Parametric and Statistical Study of the Wing Geometry of 75 Species of Odonata
Abstract
:1. Introduction
2. Materials and Methods
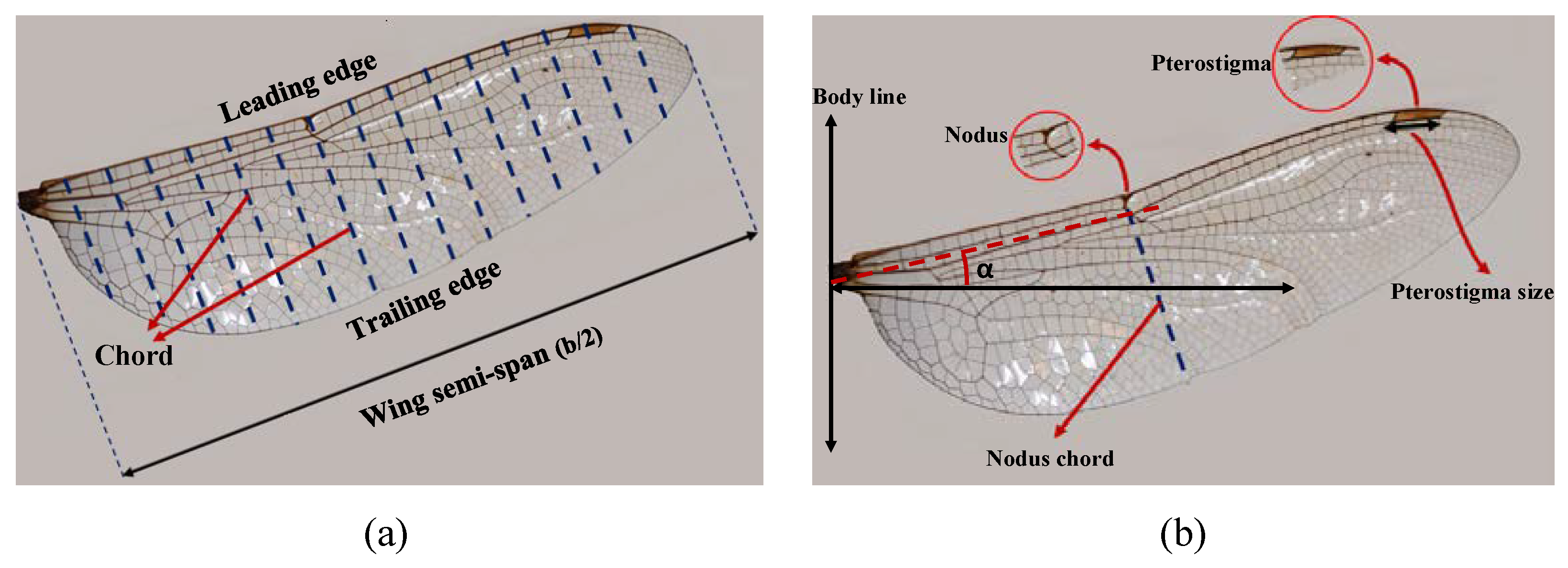
Wing Geometry and Parameter Definition
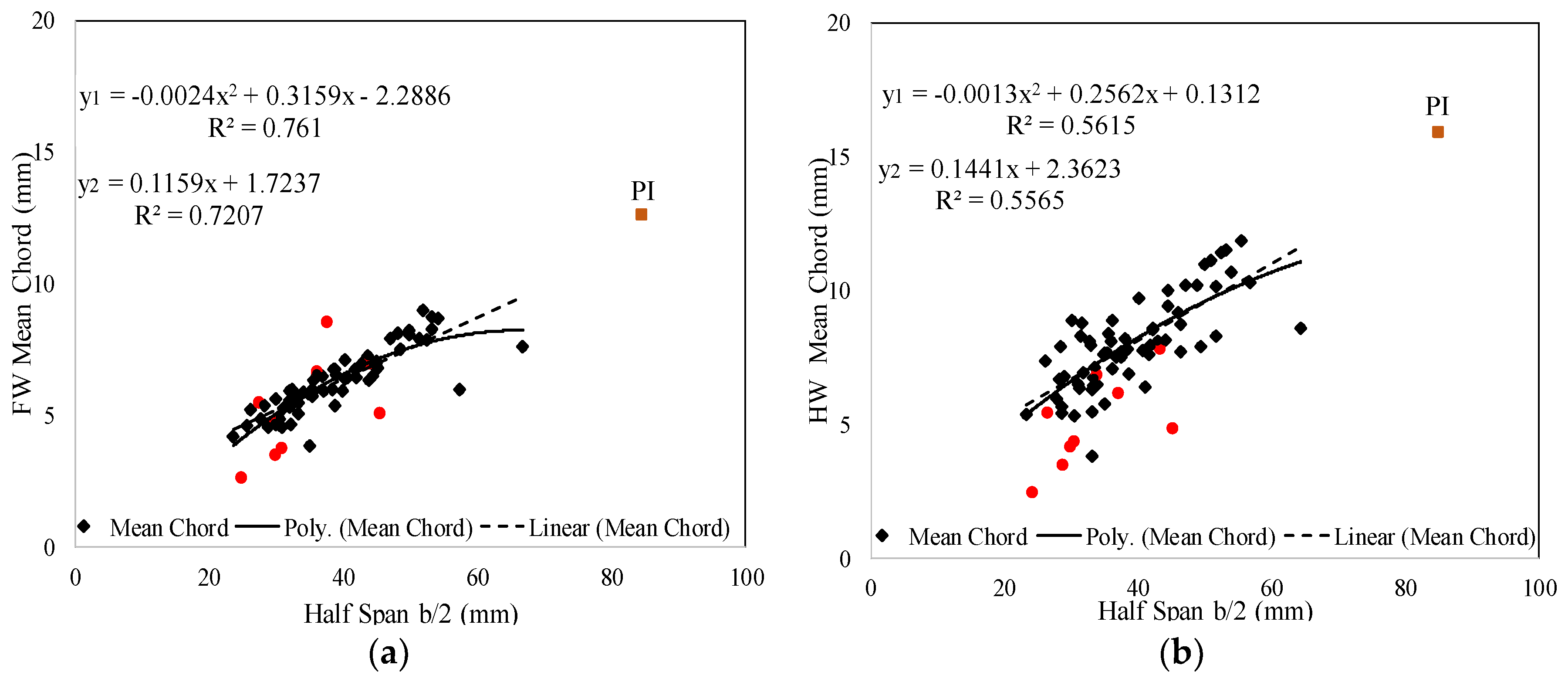
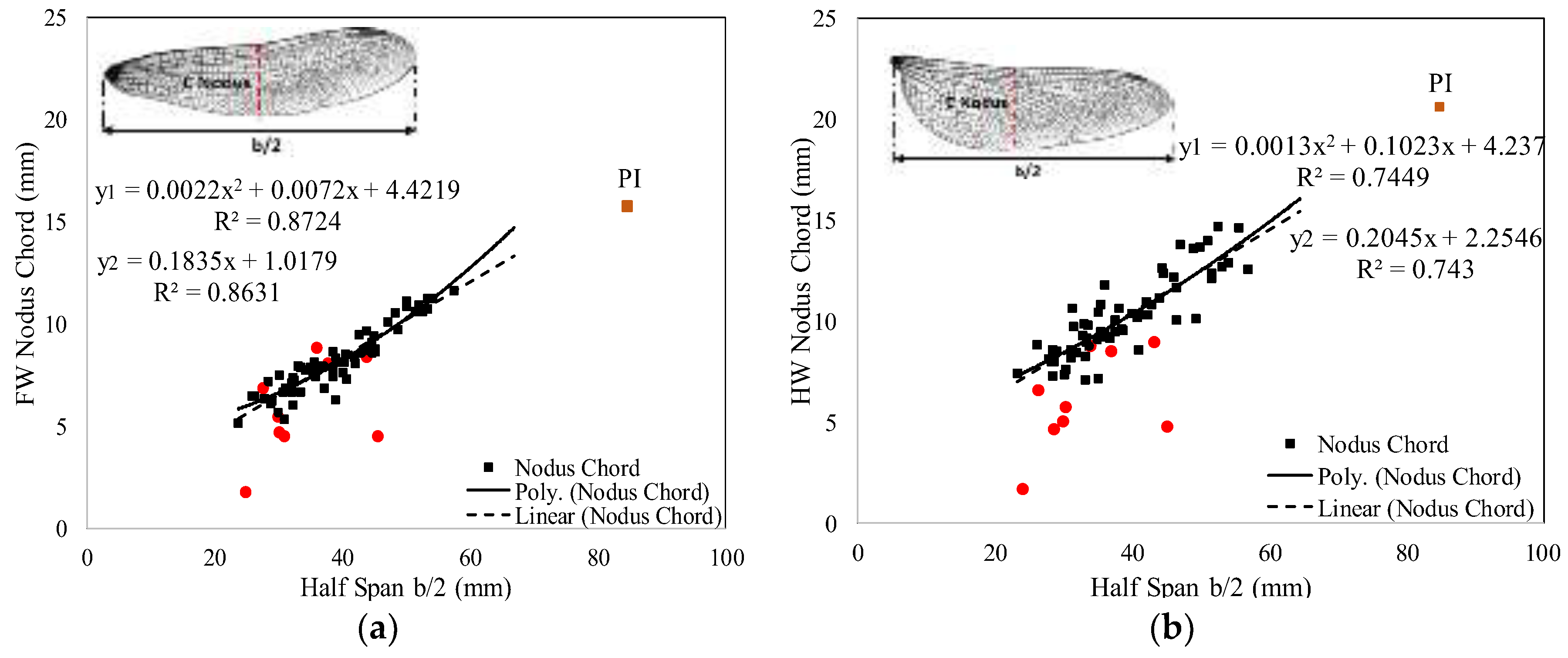
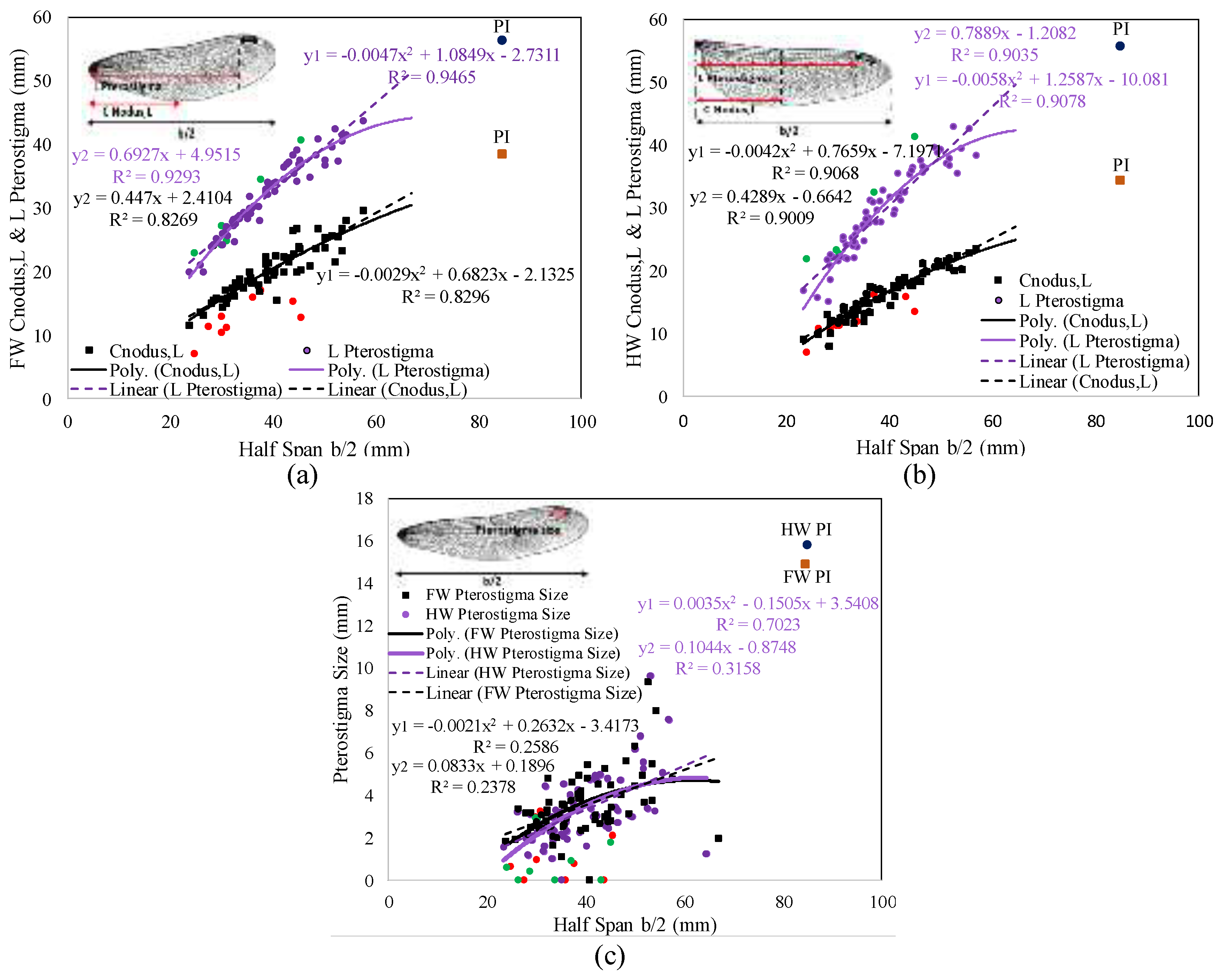
3. Results
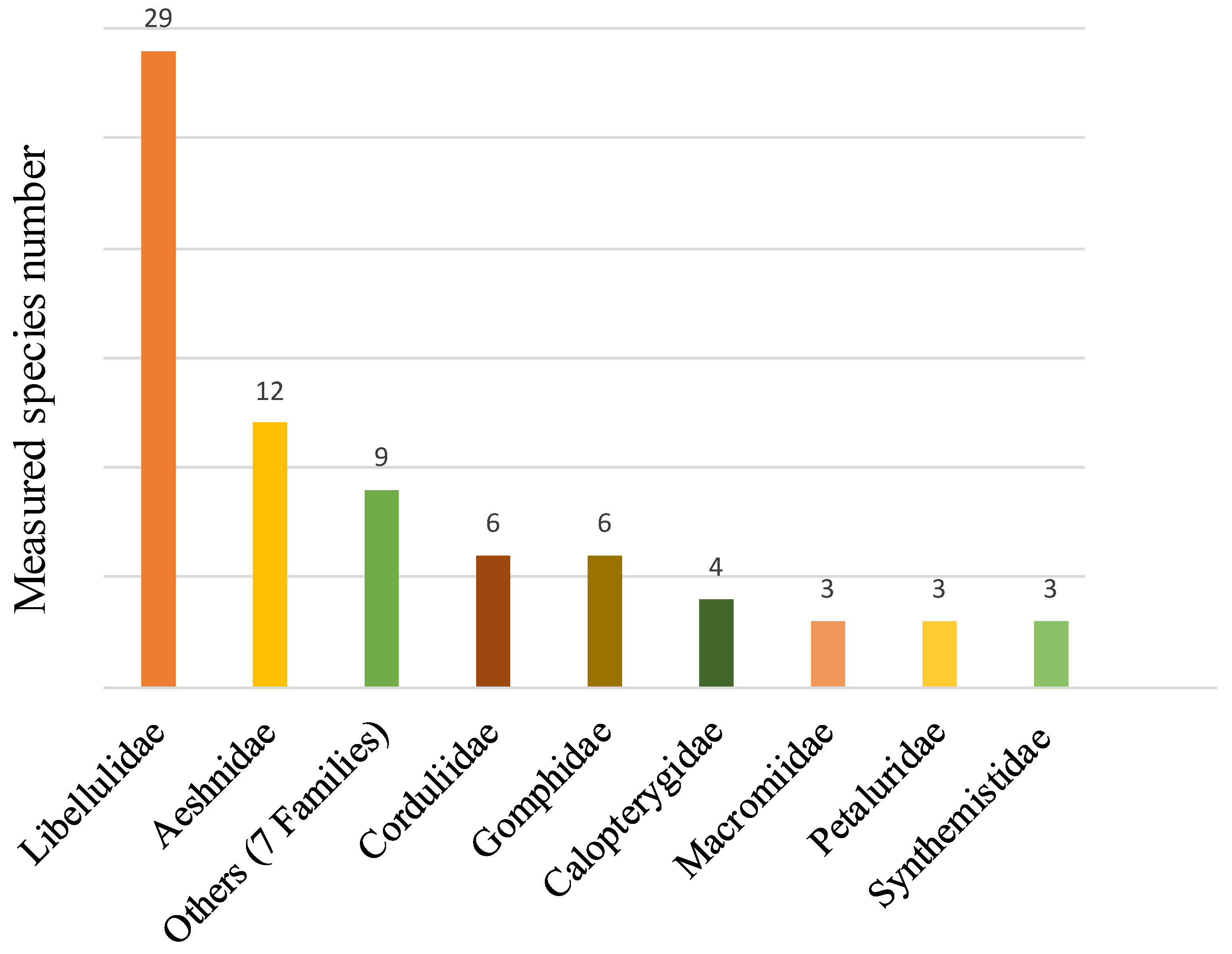
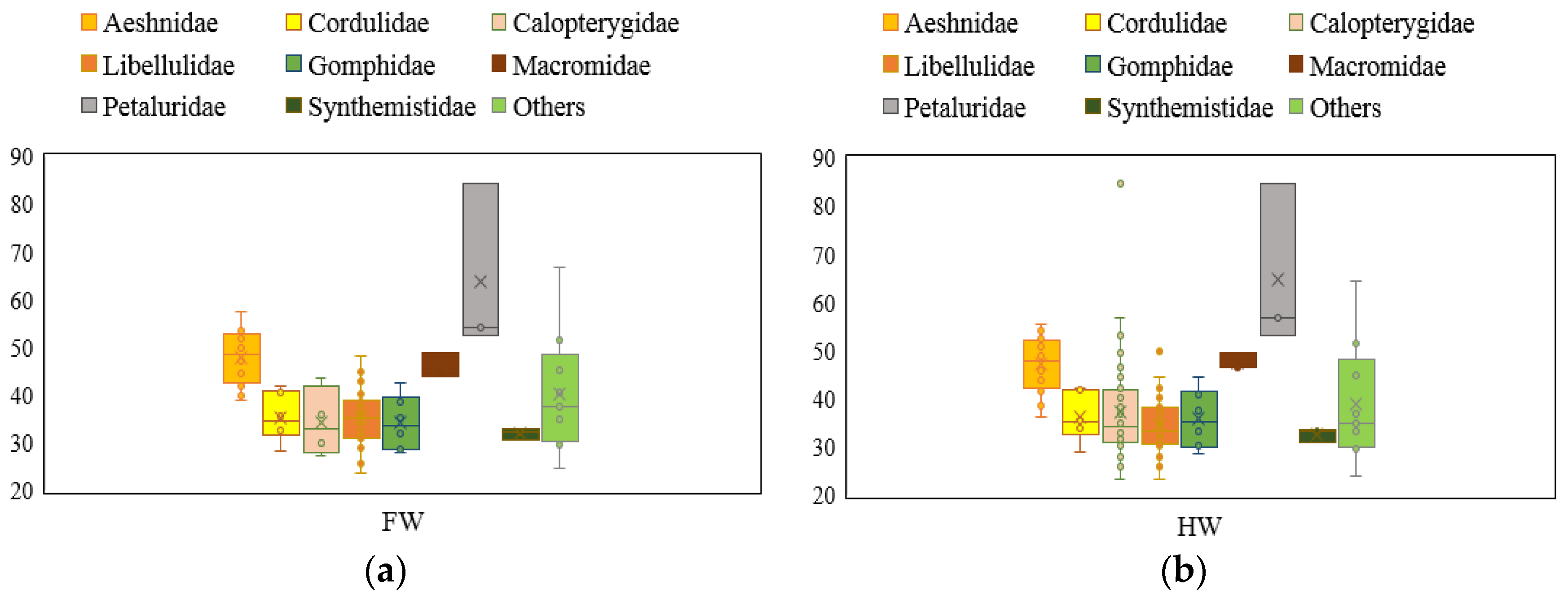
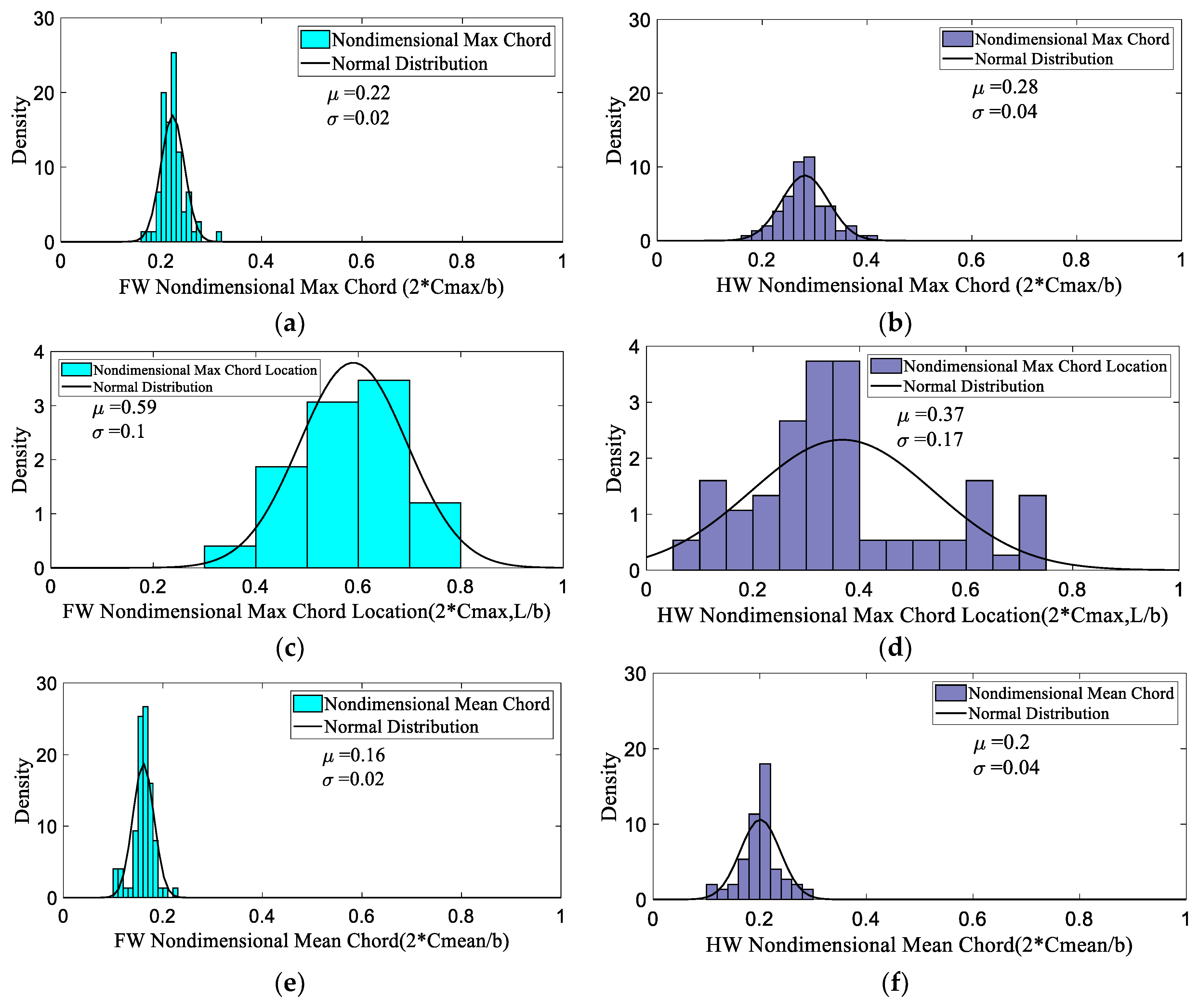
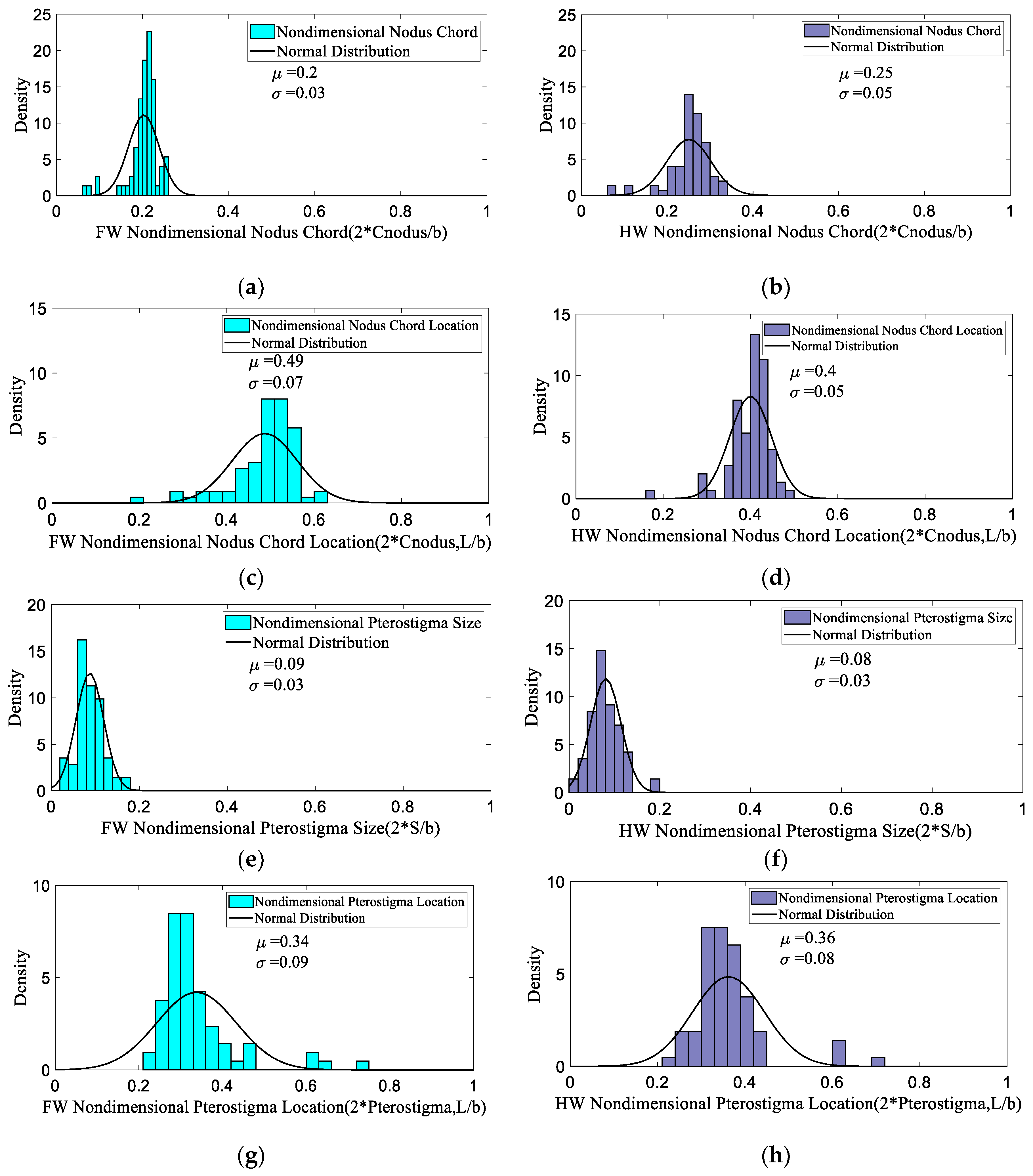
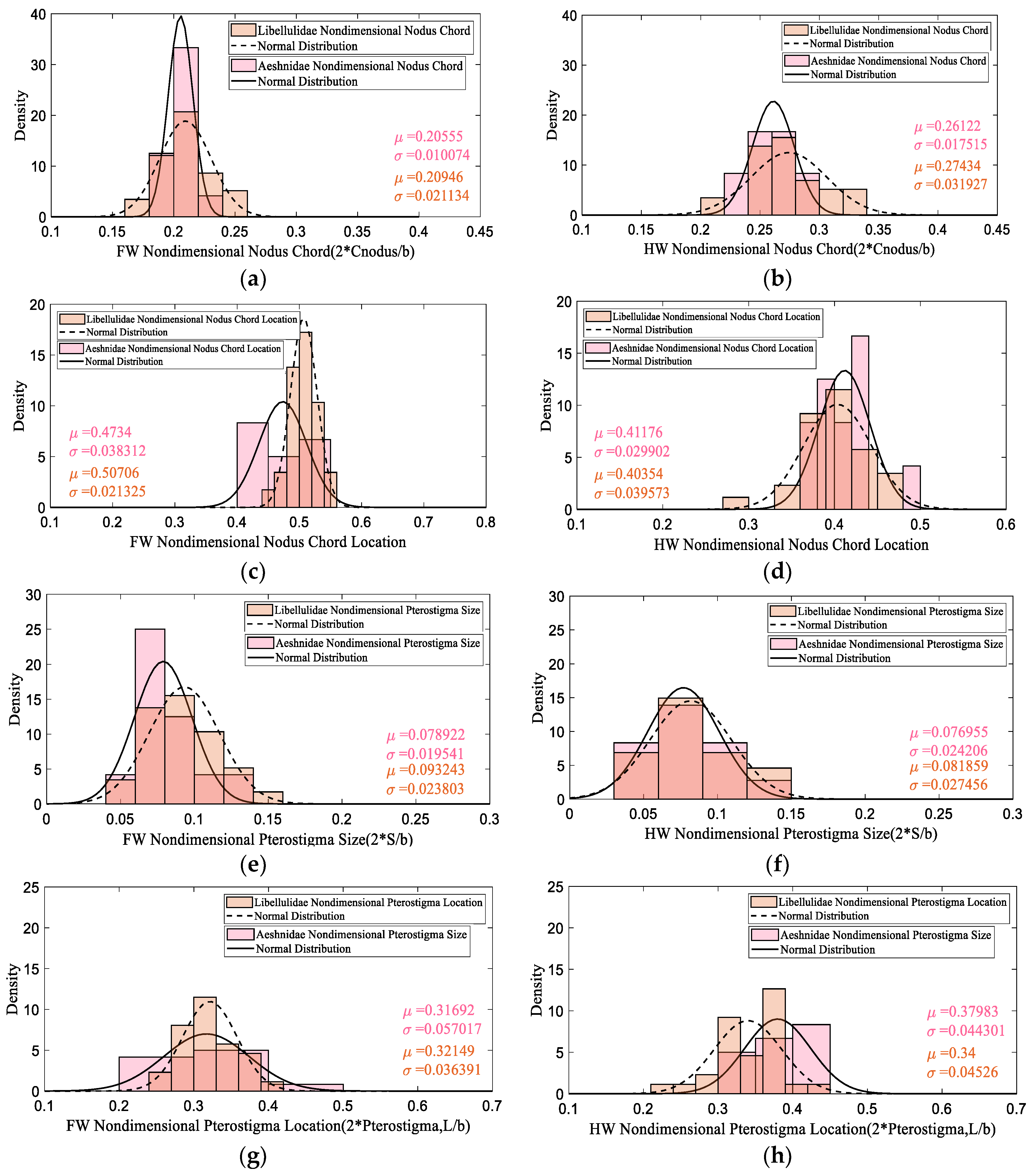
3.1. Database Analysis
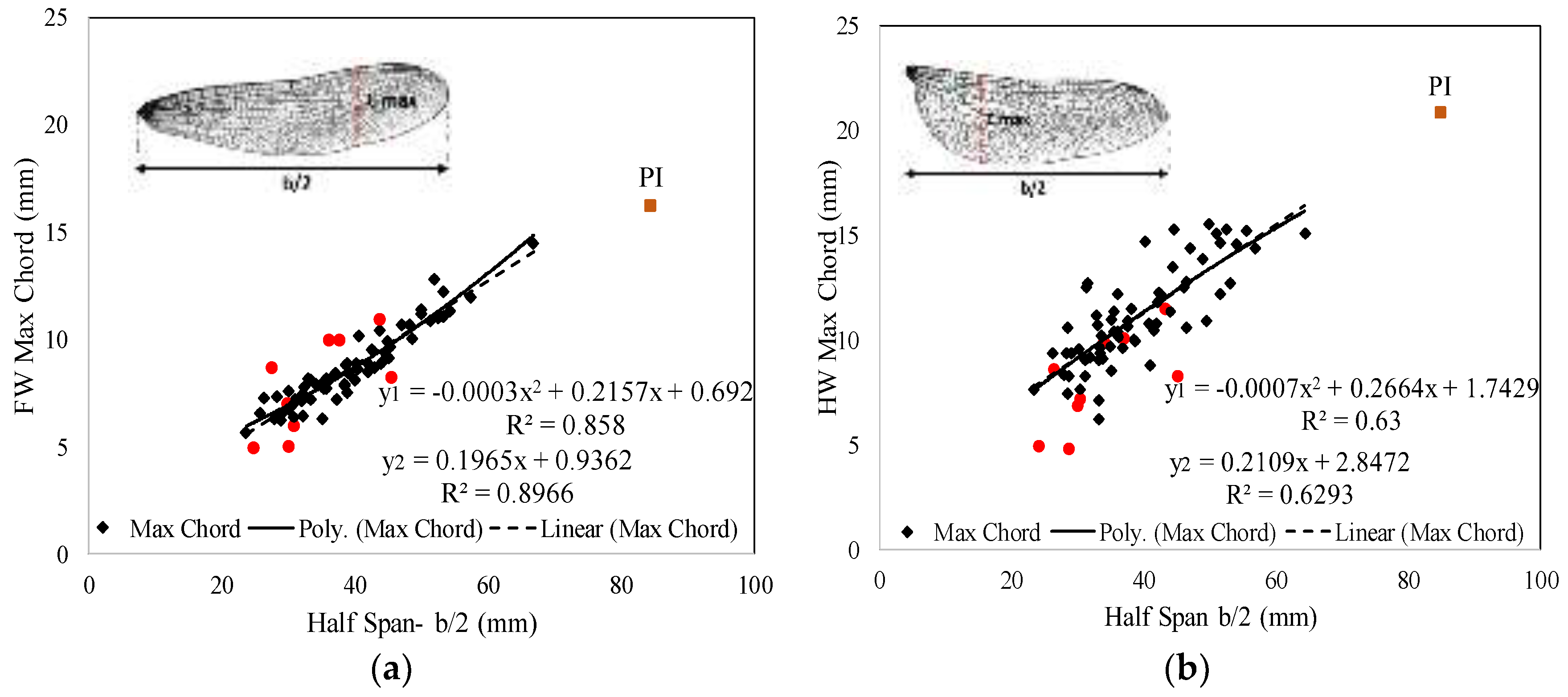
3.2. Regression Analysis
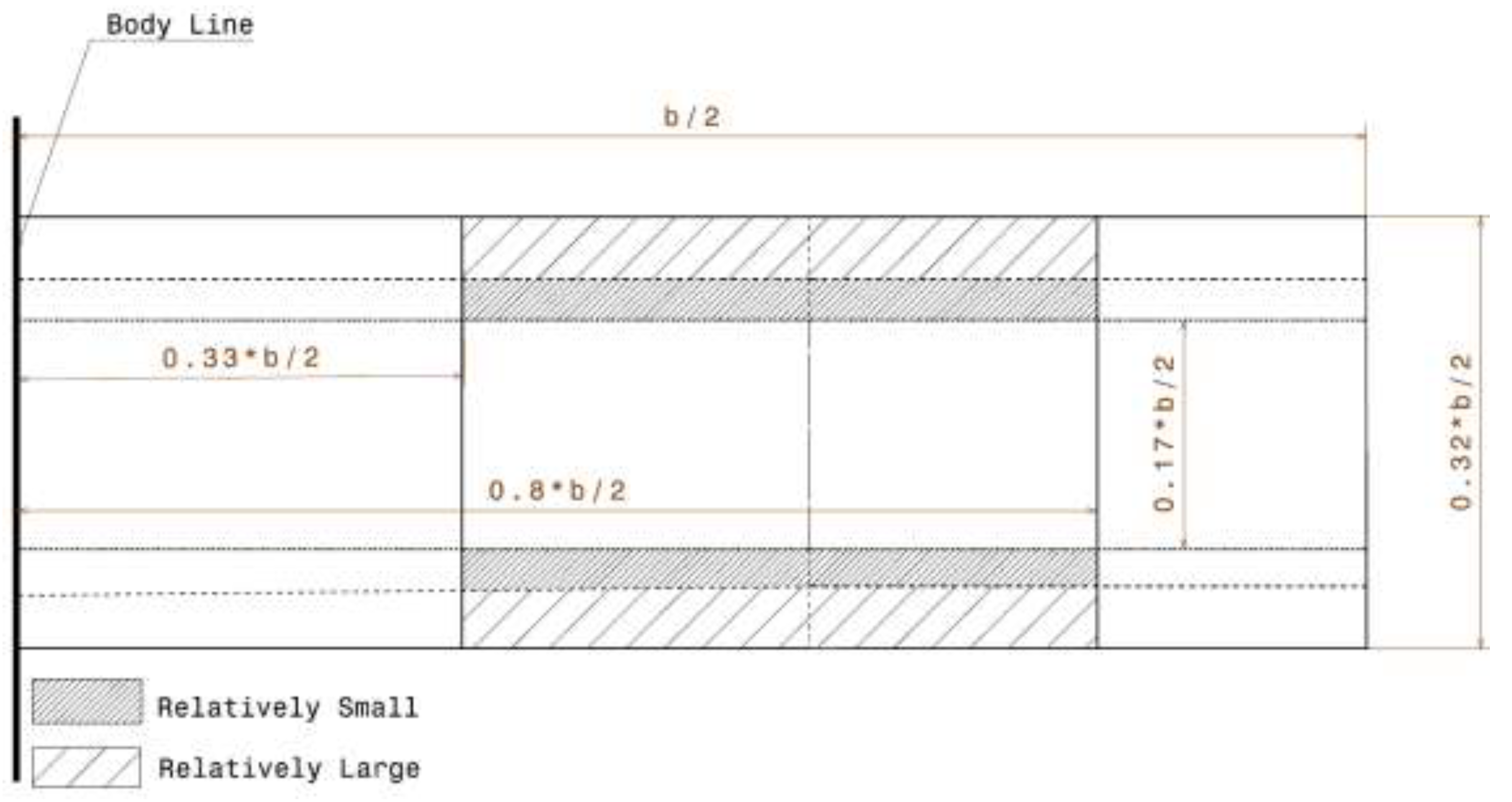
3.3. Design Approach
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- Hassall, C. Strong geographical variation in wing aspect ratio of a damselfly, Calopteryx maculata (Odonata: Zygoptera). PeerJ 2015, 3, e1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saharon, D.; Luttges, M. Visualization of unsteady separated flow produced by mechanically driven dragonfly wing kinematics model. In Proceedings of the 26th Aerospace Sciences Meeting, Reno, NV, USA, 9–12 January 1989; p. 569. [Google Scholar]
- San Ha, N.; Truong, Q.T.; Goo, N.S.; Park, H.C. Corrigendum: Relationship between wingbeat frequency and resonant frequency of the wing in insects (2013 Bioinspir. Biomim. 8 046008). Bioinspir. Biomim. 2015, 10, 019501. [Google Scholar]
