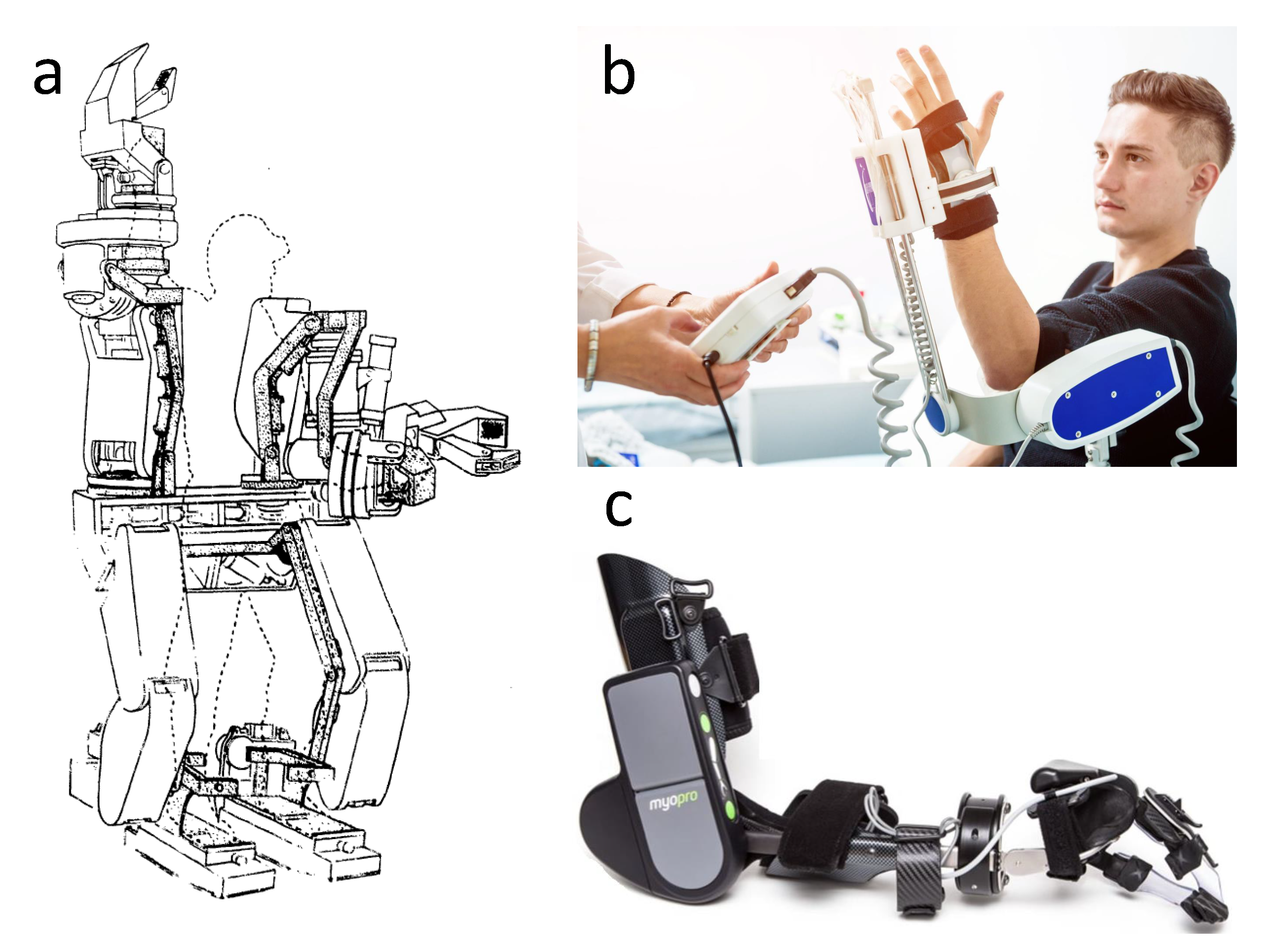
Upper Limb Bionic Orthoses: General Overview and Forecasting Changes
Abstract
:1. Introduction
2. Medical Device Regulations
3. Mechanical Construction
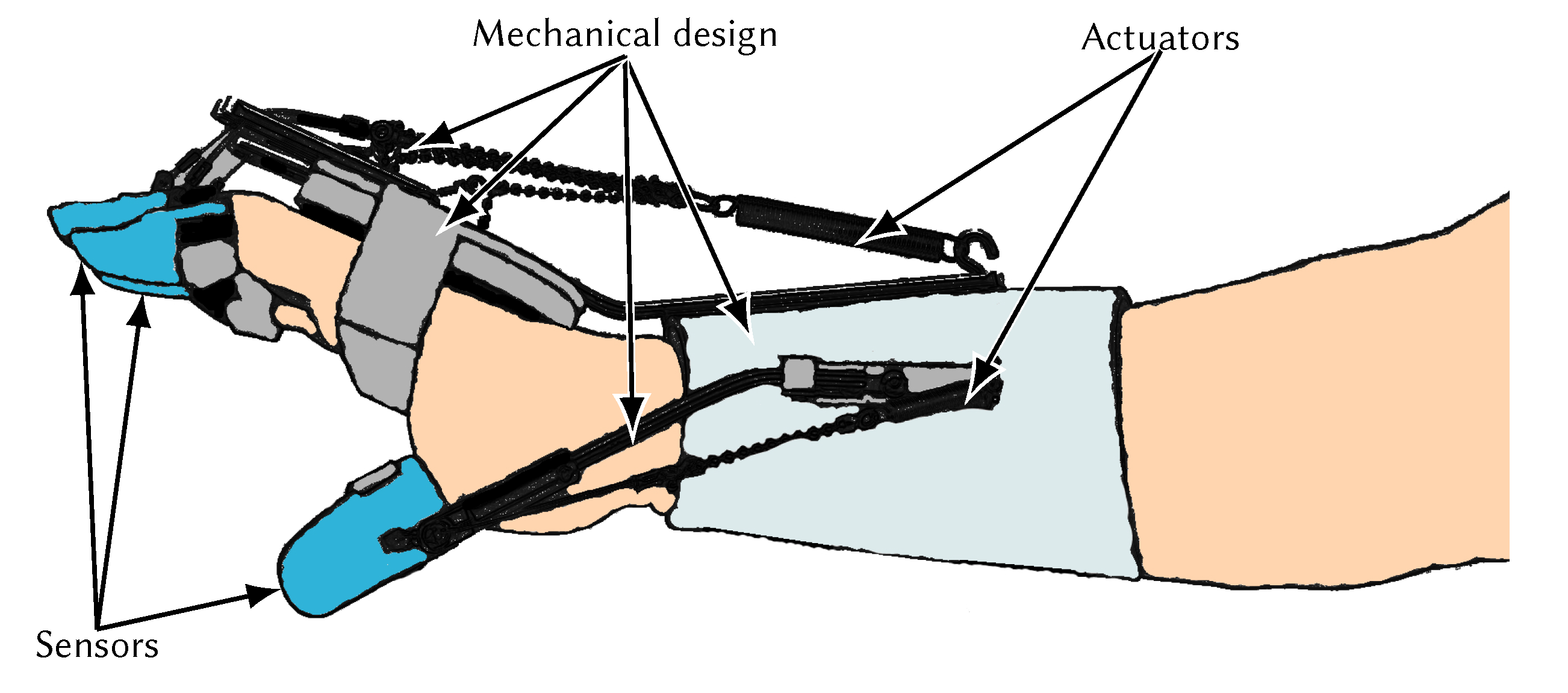
4. Actuators Overview

| Power Density (W/kg) | Torque (Nm) | Dimensions (mm) | Weight (g) | References | |
|---|---|---|---|---|---|
| Micro Servos Expert Electronics SL260 | 110 | 0.109 | 21.6 × 11.2 × 19.1 | 9.1 | [105] |
| Coreless motor MicroMo 2224-012SR | 675 | 0.728 | ϕ22 × 51.3 | 6 | [105] |
| Artificial muscle Festo | 46 | - | 260 × 30 × 30 | 136 | [106] |
| Brushless motor Maxon Motors | 125 | 9.8 | 60 × 59 × 56 | 80 | [107] |
| Pololu Micro Metal Gearmotor | 914 | 0.89 | 10 × 12 × 29.5 | 10.5 | [108] |
| Pololu Micro Metal Gearmotor | 136 | 1.57 | ϕ25 × 68 | 106 | [108] |
| Power Density (W/kg) | Average Efficiency (%) | Density (kg/m3) | Product Life Cycle (Number of Cycles) | References | |
|---|---|---|---|---|---|
| SMA | 1000–50,000 | < 5 | 6450 | 107 | [71,109] |
| CNT | 10–270 | > 22 | 1000 | 140,000 33% reduction | [71,114,120,121] |
| Elastomer | 500–5000 | 25 | 1000 | 107 | [71,110,111] |
| MRF | 690 | NDA * | 3000 | NDA * | [116] |
| Ultrasonic motor | 36 | 18–80 | 1620 | NDA * | [117,122] |
5. Sensory System
6. Control System Feedback
7. Modern Computer Methods in Medical Engineering
7.1. Machine Learning
7.2. Multimedia Systems
8. Summary and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jefferson, G. The Mind of Mechanical Man. BMJ 1949, 1, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Čapek, K. Rossumovi Univerzální Roboti (Rossum’s Universal Robots); Animedia Company: Prague, Czech, 2014; p. 89. [Google Scholar]
- Kinyon, K. The Phenomenology of Robots: Confrontations with Death in Karel Čapek’s “R.U.R.”. Sci. Fict. Stud. 1999, 26, 379–400. [Google Scholar]
- Hockstein, N.G.; Gourin, C.G.; Faust, R.A.; Terris, D.J. A history of robots: From science fiction to surgical robotics. J. Robot. Surg. 2007, 1, 113–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andry, N. L’Orthopédie ou l’art de Prévenir et de Corriger dans les Enfans, les Difformités du Corps; Chez La Veuve Alix: Paris, France, 1741; p. 348. [Google Scholar]
- Lovett, R.W.; Boston, M.D. The History of Scoliosis. J. Bone Jt. Surg. 1913, 2, 54–62. [Google Scholar]
- Fick, B.R.; Makinson, J.B. Final Report on Hardiman I Prototype for Machine Augmentation of Human Strength and Endurance; Technical Report; General Electric Company: New York, NY, USA, 1971. [Google Scholar]
- Boldt, K. Three Axis Mechanical Joint for a Power Assist Device. U.S. Patent 5,282,460, 1 February 1994. [Google Scholar]
- Salter, R.B.; Hamilton, H.W.; Wedge, J.H.; Tile, M.; Torode, I.P.; O’Driscoll, S.W.; Murnaghan, J.J.; Saringer, J.H. Clinical application of basic research on continuous passive motion for disorders and injuries of synovial joints: A preliminary report of a feasibility study. J. Orthop. Res. 1983, 1, 325–342. [Google Scholar] [CrossRef]
- Díaz, I.; Gil, J.J.; Sánchez, E. Lower-Limb Robotic Rehabilitation: Literature Review and Challenges. J. Robot. 2011, 2011. [Google Scholar] [CrossRef]
- Lum, P.S.; Burgar, C.G.; Van Der Loos, M.; Shor, P.C.; Majmundar, M.; Yap, R. MIME robotic device for upper-limb neurorehabilitation in subacute stroke subjects: A follow-up study. J. Rehabil. Res. Dev. 2006, 43, 631–642. [Google Scholar] [CrossRef]
