb Deformities in Children. Applied Sciences. 2020; 10(15):5181. https://doi.org/10.3390/app10155181
Chicago/Turabian StyleFrizziero, Leonardo, Gian Maria Santi, Alfredo Liverani, Francesca Napolitano, Paola Papaleo, Elena Maredi, Giovanni Luigi Di Gennaro, Paola Zarantonello, Stefano Stallone, Stefano Stilli, and et al. 2020. "Computer-Aided Surgical Simulation for Correcting Complex Limb Deformities in Children" Applied Sciences 10, no. 15: 5181. https://doi.org/10.3390/app10155181
APA StyleFrizziero, L., Santi, G. M., Liverani, A., Napolitano, F., Papaleo, P., Maredi, E., Gennaro, G. L. D., Zarantonello, P., Stallone, S., Stilli, S., & Trisolino, G. (2020). Computer-Aided Surgical Simulation for Correcting Complex Limb Deformities in Children. Applied Sciences, 10(15), 5181. https://doi.org/10.3390/app10155181