- Chen, J.-S.; Chen, J.-Y.; Chou, Y.-F. On the natural frequencies and mode shapes of dragonfly wings. J. Sound Vib. 2008, 313, 643–654. [Google Scholar] [CrossRef]
- Rüppell, G.; Hilfert, D. The flight of the relict dragonfly Epiophlebia superstes (Selys) in comparison with that of the modern Odonata (Anisozygoptera: Epiophlebiidae). Odonatologica 1993, 22, 295–309. [Google Scholar]
- Stanford, B.; Lacore, D.; Albertani, R.; Parker, G.; Walker, R.; Curtis, D. Proper Orthogonal Decomposition of Flexible Clap and Fling Motions via High-Speed Deformation Measurements. In Proceedings of the 48th AIAA Aerospace Sciences Meeting Including the New Horizons Forum and Aerospace Exposition, Orlando, FL, USA, 4–7 January 2010; p. 1026. [Google Scholar]
- Bomphrey, R.J.; Nakata, T.; Henningsson, P.; Lin, H.-T. Flight of the dragonflies and damselflies. Phil. Trans. R. Soc. B 2016, 371, 20150389. [Google Scholar] [CrossRef] [Green Version]
- Wakeling, J.; Ellington, C.P. Dragonfly flight. I. Gliding flight and steady-state aerodynamic forces. J. Exp. Biol. 1997, 200, 543–556. [Google Scholar]
- Sun, X.; Gong, X.; Huang, D. A review on studies of the aerodynamics of different types of maneuvers in dragonflies. Arch. Appl. Mech. 2017, 87, 521–554. [Google Scholar] [CrossRef]
