Measuring Gait-Event-Related Brain Potentials (gERPs) during Instructed and Spontaneous Treadmill Walking: Technical Solutions and Automated Classification through Artificial Neural Networks
Abstract
:1. Introduction
Aim of the Present Study
- Can gait-event-related brain potentials (gERPs) be analyzed based on human gait markers decoded by force plates?
- Can the analysis of gERPs be automated by applying machine learning approaches based on artificial neural networks (ANNs), especially long short-term memory cells (LSTM) [24]?
2. Materials and Methods
2.1. Structural Design and System Overview
2.2. EEG System
2.3. Pressure Plate and Treadmill
2.4. Temporal Synchronization Unit and Data Merging
2.5. Experimental Design and Set-Up
2.6. Data Fusion and Data Preprocessing
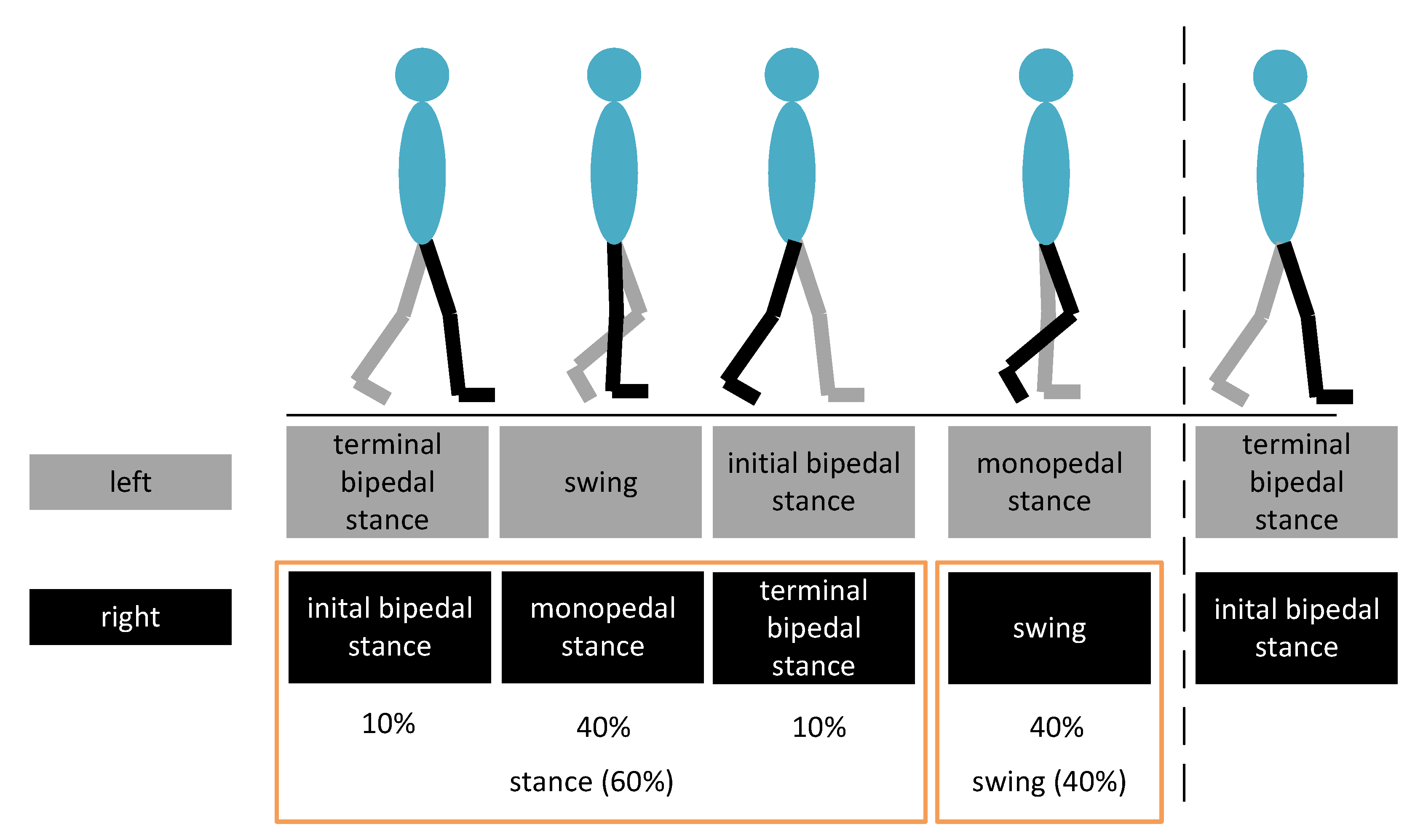
2.7. Data Evaluation and Marker Extraction
2.8. Data Analysis
2.9. Neural Networks and Data Classification
- class 1: left single support
- class 2: right single support
- class 3: double support
- Input layer of size 2 × 1100 (according to the number of EEG channels and window size)
- LSTM layer with 10 LSTM units (returning whole sequence)
- LSTM layer with 10 LSTM units (returning only the last value of the sequence)
- Dense layer with 64 neurons
- Dense layer with three neurons (according to the number of classes)
- Softmax layer
- Classification layer
3. Results
3.1. Gait-Event-Related Brain Potentials (gERPs)
3.2. Frequency Analysis (ERSP)
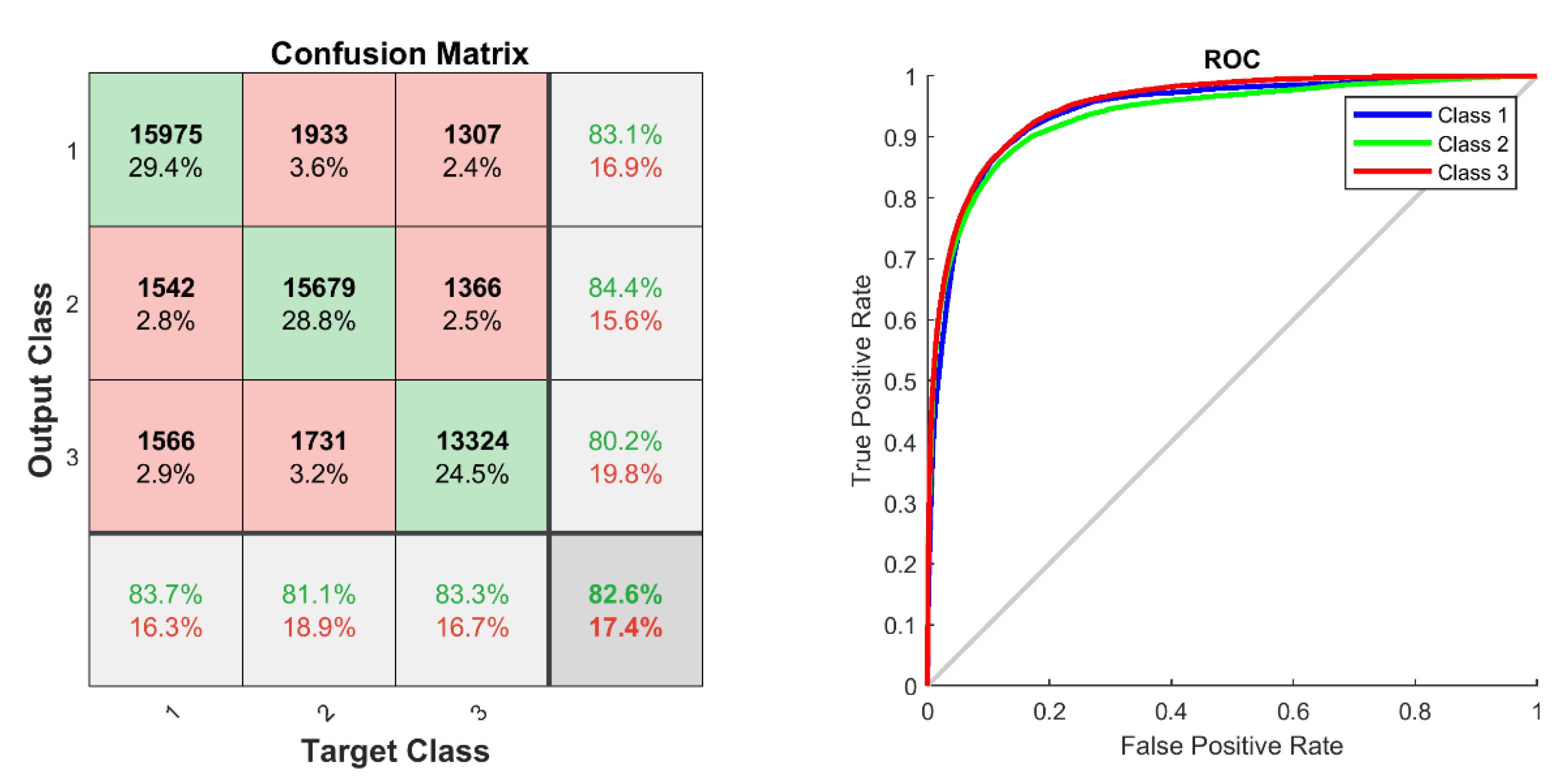
3.3. Neural Network
