Bioactive Healing Abutment as a Potential Tool for the Treatment of Peri-Implant Disease—In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
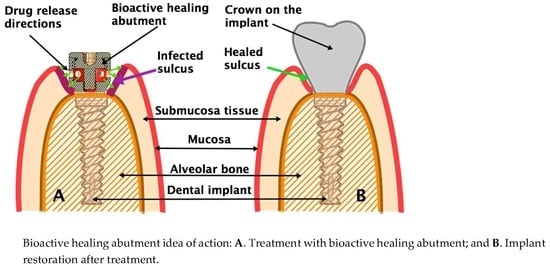
2.1. Bioactive Healing Abutment
2.2. Bacterial Strains
2.3. Reagents
2.4. Medium and Bacterial Culture Preparation
2.4.1. Disk Diffusion Assay
2.4.2. Antimicrobial Effect of Bioactive Healing Abutment
2.4.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wychowański, P.; Woliński, J.; Kacprzak, M.; Tomkiewicz, W.; Bartlomiej, I.; Szubińska-Lelonkiewicz, D.; Wojtowicz, A.; Nevins, M. Immediate palatal molar implants: A simple, safe, minimally invasive technique. Int. J. Periodontics Restor. Dent. 2017, 37, e297–e301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wychowanski, P.; Starzynska, A.; Wolinski, J.; Ksieradzki, M.; Fiedor, P. New Surgical Technique Using Xenograft as a Microinvasive Method to Avoid Extensive Bone Reconstruction in Patients With Compromised General Health: Promising Surgical Methodology and First Clinical Results. Transplant. Proc. 2020. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Siles, M.; Muñoz-Cámara, D.; Salazar-Sánchez, N.; Ballester-Ferrandis, J.; Camacho-Alonso, F. Incidence of peri-implantitis and oral quality of life in patients rehabilitated with implants with different neck designs: A 10-year retrospective study. J. Cranio Maxillofac. Surg. 2015, 43, 2168–2174. [Google Scholar] [CrossRef]
- Albrektsson, T.; Isidor, F. Concensus report of session IV. In Proceedings of the 1st European Workshop on Peri-Odontology; Lang, N.P., Karring, T., Eds.; Quintessencep: London, UK, 1994; pp. 365–369. [Google Scholar]
- Smeets, R.; Henningsen, A.; Jung, O.; Heiland, M.; Hammächer, C.; Stein, J.M. Definition, etiology, prevention and treatment of peri-implantitis—A review. Head Face Med. 2014, 10, 34. [Google Scholar] [CrossRef] [Green Version]
- Botero, J.E.; Gonzalez, A.M.; Mercado, R.A.; Olave, G.; Contreras, A. Subgingival Microbiota in Peri-Implant Mucosa Lesions and Adjacent Teeth in Partially Edentulous Patients. J. Periodontol. 2005, 76, 1490–1495. [Google Scholar] [CrossRef]
- Hultin, M.; Gustafsson, A.; Hallström, H.; Johansson, L.Å.; Ekfeldt, A.; Klinge, B. Microbiological findings and host response in patients with peri-implantitis. Clin. Oral Implant. Res. 2002, 13, 349–358. [Google Scholar] [CrossRef]
- Shibli, J.A.; Melo, L.; Ferrari, D.S.; Figueiredo, L.C.; Faveri, M.; Feres, M. Composition of supra- and subgingival biofilm of subjects with healthy and diseased implants. Clin. Oral Implant. Res. 2008, 19, 975–982. [Google Scholar] [CrossRef]
- Mombelli, A.; Décaillet, F. The characteristics of biofilms in peri-implant disease. J. Clin. Periodontol. 2011, 38, 203–213. [Google Scholar] [CrossRef] [Green Version]
- Persson, G.R.; Samuelsson, E.; Lindahl, C.; Renvert, S. Mechanical non-surgical treatment of peri-implantitis: A single-blinded randomized longitudinal clinical study. II. Microbiological results. J. Clin. Periodontol. 2010, 37, 563–573. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Speziale, P.; Montanaro, L.; Costerton, J.W. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials 2012, 33, 5967–5982. [Google Scholar] [CrossRef]