- Brodsky, A.K. The Evolution of Insect Flight; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Wootton, R.J.; Newman, D.J. Evolution, diversification, and mechanics of dragonfly wings. In Dragonflies and Damselflies: Model Organisms for Ecological and Evolutionary Research; Oxford University Press: Oxford, UK, 2008; pp. 261–274. [Google Scholar]
- Sun, J.; Bhushan, B. The structure and mechanical properties of dragonfly wings and their role on flyability. Comptes Rendus Mécanique 2012, 340, 3–17. [Google Scholar] [CrossRef]
- Zhang, S.; Sunami, Y.; Hashimoto, H. Deformation behavior of dragonfly-inspired nodus structured wing in gliding flight through experimental visualization approach. Sci. Rep. 2018, 8, 5751. [Google Scholar] [CrossRef]
- Rajabi, H.; Darvizeh, A. Experimental investigations of the functional morphology of dragonfly wings. Chin. Phys. B 2013, 22, 088702. [Google Scholar] [CrossRef]
- Wootton, R.J. Functional morphology of insect wings. Annu. Rev. Entomol. 1992, 37, 113–140. [Google Scholar] [CrossRef]
- Kesel, A.B. Aerodynamic characteristics of dragonfly wing sections compared with technical aerofoils. J. Exp. Biol. 2000, 203, 3125–3135. [Google Scholar] [PubMed]
- Lindley, R. Some armchair thoughts on the dragonfly wing. Odonatologica 1978, 7, 323–351. [Google Scholar]
- Chen, Y.; Skote, M.; Zhao, Y.; Huang, W. Dragonfly (Sympetrum flaveolum) flight: Kinematic measurement and modelling. J. Fluids Struct. 2013, 40, 115–126. [Google Scholar] [CrossRef]