- Housman, S.J.; Le, V.; Rahman, T.; Sanchez, R.J.; Remkensrneyer, D.J. Arm-training with T-WREX after chronic stroke: Preliminary results of a randomized controlled trial. In Proceedings of the 2007 IEEE 10th International Conference on Rehabilitation Robotics (ICORR ’07), Noordwijk, The Netherlands, 13–15 June 2007; pp. 562–568. [Google Scholar] [CrossRef]
- Roceso. Arm and Hand Rehabilitation Devices; Roceso Technologies: Singapore, 2020. [Google Scholar]
- Bioservo. Keeping People Strong Healthy and Motivated; Bioservo Technologies: Kista, Sweden, 2020. [Google Scholar]
- Myomo. Medical Robotics Solutions for Stroke, BPI, Upper Limb Paralysis; Myomo: Cambridge, MA, USA, 2020. [Google Scholar]
- Brokaw, E.B.; Black, I.; Holley, R.J.; Lum, P.S. Hand Spring Operated Movement Enhancer (HandSOME): A Portable, Passive Hand Exoskeleton for Stroke Rehabilitation. IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 19, 391–399. [Google Scholar] [CrossRef]
- Gassert, R.; Dietz, V. Rehabilitation robots for the treatment of sensorimotor deficits: A neurophysiological perspective. J. NeuroEng. Rehabil. 2018, 15, 46. [Google Scholar] [CrossRef] [Green Version]
- Farmer, S.E.; Durairaj, V.; Swain, I.; Pandyan, A.D. Assistive Technologies: Can They Contribute to Rehabilitation of the Upper Limb After Stroke? Arch. Phys. Med. Rehabil. 2014, 95, 968–985. [Google Scholar] [CrossRef]
- Prange, G.B.; Jannink, M.J.a.; Groothuis-Oudshoorn, C.G.M.; Hermens, H.J.; IJzerman, M.J. Systematic review of the effect of robot-aided therapy on recovery of the hemiparetic arm after stroke. J. Rehabil. Res. Dev. 2006, 43, 171–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frisoli, A.; Procopio, C.; Chisari, C.; Creatini, I.; Bonfiglio, L.; Bergamasco, M.; Rossi, B.; Carboncini, M. Positive effects of robotic exoskeleton training of upper limb reaching movements after stroke. J. NeuroEng. Rehabil. 2012, 9, 36. [Google Scholar] [CrossRef] [Green Version]
- Kwakkel, G.; Kollen, B.J.; Krebs, H.I. Effects of Robot-Assisted Therapy on Upper Limb Recovery after Stroke: A Systematic Review. Neurorehabil. Neural Repair 2008, 22, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Song, A.; Pan, L.; Li, H.; Liang, Z.; Zhu, S.; Xu, B.; Li, J. Adaptive Hierarchical Control for the Muscle Strength Training of Stroke Survivors in Robot-aided Upper-limb Rehabilitation. Int. J. Adv. Robot. Syst. 2012, 9, 1. [Google Scholar] [CrossRef]
- Burgar, C.G.; Lum, P.S.; Shor, P.C.; Machiel Van der Loos, H.F. Development of robots for rehabilitation therapy: The Palo Alto VA/Stanford experience. J. Rehabil. Res. Dev. 2000, 37, 647–663. [Google Scholar]
- Tomić, T.J.D.; Savić, A.M.; Vidaković, A.S.; Rodić, S.Z.; Isaković, M.S.; Rodríguez-de Pablo, C.; Keller, T.; Konstantinović, L.M. ArmAssist Robotic System versus Matched Conventional Therapy for Poststroke Upper Limb Rehabilitation: A Randomized Clinical Trial. BioMed Res. Int. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wu, H. Development, Dynamic Modeling, and Multi-Modal Control of a Therapeutic Exoskeleton for Upper Limb Rehabilitation Training. Sensors 2018, 18, 3611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Fu, Q.; Guo, S.; Fu, Y. Coordinative Motion-based Bilateral Rehabilitation Training System with Exoskeleton and Haptic Devices for Biomedical Application. Micromachines 2018, 10, 8. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.H.; Rahman, M.J.; Cristobal, O.L.; Saad, M.; Kenné, J.P.; Archambault, P.S. Development of a whole arm wearable robotic exoskeleton for rehabilitation and to assist upper limb movements. Robotica 2015, 33, 19–39. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://myomo.com/what-is-a-myopro-orthosis/ (accessed on 29 June 2020).