- Salvi, G.E.; Fürst, M.M.; Lang, N.P.; Persson, G.R. One-year bacterial colonization patterns of Staphylococcus aureus and other bacteria at implants and adjacent teeth. Clin. Oral Implant. Res. 2008, 19, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Carcuac, O.; Derks, J.; Charalampakis, G.; Abrahamsson, I.; Wennström, J.; Berglundh, T. Adjunctive Systemic and Local Antimicrobial Therapy in the Surgical Treatment of Peri-implantitis: A Randomized Controlled Clinical Trial. J. Dent. Res. 2016, 95, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Grusovin, M.G.; Worthington, H.V. Treatment of peri-implantitis: What interventions are effective? A Cochrane systematic review. Eur. J. Oral Implantol. 2012, 5, S21–S41. [Google Scholar] [PubMed]
- Romanos, G.E.; Weitz, D. Therapy of periimplant diseases. Where is the evidence? J. Evid. Based Dent. Pract. 2012, 12, 204–208. [Google Scholar] [CrossRef]
- Nguyen-Hieu, T.; Borghetti, A.; Aboudharam, G. Peri-implantitis: From diagnosis to therapeutics. J. Investig. Clin. Dent. 2012, 3, 79–94. [Google Scholar] [CrossRef]
- Renvert, S.; Samuelsson, E.; Lindahl, C.; Persson, G.R. Mechanical non-surgical treatment of peri-implantitis: A double-blind randomized longitudinal clinical study. I: Clinical results. J. Clin. Periodontol. 2009, 36, 604–609. [Google Scholar] [CrossRef]
- Tastepe, C.S.; Van Waas, R.; Liu, Y.; Wismeijer, D. Air powder abrasive treatment as an implant surface cleaning method: A literature review. Int. J. Oral Maxillofac. Implant. 2012, 27, 1461–1473. [Google Scholar]
- Lerario, F.; Roncati, M.; Gariffo, A.; Attorresi, E.; Lucchese, A.; Galanakis, A.; Palaia, G.; Romeo, U. Non-surgical periodontal treatment of peri-implant diseases with the adjunctive use of diode laser: Preliminary clinical study. Lasers Med. Sci. 2015, 31, 1–6. [Google Scholar] [CrossRef]
- Al Amri, M.; Kellesarian, S.V.; Ahmed, A.; Al-Kheraif, A.A.; Romanos, G.E.; Javed, F. Efficacy of periimplant mechanical debridement with and without adjunct antimicrobial photodynamic therapy in patients with type 2 diabetes mellitus. Photodiagnosis Photodyn. Ther. 2016, 14, 166–169. [Google Scholar] [CrossRef]
- Verdugo, F.; Laksmana, T.; Uribarri, A.; Information, P.E.K.F.C. Systemic antibiotics and the risk of superinfection in peri-implantitis. Arch. Oral Boil. 2016, 64, 39–50. [Google Scholar] [CrossRef]
- Sullivan, Å.; Edlund, C.; Nord, C.E. Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect. Dis. 2001, 1, 101–114. [Google Scholar] [CrossRef]
- Rashid, M.U.; Weintraub, A.; Nord, C.E. Effect of new antimicrobial agents on the ecological balance of human microflora. Anaerobe 2012, 18, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Steinebrunner, L.; Wolfart, S.; Bossmann, K.; Kern, M. In vitro evaluation of bacterial leakage along the implant-abutment interface of different implant systems. Int. J. Oral Maxillofac. Implant. 2005, 20, 875–881. [Google Scholar]
- Renvert, S.; Lessem, J.; Dahlén, G.; Renvert, H.; Lindahl, C. Mechanical and repeated antimicrobial therapy using a local drug delivery system in the treatment of peri-implantitis: A randomized clinical trial. J. Periodontol. 2008, 79, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Roos-Jansåker, A.-M.; Persson, G.R.; Lindahl, C.; Renvert, S. Surgical treatment of peri-implantitis using a bone substitute with or without a resorbable membrane: A 5-year follow-up. J. Clin. Periodontol. 2014, 41, 1108–1114. [Google Scholar] [CrossRef]