- Rajabi, H.; Ghoroubi, N.; Stamm, K.; Appel, E.; Gorb, S. Dragonfly wing nodus: A one-way hinge contributing to the asymmetric wing deformation. Acta Biomater. 2017, 60, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Norberg, R.Å. The pterostigma of insect wings an inertial regulator of wing pitch. J. Comp. Physiol. 1972, 81, 9–22. [Google Scholar] [CrossRef]
- Roskam, J.; Lan, C.-T.E. Airplane Aerodynamics and Performance; DARcorporation: Lawrence, KS, USA, 1997. [Google Scholar]
- Laliberté, J.F.; Kraemer, K.L.; Dawson, J.W.; Miyata, D. Design and manufacturing of biologically inspired micro aerial vehicle wings using rapid prototyping. Int. J. Micro Air Veh. 2013, 5, 15–38. [Google Scholar] [CrossRef] [Green Version]
- Levy, D.-E.; Seifert, A. Parameter study of simplified dragonfly airfoil geometry at Reynolds number of 6000. J. Theor. Biol. 2010, 266, 691–702. [Google Scholar] [CrossRef]
- Packer, S.; Riservato, E.; Aggio, C. The Status and Distribution of Dragonflies of the Mediterranean Basin; IUCN: Gland, Switzerland; Malaga, Spain, 2009. [Google Scholar]
- Marden, J. Large-scale changes in thermal sensitivity of flight performance during adult maturation in a dragonfly. J. Exp. Biol. 1995, 198, 2095–2102. [Google Scholar]
- Chitsaz, N.; Marian, R.; Chahl, J. Experimental method for 3D reconstruction of Odonata wings (methodology and dataset). PLoS ONE 2020, 15, e0232193. [Google Scholar] [CrossRef]