- Available online: https://www.shutterstock.com/pl/image-photo/patient-on-cpm-continuous-passive-range-1154961649?fbclid=IwAR09jNTzgSIUafTXdMD-6aOrUawSfCI_dyw959PKIEDFoB2qviJKBjjc8iM (accessed on 29 June 2020).
- U.S. Food and Drug Administration. Overview of Device Regulation; FDA: Silver Spring, MD, USA, 2011. [Google Scholar]
- Japan Quality Assurance Organization. Overview | Pharmaceuticals and Medical Device Law (PMDL) (Mandatory Medical Device Approval) | Mandatory Approvals | Testing and Certification of Electrical and Electronic Products; JQA: Tokyo, Japan, 2020. [Google Scholar]
- Fikriah, N.S.; Saripan, H.; Ismail, Z. The Medical Device Regulation for Humanoid Robotics: Does One Size Fits All? Procedia Comput. Sci. 2015, 76, 381–387. [Google Scholar] [CrossRef] [Green Version]
- Food and Drug Administration. Factors to Consider Regarding BenefitRisk in Medical Device Product Availability, Compliance, and Enforcement Decisions; Food and Drug Administration: Muntinlupa, Philippines, 2016. [Google Scholar]
- Tamura, A. Understanding Japanese Medical Device Requirements. In 2011 AHC Workshop on Medical Devices: Implementation of GHTF Documents; Asian-Pacific Economic Cooperation: Seoul, Korea, 2011. [Google Scholar]
- Ciarkowski, A.A. FDA regulatory requirements for medical devices with control algorithms. In Proceedings of the 2000 American Control Conference ACC (IEEE Cat. No.00CH36334), Chicago, IL, USA, 28–30 June 2000; Volume 5, pp. 3497–3500. [Google Scholar] [CrossRef]
- The Council of the European Communities. Council Directive 93/42/EEC of 14 June 1993 Concerning Medical Devices; The Council of the European Communities: Brussels, Belgium, 1993. [Google Scholar]
- U.S. Government Printing Office. eCFR—Code of Federal Regulations. In Electronic Code of Federal Regulations; U.S. Government Printing Office: Washington, DC, USA, 2010. [Google Scholar]
- Sejm Rzeczypospolitej Polskiej. Ustawa o Wyrobach Medycznych; Sejm Rzeczypospolitej Polskiej: Warsaw, Poland, 2018. [Google Scholar]
- European Commission. Dyrektywa Maszynowa; European Commission: Brussels, Belgium, 2006. [Google Scholar]
- European Commision. Council Directive 73/23/EEC of 19 February 1973 on the Harmonization of the Laws of Member States Relating to Electrical Equipment Designed for Use within Certain Voltage Limits; European Commission: Brussels, Belgium, 1973. [Google Scholar]
- ISO/TC 299. IEC 80601-2-78:2019. In Medical Electrical Equipment—Part 2-78: Particular Requirements for Basic Safety and Essential Performance of Medical Robots for Rehabilitation, Assessment, Compensation or Alleviation; ISO: Geneva, Switzerland, 2019. [Google Scholar]
- Chen, C.W.; Lin, C.C.K.; Ju, M.S. Hand orthosis controlled using brain-computer interface. J. Med. Biol. Eng. 2009, 29, 234–241. [Google Scholar]
- Rupp, R.; Rohm, M.; Schneiders, M.; Kreilinger, A.; Muller-Putz, G.R. Functional Rehabilitation of the Paralyzed Upper Extremity After Spinal Cord Injury by Noninvasive Hybrid Neuroprostheses. Proc. IEEE 2015, 103, 954–968. [Google Scholar] [CrossRef]
- Vorobyev, A.A.; Andryushchenko, F.A.; Ponomareva, O.A.; Solovyeva, I.O.; Krivonozhkina, P.S. The Development and Clinical Testing of “EXAR”, Passive Upper Limb Exoskeleton. Sovrem. Tehnol. Med. 2016, 8, 90–97. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Zhang, X.; Fu, R.; Sun, G. Study of the Home-Auxiliary Robot Based on BCI. Sensors 2018, 18, 1779. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Sun, S.; Zhang, S.; Chen, Y.; Li, C.; Chen, S. A Brain-Machine Interface Based on ERD/ERS for an Upper-Limb Exoskeleton Control. Sensors 2016, 16, 2050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhary, U.; Birbaumer, N.; Ramos-Murguialday, A. Brain–computer interfaces for communication and rehabilitation. Nat. Rev. Neurol. 2016, 12, 513–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wairagkar, M.; Zoulias, I.; Oguntosin, V.; Hayashi, Y.; Nasuto, S. Movement intention based Brain Computer Interface for Virtual Reality and Soft Robotics rehabilitation using novel autocorrelation analysis of EEG. In Proceedings of the 2016 6th IEEE International Conference on Biomedical Robotics and Biomechatronics (BioRob), Singapore, 26–29 June 2016; p. 685. [Google Scholar]
- Fleischer, C.; Hommel, G. A Human–Exoskeleton Interface Utilizing Electromyography. IEEE Trans. Robot. 2008, 24, 872–882. [Google Scholar] [CrossRef]
- Struktura i własności stali 316L. 2007. Available online: http://www.stalenierdzewne.pl/1092/struktura-i-wlasnosci-stali-316l (accessed on 29 June 2020).