- Helovuo, H.; Hakkarainen, K.; Paunio, K. Changes in the prevalence of subgingival enteric rods, staphylococci and yeasts after treatment with penicillin and erythromycin. Oral Microbiol. Immunol. 1993, 8, 75–79. [Google Scholar] [CrossRef]
- Bottino, M.C.; Munchow, E.; Albuquerque, M.T.P.; Kamocki, K.; Shahi, R.; Gregory, R.L.; Chu, T.-M.G.; Pankajakshan, D. Tetracycline-incorporated polymer nanofibers as a potential dental implant surface modifier. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 105, 2085–2092. [Google Scholar] [CrossRef]
- Sharma, S.; Bano, S.; Ghosh, A.S.; Mandal, M.; Kim, H.-W.; Dey, T.; Kundu, S.C. Silk fibroin nanoparticles support in vitro sustained antibiotic release and osteogenesis on titanium surface. Nanomed. Nanotechnol. Boil. Med. 2016, 12, 1193–1204. [Google Scholar] [CrossRef]
- Zhong, X.; Song, Y.; Yang, P.; Wang, Y.; Jiang, S.; Zhang, X.; Li, C. Titanium Surface Priming with Phase-Transited Lysozyme to Establish a Silver Nanoparticle-Loaded Chitosan/Hyaluronic Acid Antibacterial Multilayer via Layer-by-Layer Self-Assembly. PLoS ONE 2016, 11, e0146957. [Google Scholar] [CrossRef]
- Adams, C.S.; Antoci, V.; Harrison, G.; Patal, P.; Freeman, T.; Shapiro, I.M.; Parvizi, J.; Hickok, N.J.; Radin, S.; Ducheyne, P. Controlled release of vancomycin from thin sol-gel films on implant surfaces successfully controls osteomyelitis. J. Orthop. Res. 2009, 27, 701–709. [Google Scholar] [CrossRef]
- Xing, R.; Witsø, I.L.; Jugowiec, D.; Tiainen, H.; Shabestari, M.; Lyngstadaas, S.P.; Lönn-Stensrud, J.; Haugen, H.J. Antibacterial effect of doxycycline-coated dental abutment surfaces. Biomed. Mater. 2015, 10, 55003. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Liqi, Z.; Jian, L.; Qinghua, Y.; Qian, Y. Doxycycline Induces Mitophagy and Suppresses Production of Interferon-β in IPEC-J2 Cells. Front. Cell. Infect. Microbiol. 2017, 7, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arcos, D.; Vallet-Regí, M. Bioceramics for drug delivery. Acta Mater. 2013, 61, 890–911. [Google Scholar] [CrossRef]
- Parent, M.; Magnaudeix, A.; Delebassée, S.; Sarre, E.; Damia, C.; Trecant, M.V.; Champion, E. Hydroxyapatite microporous bioceramics as vancomycin reservoir: Antibacterial efficiency and biocompatibility investigation. J. Biomater. Appl. 2016, 31, 488–498. [Google Scholar] [CrossRef]
- Molina-Manso, D.; Manzano, M.; Doadrio, J.C.; Del Prado, G.; Ortiz-Pérez, A.; Vallet-Regí, M.; Gómez-Barrena, E.; Esteban, J. Usefulness of SBA-15 mesoporous ceramics as a delivery system for vancomycin, rifampicin and linezolid: A preliminary report. Int. J. Antimicrob. Agents 2012, 40, 252–256. [Google Scholar] [CrossRef]
- Manzano, M.; Vallet-Regi, M. Revisiting bioceramics: Bone regenerative and local drug delivery systems. Prog. Solid State Chem. 2012, 40, 17–30. [Google Scholar] [CrossRef]
- Kang, J.; Jang, Y.; Kim, D.; Park, J. Antimicrobial Effectiveness of Cetylpyridinium Chloride and Zinc Chloride–Containing Mouthrinses on Bacteria of Halitosis and Peri-implant Disease. Int. J. Oral Maxillofac. Implant. 2015, 30. [Google Scholar] [CrossRef] [Green Version]