- Chitsaz, N.; Chahl, J. Current knowledge of corrugated dragonfly wing structures and future measurement methodology. In Proceedings of the AIAC18: 18th Australian International Aerospace Congress (2019): HUMS—11th Defence Science and Technology (DST) International Conference on Health and Usage Monitoring (HUMS 2019): ISSFD—27th International Symposium on Space Flight Dynamics (ISSFD), Melbourne, Australia, 24–28 February 2019; Engineers Australia, Royal Aeronautical Society: Melbourne, Australia, 2019; pp. 412–417. [Google Scholar]
- Combes, S.; Daniel, T. Flexural stiffness in insect wings II. Spatial distribution and dynamic wing bending. J. Exp. Biol. 2003, 206, 2989–2997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stavenga, D.; Stowe, S.; Siebke, K.; Zeil, J.; Arikawa, K. Butterfly wing colours: Scale beads make white pierid wings brighter. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2004, 271, 1577–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindsey, E.; Altizer, S. Sex differences in immune defenses and response to parasitism in monarch butterflies. Evol. Ecol. 2009, 23, 607–620. [Google Scholar] [CrossRef]
- Anholt, B.; Marden, J.H.; Jenkins, D. Patterns of mass gain and sexual dimorphism in adult dragonflies (Insecta: Odonata). Can. J. Zool. 1991, 69, 1156–1163. [Google Scholar] [CrossRef]