- Maciejasz Pawełand Eschweiler, J.; Gerlach-Hahn, K.; Jansen-Troy, A.; Leonhardt, S. A survey on robotic devices for upper limb rehabilitation. J. NeuroEng. Rehabil. 2014, 11, 3. [Google Scholar] [CrossRef] [Green Version]
- Bos, R.A.; Haarman, C.J.W.; Stortelder, T.; Nizamis, K.; Herder, J.L.; Stienen, A.H.A.; Plettenburg, D.H. A structured overview of trends and technologies used in dynamic hand orthoses. J. NeuroEng. Rehabil. 2016, 13, 62. [Google Scholar] [CrossRef] [Green Version]
- Biagiotti, L.; Lotti, F.; Melchiorri, C.; Vassura, G. How Far Is the Human Hand? Technical Report; University of Bologna: Bologna, Italy, 2004. [Google Scholar]
- Controzzi, M.; Cipriani, C.; Carrozza, M.C. Design of artificial hands: A review. In The Human Hand as an Inspiration for Robot Hand Development; Springer: Cham, Switzerland, 2014; Volume 95, pp. 219–246. [Google Scholar] [CrossRef]
- Grebenstein, M.; Albu-Schäffer, A.; Bahls, T.; Chalon, M.; Eiberger, O.; Friedl, W.; Gruber, R.; Haddadin, S.; Hagn, U.; Haslinger, R.; et al. The DLR hand arm system. In Proceedings of the 2011 IEEE International Conference on Robotics and Automation, Shanghai, China, 9–13 May 2011; pp. 3175–3182. [Google Scholar] [CrossRef]
- Bridgwater, L.B.; Ihrke, C.A.; Diftler, M.A.; Abdallah, M.E.; Radford, N.A.; Rogers, J.M.; Yayathi, S.; Askew, R.S.; Linn, D.M. The robonaut 2 hand—Designed to do work with tools. In Proceedings of the 2012 IEEE International Conference on Robotics and Automation, Saint Paul, MN, USA, 14–18 May 2012; pp. 3425–3430. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, M.; Sato, R.; Higashihara, T.; Ogasawara, T.; Kawashima, N. Rehand: Realistic electric prosthetic hand created with a 3D printer. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; Volume 40, pp. 2470–2473. [Google Scholar] [CrossRef]
- Heo, P.; Gu, G.M.; Lee, S.j.; Rhee, K.; Kim, J. Current hand exoskeleton technologies for rehabilitation and assistive engineering. Int. J. Precis. Eng. Manuf. 2012, 13, 807–824. [Google Scholar] [CrossRef]
- Iqbal, J.; Khan, H.; Tsagarakis, N.G.; Caldwell, D.G. A novel exoskeleton robotic system for hand rehabilitation—Conceptualization to prototyping. Biocybern. Biomed. Eng. 2014, 34, 79–89. [Google Scholar] [CrossRef]
- Wege, A.; Hommel, G. Development and control of a hand exoskeleton for rehabilitation of hand injuries. In Proceedings of the 2005 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Edmonton, AB, Canada, 2–6 August 2005; pp. 3461–3466. [Google Scholar] [CrossRef]
- Ueki, S.; Kawasaki, H.; Ito, S.; Nishimoto, Y.; Abe, M.; Aoki, T.; Ishigure, Y.; Ojika, T.; Mouri, T. Development of a hand-assist robot with multi-degrees-of-freedom for rehabilitation therapy. IEEE/ASME Trans. Mechatron. 2012, 17, 136–146. [Google Scholar] [CrossRef]
- Shields, B.L.; Main, J.A.; Peterson, S.W.; Strauss, A.M. An anthropomorphic hand exoskeleton to prevent astronaut hand fatigue during extravehicular activities. IEEE Trans. Syst. Man Cybern. Part A Syst. Hum. 1997, 27, 668–673. [Google Scholar] [CrossRef] [PubMed]
- In, H.K.; Cho, K.J.; Kim, K.R.; Lee, B.S. Jointless structure and under-actuation mechanism for compact hand exoskeleton. In Proceedings of the IEEE International Conference on Rehabilitation Robotics, Zurich, Switzerland, 29 June–1 July 2011. [Google Scholar] [CrossRef]
- In, H.K.; Cho, K.J. Evaluation of the antagonistic tendon driven system for SNU Exo-Glove. In Proceedings of the 2012 9th International Conference on Ubiquitous Robots and Ambient Intelligence (URAI 2012), Daejeon, South Korea, 26–28 November 2012; pp. 507–509. [Google Scholar] [CrossRef]
- Cempini, M.; Cortese, M.; Vitiello, N. A powered finger-thumb wearable hand exoskeleton with self-aligning joint axes. IEEE/ASME Trans. Mechatron. 2015, 20, 705–716. [Google Scholar] [CrossRef]
- Baker, M.D.; McDonough, M.K.; McMullin, E.M.; Swift, M.; BuSha, B.F. Orthotic hand-assistive exoskeleton. In Proceedings of the 2011 IEEE 37th Annual Northeast Bioengineering Conference (NEBEC), Troy, NY, USA, 1–3 April 2011. [Google Scholar] [CrossRef]
- Saebo Incorporated. SaeboFlex; Saebo Incorporated: Charlotte, NC, USA, 2020. [Google Scholar]
- Rakib, M.I.; Choudhury, I.A.; Hussain, S.; Osman, N.A.A. Design and biomechanical performance analysis of a user-friendly orthotic device. Mater. Des. (1980–2015) 2015, 65, 716–725. [Google Scholar] [CrossRef]
- The Aluminum Association Inc. . International Alloy Designations and Chemical Composition Limits for Wrought Aluminum and Wrought Aluminum Alloys; Technical Report; Arlington: Quezon City, Philippines, 2006. [Google Scholar]
- Zhang, R.X.; Ni, Q.Q.; Natsuki, T.; Iwamoto, M. Mechanical properties of composites filled with SMA particles and short fibers. Compos. Struct. 2007, 79, 90–96. [Google Scholar] [CrossRef] [Green Version]
- Madden, J.D.W.; Vandesteeg, N.A.; Anquetil, P.A.; Madden, P.G.A.; Takshi, A.; Pytel, R.Z.; Lafontaine, S.R.; Wieringa, P.A.; Hunter, I.W. Artificial Muscle Technology: Physical Principles and Naval Prospects. IEEE J. Ocean. Eng. 2004, 29, 706–728. [Google Scholar] [CrossRef]
- JASZKIEWICZ, A.; BLEDZKI, A.K.; FRANCISZCZAK, P. Improving the mechanical performance of PLA composites with natural, man-made cellulose and glass fibers—A comparison to PP counterparts. Polimery 2013, 58, 435–442. [Google Scholar] [CrossRef]
- Duda, A.; Penczek, S. Polilaktyd [poli(kwas mlekowy)]: Synteza, właściwości i zastosowania. Polimery 2003, 48, 16–27. [Google Scholar] [CrossRef] [Green Version]
- Cantrell, J.T.; Rohde, S.; Damiani, D.; Gurnani, R.; DiSandro, L.; Anton, J.; Young, A.; Jerez, A.; Steinbach, D.; Kroese, C.; et al. Experimental characterization of the mechanical properties of 3D-printed ABS and polycarbonate parts. Rapid Prototyp. J. 2017, 23, 811–824. [Google Scholar] [CrossRef]