- Carcuac, O.; Abrahamsson, I.; Charalampakis, G.; Berglundh, T. The effect of the local use of chlorhexidine in surgical treatment of experimental peri-implantitis in dogs. J. Clin. Periodontol. 2015, 42, 196–203. [Google Scholar] [CrossRef]
- Paolantonio, M.; D’Angelo, M.; Grassi, F.; Perinetti, G.; Piccolomini, R.; Pizzo, G.; Annunziata, M.; D’Archivio, D.; D’Ercole, S.; Nardi, G.; et al. Clinical and Microbiologic Effects of Subgingival Controlled-Release Delivery of Chlorhexidine Chip in the Treatment of Periodontitis: A Multicenter Study. J. Periodontol. 2008, 79, 271–282. [Google Scholar] [CrossRef]
- Vandana, K.L.; Kalsi, R.; Prakash, S. Effect of local drug delivery in chronic periodontitis patients: A meta-analysis. J. Indian Soc. Periodontol. 2011, 15, 304–309. [Google Scholar] [CrossRef]
- Agarwal, V.; Sachdeva, S. Evaluation of commercially available biodegradable tetracycline fiber therapy in chronic periodontitis. J. Indian Soc. Periodontol. 2011, 15, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Matesanz-Pérez, P.; García-Gargallo, M.; Figuero, E.; Bascones-Martinez, A.; Sanz, M.; Herrera, D. A systematic review on the effects of local antimicrobials as adjuncts to subgingival debridement, compared with subgingival debridement alone, in the treatment of chronic periodontitis. J. Clin. Periodontol. 2013, 40, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Wychowanski, P.; Osiak, M.; Morawiec, T.; Laubitz, D.; Czerniuk, M.; Wolinski, J. The Bioactive Healing Abutment (BHA) for controlling microflora in periimplantitis. Clin. Oral Implant. Res. 2019, 30, 247. [Google Scholar] [CrossRef]





| Antibiotic | Clindamycin 2 µg | Tetracycline 30 µg | ||
|---|---|---|---|---|
| Bacterial strain | Staphylococus aureus | Staphylococus epidermidis | Staphylococus aureus | Staphylococus epidermidis |
| Paper disc (mm) | 19.9 ± 2.3 | 31 ± 0.6 | 15.2 ± 2.7 | 11.3 ± 0.3 |
| Bioactive healing abutment (mm) | 24.4 ± 2.5 | 34.7 ± 2.3 | 18.8 ± 3.4 | 13 ± 3.0 |
| Difference (mm) | 4.5 ± 0.1 | 3.7 ± 2.4 | 3.7 ± 0.7 | 1.7 ± 3.0 |
| p | 0.0000043 | 0.2018 | 0.00041 | 0.6164 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwańczyk, B.; Wychowański, P.; Minkiewicz-Zochniak, A.; Strom, K.; Jarzynka, S.; Olędzka, G. Bioactive Healing Abutment as a Potential Tool for the Treatment of Peri-Implant Disease—In Vitro Study. Appl. Sci. 2020, 10, 5376. https://doi.org/10.3390/app10155376
Iwańczyk B, Wychowański P, Minkiewicz-Zochniak A, Strom K, Jarzynka S, Olędzka G. Bioactive Healing Abutment as a Potential Tool for the Treatment of Peri-Implant Disease—In Vitro Study. Applied Sciences. 2020; 10(15):5376. https://doi.org/10.3390/app10155376
Chicago/Turabian StyleIwańczyk, Bartłomiej, Piotr Wychowański, Anna Minkiewicz-Zochniak, Kamila Strom, Sylwia Jarzynka, and Gabriela Olędzka. 2020. "Bioactive Healing Abutment as a Potential Tool for the Treatment of Peri-Implant Disease—In Vitro Study" Applied Sciences 10, no. 15: 5376. https://doi.org/10.3390/app10155376
APA StyleIwańczyk, B., Wychowański, P., Minkiewicz-Zochniak, A., Strom, K., Jarzynka, S., & Olędzka, G. (2020). Bioactive Healing Abutment as a Potential Tool for the Treatment of Peri-Implant Disease—In Vitro Study. Applied Sciences, 10(15), 5376. https://doi.org/10.3390/app10155376