- Kulfan, B.M. Universal parametric geometry representation method. J. Aircr. 2008, 45, 142–158. [Google Scholar] [CrossRef]
- Raymer, D. Aircraft Design: A Conceptual Approach; American Institute of Aeronautics and Astronautics, Inc.: Reston, VA, USA, 2012. [Google Scholar]
- Walker, S.M.; Thomas, A.L.; Taylor, G.K. Photogrammetric reconstruction of high-resolution surface topographies and deformable wing kinematics of tethered locusts and free-flying hoverflies. J. R. Soc. Interface 2009, 6, 351–366. [Google Scholar] [CrossRef] [Green Version]
- Chitsaz, N.; Marian, R.; Chahl, J. Bio-inspired flapping-wing micro air vehicles material properties and evolutionary fabrication. In Proceedings of the AIAC18: 18th Australian International Aerospace Congress (2019): HUMS—11th Defence Science and Technology (DST) International Conference on Health and Usage Monitoring (HUMS 2019): ISSFD—27th International Symposium on Space Flight Dynamics (ISSFD), Melbourne, Australia, 24–28 February 2019; Engineers Australia, Royal Aeronautical Society: Melbourne, Australia, 2019; pp. 425–430. [Google Scholar]
- Gaurav, S. Investigation of fluid flow and aerodynamic performance of a corrugated dragonfly wing section: A Review. Int. J. Res. Aeronaut. Mech. Eng. 2016, 4, 1–9. [Google Scholar]
- Steffen, C. Fluid-Structure Interaction Analysis of a Dragonfly Wing. Master‘s Thesis, Nanyang Technological University, Singapore, 2016. [Google Scholar]