- Artemenko, S.E.; Kadykova, Y.A. Polymer composite materials based on carbon, basalt, and glass fibres. Fibre Chem. 2008, 40, 30–32. [Google Scholar] [CrossRef]
- Concilio, A.; Lecce, L. Shape Memory Alloy Engineering: For Aerospace, Structural and Biomedical Applications; Elsevier: Oxford, UK, 2014; p. 448. [Google Scholar]
- Gupta, A.; O’Malley, M.K. Design of a haptic arm exoskeleton for training and rehabilitation. IEEE/ASME Trans. Mechatron. 2006, 11, 280–289. [Google Scholar] [CrossRef]
- Baronio, G.; Harran, S.; Signoroni, A. A Critical Analysis of a Hand Orthosis Reverse Engineering and 3D Printing Process. Appl. Bionics Biomech. 2016, 2016, 8347478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, L.; Phan, A.; Allison, G. Design and fabrication of a three dimensional printable non-assembly articulated hand exoskeleton for rehabilitation. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 4627–4630. [Google Scholar]
- Bataller, A.; Cabrera, J.A.; Clavijo, M.; Castillo, J.J. Evolutionary synthesis of mechanisms applied to the design of an exoskeleton for finger rehabilitation. Mech. Mach. Theory 2016, 105, 31–43. [Google Scholar] [CrossRef]
- Agarwal, P.; Neptune, R.R.; Deshpande, A.D. A Simulation Framework for Virtual Prototyping of Robotic Exoskeletons. J. Biomech. Eng. 2016, 138, 61004. [Google Scholar] [CrossRef] [PubMed]
- Conti, R.; Meli, E.; Ridolfi, A. A novel kinematic architecture for portable hand exoskeletons. Mechatronics 2016, 35, 192–207. [Google Scholar] [CrossRef]
- Zuniga, J.; Katsavelis, D.; Peck, J.; Stollberg, J.; Petrykowski, M.; Carson, A.; Fernandez, C. Cyborg beast: A low-cost 3d-printed prosthetic hand for children with upper-limb differences. BMC Res. Notes 2015, 8, 10. [Google Scholar] [CrossRef] [Green Version]
- Silva, K.; Rand, S.; Cancel, D.; Chen, Y.; Kathirithamby, R.; Stern, M. Three-Dimensional (3-D) Printing: A Cost-Effective Solution for Improving Global Accessibility to Prostheses. PM&R 2015, 7, 1312–1314. [Google Scholar]
- Gopura, R.; Bandara, D.S.V.; Kiguchi, K.; Mann, G.K.I. Developments in hardware systems of active upper-limb exoskeleton robots: A review. Robot. Auton. Syst. 2016, 75, 203–220. [Google Scholar] [CrossRef]
- Veale, A.J.; Xie, S.Q. Towards compliant and wearable robotic orthoses: A review of current and emerging actuator technologies. Med. Eng. Phys. 2016, 38, 317–325. [Google Scholar] [CrossRef]
- Kalantari, O.; Ghaffari, A.S. Prototype Construction of the Wearable Soft Orthotic Exoskeleton for Upper Limb Rehabilitation of Post-Stroke Patients. J. Life Sci. Technol. 2016. [Google Scholar] [CrossRef]
- Bogue, R. Robotic exoskeletons: A review of recent progress. Ind. Robot Int. J. 2015, 42, 5–10. [Google Scholar] [CrossRef]
- Stewart, A.M.; Pretty, C.G.; Adams, M.; Chen, X. Review of Upper Limb Hybrid Exoskeletons. IFAC-PapersOnLine 2017, 50, 15169–15178. [Google Scholar] [CrossRef]
- Shahid, T.; Gouwanda, D.; Nurzaman, S.G.; Gopalai, A.A. Moving toward Soft Robotics: A Decade Review of the Design of Hand Exoskeletons. Biomimetics 2018, 3, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirvakili, S.M.; Hunter, I.W. Artificial Muscles: Mechanisms, Applications, and Challenges. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef] [PubMed]
- López-Larraz, E.; Trincado-Alonso, F.; Rajasekaran, V.; Pérez-Nombela, S.; Del-Ama, A.J.; Aranda, J.; Minguez, J.; Gil-Agudo, A.; Montesano, L. Control of an Ambulatory Exoskeleton with a Brain–Machine Interface for Spinal Cord Injury Gait Rehabilitation. Front. Neurosci. 2016, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.T.; Cheng, H.; Rui, H.; Lin, X.; Duong, M.K.; Chen, Q. Evaluation of a Fuzzy-Based Impedance Control Strategy on a Powered Lower Exoskeleton. Int. J. Soc. Robot. 2016, 8, 103–123. [Google Scholar] [CrossRef]
- Guan, X.; Ji, L.; Wang, R. Development of Exoskeletons and Applications on Rehabilitation. MATEC Web Conf. 2016, 40, 2004. [Google Scholar] [CrossRef] [Green Version]
- Yun, D.; Khan, A.M.; Yan, R.J.; Ji, Y.; Jang, H.; Iqbal, J.; Zuhaib, K.M.; Ahn, J.Y.; Han, J.; Han, C. Handling subject arm uncertainties for upper limb rehabilitation robot using robust sliding mode control. Int. J. Precis. Eng. Manuf. 2016, 17, 355–362. [Google Scholar] [CrossRef]
- Sergi, F.; Erwin, A.C.; OMalley, M.K. Interaction Control Capabilities of an MR-Compatible Compliant Actuator for Wrist Sensorimotor Protocols During fMRI. IEEE/ASME Trans. Mechatron. 2015, 20, 2678–2690. [Google Scholar] [CrossRef]
- Ates, S.; Mora-Moreno, I.; Wessels, M.; Stienen, A.H.A. Combined active wrist and hand orthosis for home use: Lessons learned. In Proceedings of the 2015 IEEE International Conference on Rehabilitation Robotics (ICORR), Singapore, 11–14 August 2015; pp. 398–403. [Google Scholar]
- Ripel, T.; Krejsa, J.; Hrbacek, J.; Cizmar, I. Active Elbow Orthosis. Int. J. Adv. Robot. Syst. 2014, 11, 1. [Google Scholar] [CrossRef] [Green Version]
- Bancud, K.; Kutilek, P.; Krivanek, V. Design of powered wearable elbow brace for rehabilitation applications at clinic and home. In Proceedings of the 2019 European Conference on Mobile Robots (ECMR), Prague, Czech Republic, 4–6 September 2019. [Google Scholar] [CrossRef]
- Ben, I.A.; Bouteraa, Y.; Rekik, C. Design and development of 3D printed myoelectric robotic exoskeleton for hand rehabilitation. Int. J. Smart Sens. Intell. Syst. 2017, 10, 341–366. [Google Scholar] [CrossRef] [Green Version]
- Manian, Y.; Modi, S.; Chandak, T.; Gupta, S.; Sheeba, P.S. Exoskeleton Arm with Pneumatic Muscle Actuation. Int. J. Adv. Eng. Innov. Technol. (IJAEIT) 2014, 4–10. [Google Scholar]
- Groenhuis, V.; Chandrapal, M.; Stramigioli, S.; Chen, X. Controlling pneumatic artificial muscles in exoskeletons with surface electromyography. In Proceedings of the 14th Mechatronics Forum International Conference (Mechatronics 2014), Karlstad, Sweden, 6–18 June 2014; pp. 451–457. [Google Scholar]
- Ba, D.X.; Dinh, T.Q.; Ahn, K.K. An Integrated Intelligent Nonlinear Control Method for a Pneumatic Artificial Muscle. IEEE/ASME Trans. Mechatron. 2016, 21, 1835–1845. [Google Scholar] [CrossRef]
- Andrikopoulos, G.; Nikolakopoulos, G.; Manesis, S. Advanced Nonlinear PID-Based Antagonistic Control for Pneumatic Muscle Actuators. IEEE Trans. Ind. Electron. 2014, 61, 6926–6937. [Google Scholar] [CrossRef]
- Williams, M.R.; Walter, W. Development of a prototype over-actuated biomimetic prosthetic hand. PLoS ONE 2015, 10, e0118817. [Google Scholar] [CrossRef]
- FESTO. Fluidic Muscle DMSP/MAS; FESTO: Esslingen, Germany, 2017. [Google Scholar]
- Schabowsky, C.N.; Godfrey, S.B.; Holley, R.J.; Lum, P.S. Development and pilot testing of HEXORR: Hand EXOskeleton rehabilitation robot. J. NeuroEng. Rehabil. 2010, 7, 36. [Google Scholar] [CrossRef] [Green Version]
- Edwards, R.; Lanfontant, K.; Sujumnong, N.; Wormley, J. Vision-Based Intelligent Prosthetic Robotic Arm; Technical Report; Worcester Polytechnic Institute: Worcester, MA, USA, 2009. [Google Scholar]
- Zhang, J.; Yin, Y. SMA-based bionic integration design of self-sensor-actuator-structure for artificial skeletal muscle. Sens. Actuators A Phys. 2012, 181, 94–102. [Google Scholar] [CrossRef]
- Zhao, H.; Jalving, J.; Huang, R.; Knepper, R.; Ruina, A.; Shepherd, R. A Helping Hand: Soft Orthosis with Integrated Optical Strain Sensors and EMG Control. IEEE Robot. Autom. Mag. 2016, 23, 55–64. [Google Scholar] [CrossRef]
- Wang, T.; Farajollahi, M.; Choi, Y.S.; Lin, I.T.; Marshall, J.E.; Thompson, N.M.; Kar-Narayan, S.; Madden, J.D.W.; Smoukov, S.K. Electroactive polymers for sensing. Interface Focus 2016, 6, 20160026. [Google Scholar] [CrossRef]
- Benslimane, M.Y.; Kiil, H.E.; Tryson, M.J. Dielectric electro-active polymer push actuators: Performance and challenges. Polym. Int. 2010, 59, 415–421. [Google Scholar] [CrossRef]
- Brochu, P.; Pei, Q. Advances in Dielectric Elastomers for Actuators and Artificial Muscles. Macromol. Rapid Commun. 2010, 31, 10–36. [Google Scholar] [CrossRef] [PubMed]
- Sinha, N.; Yeow, J.W. Carbon Nanotubes for Biomedical Applications. IEEE Trans. Nanobiosci. 2005, 4, 180–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unluhisarcikli, O.; Weinberg, B.; Sivak, M.; Mirelman, A.; Bonato, P.; Mavroidis, C. A robotic hand rehabilitation system with interactive gaming using novel Electro-Rheological Fluid based actuators. In Proceedings of the 2010 IEEE International Conference on Robotics and Automation, Anchorage, AK, USA, 3–7 May 2010; pp. 1846–1851. [Google Scholar]
- Winter, S.H.; Bouzit, M. Use of Magnetorheological Fluid in a Force Feedback Glove. IEEE Trans. Neural Syst. Rehabil. Eng. 2007, 15, 2–8. [Google Scholar] [CrossRef]
- Rocon, E.; Belda-Lois, J.M.; Ruiz, A.F.; Manto, M.; Moreno, J.C.; Pons, J.L. Design and Validation of a Rehabilitation Robotic Exoskeleton for Tremor Assessment and Suppression. IEEE Trans. Neural Syst. Rehabil. Eng. 2007, 15, 367–378. [Google Scholar] [CrossRef] [Green Version]
- Tai, K.; El-Sayed, A.R.; Shahriari, M.; Biglarbegian, M.; Mahmud, S. State of the Art Robotic Grippers and Applications. Robotics 2016, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Kim, K. A novel method of manufacturing three-dimensional ionic polymer–metal composites (IPMCs) biomimetic sensors, actuators and artificial muscles. Polymer 2002, 43, 797–802. [Google Scholar] [CrossRef]
- Gendron, D.; Bubak, G.; Ceseracciu, L.; Ricciardella, F.; Ansaldo, A.; Ricci, D. Significant strain and force improvements of single-walled carbon nanotube actuator: A metal chalcogenides approach. Sens. Actuators B Chem. 2016, 230, 673–683. [Google Scholar] [CrossRef]
- Yu, M.F. Strength and Breaking Mechanism of Multiwalled Carbon Nanotubes Under Tensile Load. Science 2000, 287, 637–640. [Google Scholar] [CrossRef] [Green Version]
- Cura, V.O.D.; Cunha, F.L.; Aguiar, M.L.; Cliquet, A., Jr. Study of the Different Types of Actuators and Mechanisms for Upper Limb Prostheses. Artif. Organs 2003, 27, 507–516. [Google Scholar] [CrossRef]
- Lee, H.; Kim, W.; Han, J.; Han, C. The technical trend of the exoskeleton robot system for human power assistance. Int. J. Precis. Eng. Manuf. 2012, 13, 1491–1497. [Google Scholar] [CrossRef]
- Kumar, S.; Wöhrle, H.; Trampler, M.; Simnofske, M.; Peters, H.; Mallwitz, M.; Kirchner, E.; Kirchner, F. Modular Design and Decentralized Control of the Recupera Exoskeleton for Stroke Rehabilitation. Appl. Sci. 2019, 9, 626. [Google Scholar] [CrossRef] [Green Version]
- Wege, A.; Kondak, K.; Hommel, G. Mechanical design and motion control of a hand exoskeleton for rehabilitation. In Proceedings of the 2005 IEEE International Conference Mechatronics and Automation, Niagara Falls, ON, Canada, 29 July–1 August 2005; Volume 1, pp. 155–159. [Google Scholar]
- Xu, K.; Zhao, J.; Qiu, D.; Wang, Y. A Pilot Study of a Continuum Shoulder Exoskeleton for Anatomy Adaptive Assistances. J. Mech. Robot. 2014, 6, 41011. [Google Scholar] [CrossRef]
- Edin, B.B.; Ascari, L.; Beccai, L.; Roccella, S.; Cabibihan, J.J.; Carrozza, M.C. Bio-inspired sensorization of a biomechatronic robot hand for the grasp-and-lift task. Brain Res. Bull. 2008, 75, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Zha, F.; Sheng, W.; Guo, W.; Qiu, S.; Wang, X.; Chen, F.; Zha, F.; Sheng, W.; Guo, W.; Qiu, S.; et al. The Exoskeleton Balance Assistance Control Strategy Based on Single Step Balance Assessment. Appl. Sci. 2019, 9, 884. [Google Scholar] [CrossRef] [Green Version]
- Mancisidor, A.; Zubizarreta, A.; Cabanes, I.; Portillo, E.; Jung, J. Virtual Sensors for Advanced Controllers in Rehabilitation Robotics. Sensors 2018, 18, 785. [Google Scholar] [CrossRef] [Green Version]