- Patel, J.K.; Read, C.B. Handbook of the Normal Distribution; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Wilson, K. Dragonfly giants. Agrion 2009, 13, 29–31. [Google Scholar]
- Rice, W.R. Analyzing tables of statistical tests. Evolution 1989, 43, 223–225. [Google Scholar] [CrossRef]
- Wootton, R. The functional morphology of the wings of Odonata. Adv. Odonatol. 1991, 5, 153–169. [Google Scholar]
- Dileo, C.; Deng, X. Design of and experiments on a dragonfly-inspired robot. Adv. Robot. 2009, 23, 1003–1021. [Google Scholar] [CrossRef] [Green Version]
- Naidu, V.; Young, J.; Lai, J. Effect of wing flexibility on dragonfly Hover flight. In Proceedings of the 19th Australasian Fluid Mechanics Conference, Melbourne, Australia, 8–11 December 2014. [Google Scholar]
- Verspui, K.; Wasscher, M.T. The damselfly and dragonfly watercolour collection of Edmond de Selys Longchamps: II Calopterygines, Cordulines, Gomphines and Aeschnines. Int. J. Odonatol. 2017, 20, 79–112. [Google Scholar] [CrossRef]



| Wing Geometry Parameter | R2 |
|---|---|
| Cmax FW = 0.219 × (b/2) | 0.86 |
| Cmax HW = 0.276 × (b/2) | 0.70 |
| Cmax,L FW = 0.58 × (b/2) | 0.62 |
| Cmax,L HW = 0.37 × (b/2) | 0.27 |
| Cmean FW = 0.16 × (b/2) | 0.73 |
| Cmean HW = 0.20 × (b/2) | 0.63 |
| b FW/HW = 1 | - |
| Cnodus,L FW = 0.489 × (b/2) | 0.78 |