- Tjahyono, A.P.; Aw, K.C.; Devaraj, H.; Surendra, W.; Haemmerle, E.; Travas-Sejdic, J. A five-fingered hand exoskeleton driven by pneumatic artificial muscles with novel polypyrrole sensors. Ind. Robot Int. J. 2013, 40, 251–260. [Google Scholar] [CrossRef]
- Kazerooni, H. The human power amplifier technology at the University of California, Berkeley. Robot. Auton. Syst. 1996, 19, 179–187. [Google Scholar] [CrossRef]
- Proietti, T.; Crocher, V.; Roby-Brami, A.; Jarrasse, N. Upper-Limb Robotic Exoskeletons for Neurorehabilitation: A Review on Control Strategies. IEEE Rev. Biomed. Eng. 2016, 9, 4–14. [Google Scholar] [CrossRef] [Green Version]
- Sakurada, T.; Kawase, T.; Takano, K.; Komatsu, T.; Kansaku, K. A BMI-based occupational therapy assist suit: Asynchronous control by SSVEP. Front. Neurosci. 2013, 7, 172. [Google Scholar] [CrossRef] [Green Version]
- Frisoli, A.; Loconsole, C.; Leonardis, D.; Banno, F.; Barsotti, M.; Chisari, C.; Bergamasco, M. A New Gaze-BCI-Driven Control of an Upper Limb Exoskeleton for Rehabilitation in Real-World Tasks. IEEE Trans. Syst. Man Cybern. Part C (Appl. Rev.) 2012, 42, 1169–1179. [Google Scholar] [CrossRef]
- Barsotti, M.; Leonardis, D.; Loconsole, C.; Solazzi, M.; Sotgiu, E.; Procopio, C.; Chisari, C.; Bergamasco, M.; Frisoli, A. A full upper limb robotic exoskeleton for reaching and grasping rehabilitation triggered by MI-BCI. In Proceedings of the 2015 IEEE International Conference on Rehabilitation Robotics (ICORR), Singapore, 11–14 August 2015; pp. 49–54. [Google Scholar]
- Brauchle, D.; Vukelić, M.; Bauer, R.; Gharabaghi, A. Brain state-dependent robotic reaching movement with a multi-joint arm exoskeleton: Combining brain-machine interfacing and robotic rehabilitation. Front. Hum. Neurosci. 2015, 9, 564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas, L.; Dicicco, M.; Matsuoka, Y. An EMG-Controlled Hand Exoskeleton for Natural Pinching. J. Robot. Mechatron. 2004, 16, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Leonardis, D.; Barsotti, M.; Loconsole, C.; Solazzi, M.; Troncossi, M.; Mazzotti, C.; Castelli, V.P.; Procopio, C.; Lamola, G.; Chisari, C.; et al. An EMG-controlled robotic hand exoskeleton for bilateral rehabilitation. IEEE Trans. Haptics 2015, 8, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Palkowski, A.; Redlarski, G.; Rzyman, G.; Krawczuk, M. Basic evaluation of limb exercises based on electromyography and classification methods. In Proceedings of the 2018 International Interdisciplinary PhD Workshop (IIPhDW), Swinoujscie, Poland, 9–12 May 2018; pp. 323–325. [Google Scholar]
- Palkowski, A.; Redlarski, G. Basic Hand Gestures Classification Based on Surface Electromyography. Comput. Math. Methods Med. 2016, 2016, 6481282. [Google Scholar] [CrossRef] [Green Version]
- Biagetti, G.; Crippa, P.; Curzi, A.; Orcioni, S.; Turchetti, C. Analysis of the EMG Signal During Cyclic Movements Using Multicomponent AM–FM Decomposition. IEEE J. Biomed. Health Inform. 2015, 19, 1672–1681. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, Y.; Zhu, Y.; Zhao, J. Estimation of pathological tremor from recorded signals based on adaptive sliding fast Fourier transform. Adv. Mech. Eng. 2016, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Gradolewski, D.; Tojza, P.M.; Jaworski, J.; Ambroziak, D.; Redlarski, G.; Krawczuk, M. Arm EMG Wavelet-Based Denoising System. In Advances in Intelligent Systems and Computing; Springer: Cham, Switzerland, 2015; Volume 317, pp. 289–296. [Google Scholar]
- Majdalawieh, O.; Gu, J.; Bai, T.; Cheng, G. Biomedical signal processing and rehabilitation engineering: A review. In Proceedings of the 2003 IEEE Pacific Rim Conference on Communications Computers and Signal Processing (PACRIM 2003) (Cat. No.03CH37490), Victoria, BC, Canada, 28–30 August 2003; Volume 2, pp. 1004–1007. [Google Scholar]
- Lotte, F.; Bougrain, L.; Cichocki, A.; Clerc, M.; Congedo, M.; Rakotomamonjy, A.; Yger, F. A review of classification algorithms for EEG-based brain–computer interfaces: A 10 year update. J. Neural Eng. 2018, 15, 31005. [Google Scholar] [CrossRef] [Green Version]
- Cantillo-Negrete, J.; Carino-Escobar, R.; Elias-Vinas, D.; Gutierrez-Martinez, J. Control signal for a mechatronic hand orthosis aimed for neurorehabilitation. In Proceedings of the 2015 Pan American Health Care Exchanges (PAHCE), Vina del Mar, Chile, 23–28 March 2015; pp. 1–4. [Google Scholar]
- Gao, L.; Cheng, W.; Zhang, J.; Wang, J. EEG classification for motor imagery and resting state in BCI applications using multi-class Adaboost extreme learning machine. Rev. Sci. Instrum. 2016, 87, 85110. [Google Scholar] [CrossRef]
- Bhagat, N.A.; Venkatakrishnan, A.; Abibullaev, B.; Artz, E.J.; Yozbatiran, N.; Blank, A.A.; French, J.; Karmonik, C.; Grossman, R.G.; O’Malley, M.K.; et al. Design and Optimization of an EEG-Based Brain Machine Interface (BMI) to an Upper-Limb Exoskeleton for Stroke Survivors. Front. Neurosci. 2016, 10, 122. [Google Scholar] [CrossRef]
- Wolpaw, J.R.; Birbaumer, N.; McFarland, D.J.; Pfurtscheller, G.; Vaughan, T.M. Brain-computer interfaces for communication and control. Clin. Neurophysiol. 2002, 113, 767–791. [Google Scholar] [CrossRef]
- Amiri, S.; Fazel-Rezai, R.; Asadpour, V. A Review of Hybrid Brain-Computer Interface Systems. Adv. Hum. Comput. Interact. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Siebert, J.; Poliński, A. Badania modelowe zastosowania pletyzmografii impedancyjnej do badania przepływu krwi w kończynach. Folia Cardiol. 1999, 6, 417–422. [Google Scholar]
- Gradolewski, D.; Redlarski, G. Wavelet-based denoising method for real phonocardiography signal recorded by mobile devices in noisy environment. Comput. Biol. Med. 2014, 52, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Redlarski, G.; Gradolewski, D.; Palkowski, A. A System for Heart Sounds Classification. PLoS ONE 2014, 9, e112673. [Google Scholar] [CrossRef] [PubMed]