| Cnodus,L HW = 0.404 × (b/2) | 0.91 |
| Cpterostigma,L FW = 0.811 × (b/2) | 0.95 |
| Cpterostigma,L HW = 0.756 × (b/2) | 0.91 |
| Nondependent Parameters (R2 > 0.7) | Dependent Parameters (R2 < 0.7) |
|---|---|
| Cmax | Cmax,L |
| Cmean FW | Pterostigma size |
| Cnodus | Cmean HW |
| Cnodus,L | - |
| Lpterostigma | - |
| Forewing | Hindwing | |||||
|---|---|---|---|---|---|---|
| Min. | Ave. | Max. | Min. | Ave. | Max. | |
| 2 × Cmax/b | 0.17 | 0.22 | 0.32 | 0.17 | 0.27 | 0.40 |
| 2 × Cmax L/b | 0.33 | 0.59 | 0.79 | 0.10 | 0.37 | 0.74 |
| 2 × Cmean/b | 0.10 | 0.16 | 0.23 | 0.10 | 0.20 | 0.30 |
| 2 × Cnodus/b | 0.06 | 0.20 | 0.25 | 0.06 | 0.247 | 0.34 |
| AR | 8.74 | 12.68 | 19.30 | 6.77 | 10.37 | 19.20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chitsaz, N.; Marian, R.; Chitsaz, A.; Chahl, J.S. Parametric and Statistical Study of the Wing Geometry of 75 Species of Odonata. Appl. Sci. 2020, 10, 5389. https://doi.org/10.3390/app10155389
Chitsaz N, Marian R, Chitsaz A, Chahl JS. Parametric and Statistical Study of the Wing Geometry of 75 Species of Odonata. Applied Sciences. 2020; 10(15):5389. https://doi.org/10.3390/app10155389
Chicago/Turabian StyleChitsaz, Nasim, Romeo Marian, Amirmasoud Chitsaz, and Javaan S. Chahl. 2020. "Parametric and Statistical Study of the Wing Geometry of 75 Species of Odonata" Applied Sciences 10, no. 15: 5389. https://doi.org/10.3390/app10155389