- Jagodnik, K.M.; Thomas, P.S.; van den Bogert, A.J.; Branicky, M.S.; Kirsch, R.F. Human-Like Rewards to Train a Reinforcement Learning Controller for Planar Arm Movement. IEEE Trans. Hum. Mach. Syst. 2016, 46, 723–733. [Google Scholar] [CrossRef] [Green Version]
- Celadon, N.; Došen, S.; Binder, I.; Ariano, P.; Farina, D. Proportional estimation of finger movements from high-density surface electromyography. J. NeuroEng. Rehabil. 2016, 13, 73. [Google Scholar] [CrossRef] [Green Version]
- Rzyman, G.; Redlarski, G.; Krawczuk, M. Komputerowo wspomagana klasyfikacja wybranych sygnałów elektromiografii powierzchniowej. Model. Inż. 2017, 62, 81–87. [Google Scholar]
- Subasi, A. Classification of EMG signals using combined features and soft computing techniques. Appl. Soft Comput. 2012, 12, 2188–2198. [Google Scholar] [CrossRef]
- Mohammad, T.K.; Hasan, M.T. Comparison between kNN and SVM for EMG Signal Classification. Int. J. Recent Innov. Trends Comput. Commun. (IJRITCC) 2015, 3, 6799–6801. [Google Scholar]
- Gokgoz, E.; Subasi, A. Comparison of decision tree algorithms for EMG signal classification using DWT. Biomed. Signal Process. Control 2015, 18, 138–144. [Google Scholar] [CrossRef]
- Rzyman, G.; Redlarski, G.; Palkowski, A.; Tojza, P.M.; Krawczuk, M.; Siebert, J. Computing methods for fast and precise body surface area estimation of selected body parts. In Proceedings of the 2018 International Interdisciplinary PhD Workshop (IIPhDW), Swinoujście, Poland, 9–12 May 2018; pp. 316–318. [Google Scholar]
- Li, C.; Rusák, Z.; Horváth, I.; Ji, L. Development of engagement evaluation method and learning mechanism in an engagement enhancing rehabilitation system. Eng. Appl. Artif. Intell. 2016, 51, 182–190. [Google Scholar] [CrossRef]
- Chestek, C.A.; Gilja, V.; Blabe, C.H.; Foster, B.L.; Shenoy, K.V.; Parvizi, J.; Henderson, J.M. Hand posture classification using electrocorticography signals in the gamma band over human sensorimotor brain areas. J. Neural Eng. 2013, 10, 026002. [Google Scholar] [CrossRef] [PubMed]
- Pazzaglia, C.; Imbimbo, I.; Tranchita, E.; Minganti, C.; Ricciardi, D.; Lo Monaco, R.; Parisi, A.; Padua, L. Comparison of virtual reality rehabilitation and conventional rehabilitation in Parkinson’s disease: A randomised controlled trial. Physiotherapy 2020, 106, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Jack, D.; Boian, R.; Merians, A.; Tremaine, M.; Burdea, G.; Adamovich, S.; Recce, M.; Poizner, H. Virtual reality-enhanced stroke rehabilitation. IEEE Trans. Neural Syst. Rehabil. Eng. 2001, 9, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Sveistrup, H. Motor rehabilitation using virtual reality. J. NeuroEng. Rehabil. 2004, 1, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majid, M.S.H.; Khairunizam, W.; Ikram, K.; Jing, L.M.; Sahyudi, B.N.; Zunaidi, I.; Ariffin, M.A.; Bakar, A.S.; Razlan, Z.M. Performance evaluation of a VR-based arm rehabilitation using movement sequence pattern. In Proceedings of the 2018 IEEE 14th International Colloquium on Signal Processing & Its Applications (CSPA), Batu Feringghi, Malaysia, 9–10 March 2018; pp. 123–128. [CrossRef]
- Maier, M.; Rubio Ballester, B.; Duff, A.; Duarte Oller, E.; Verschure, P.F.M.J. Effect of Specific Over Nonspecific VR-Based Rehabilitation on Poststroke Motor Recovery: A Systematic Meta-analysis. Neurorehabil. Neural Repair 2019, 33, 112–129. [Google Scholar] [CrossRef] [PubMed]
- Laver, K.E.; Lange, B.; George, S.; Deutsch, J.E.; Saposnik, G.; Crotty, M. Virtual reality for stroke rehabilitation. Cochrane Database Syst. Rev. 2017, 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Tensile Strength (MPa) | Yield Strength (MPa) | Density (kg/m3) | Processing Difficulty | Price (USD/kg) * | References | |
|---|---|---|---|---|---|---|
| Stainless steel | 500–700 | 200 | 8000 | Medium | 25 | [50] |
| Aluminium alloy | 510–540 | 430–480 | 2810 | Difficult | 15 | [68,69] |
| PLA | 800 | 70–100 | 900–1500 | Easy | 2 | [72,73] |
| SMA | 1000 | 200 | 6500 | Difficult | 100 | [70,71,76] |
| Carbon fibre | 2800–5000 | 840 | 1600–2000 | Very difficult | 25 | [75] |
| Sensor | Main Advantage | Main Drawback | Usefulness in Bionic Orthoses |
|---|---|---|---|
| Touch sensor | Feedback improvement | Complex | Optional |
| Forcetorque sensor | Could be estimated by the current | Difficulties in measurement in dynamic conditions | Yes |
| Encoder | Simplicity | Relatively big | Yes |
| Accelerometer | Versatility | Limited accuracy of determining device orientation | Yes |
| Inclinometer | Simple posture control | Usefull in specific conditions | Optional |
| Gyroscope | Precision | Requires additional electronics | Yes |
| Distance sensor | Protection against breakage | Need to use several sensors | Optional |
| Camera for shape recognition | Increases rehabilitation efficiency | Expensive | Optional |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rzyman, G.; Szkopek, J.; Redlarski, G.; Palkowski, A. Upper Limb Bionic Orthoses: General Overview and Forecasting Changes. Appl. Sci. 2020, 10, 5323. https://doi.org/10.3390/app10155323
Rzyman G, Szkopek J, Redlarski G, Palkowski A. Upper Limb Bionic Orthoses: General Overview and Forecasting Changes. Applied Sciences. 2020; 10(15):5323. https://doi.org/10.3390/app10155323
Chicago/Turabian StyleRzyman, Gustaw, Jacek Szkopek, Grzegorz Redlarski, and Aleksander Palkowski. 2020. "Upper Limb Bionic Orthoses: General Overview and Forecasting Changes" Applied Sciences 10, no. 15: 5323. https://doi.org/10.3390/app10155323
APA StyleRzyman, G., Szkopek, J., Redlarski, G., & Palkowski, A. (2020). Upper Limb Bionic Orthoses: General Overview and Forecasting Changes. Applied Sciences, 10(15), 5323. https://doi.org/10.3390/app10155323