4. Discussion and Conclusions
4.1. Analysis of gERPs Using Gait Markers
4.2. Implications for the Rehabilitation of Gait
4.3. Can Gait-Related EEG Data be Trained and Classified by an ANN?
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability Statement
References
- Belda-Lois, J.-M.; Mena-del Horno, S.; Bermejo-Bosch, I.; Moreno, J.C.; Pons, J.L.; Farina, D.; losa, M.; Molinari, M.; Tamburella, F.; Ramos, A.; et al. Rehabilitation of gait after stroke: A review towards a top-down approach. J. Neuroeng. Rehabil. 2011, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirelman, A.; Shema, S.; Maidan, I.; Hausdorff, J.M. Gait. Handb. Clin. Neurol. 2018, 159, 119–134. [Google Scholar] [PubMed]
- Ambrose, A.F.; Cruz, L.; Paul, G. Falls and Fractures: A systematic approach to screening and prevention. Maturitas 2015, 82, 85–93. [Google Scholar] [CrossRef]
- Amboni, M.; Barone, P.; Hausdorff, J.M. Cognitive contributions to gait and falls: Evidence and implications. Mov. Disord. Off. J. Mov. Disord. Soc. 2013, 28, 1520–1533. [Google Scholar] [CrossRef]
- Enright, P.L.; Sherrill, D.L. Reference equitations for the six-minute walk in healthy adults. Am. J. Respir. Crit. Care Med. 1998, 158, 1384–1387. [Google Scholar] [CrossRef] [Green Version]
- Allali, G.; Blumen, H.M.; Devanne, H.; Pirondini, E.; Delval, A.; van de Ville, D. Brain imaging of locomotion in neurological conditions. Neurophysiol. Clin. 2018, 48, 337–359. [Google Scholar] [CrossRef]
- Babiloni, C.; Carducci, F.; Cincotti, F.; Rossini, P.M.; Neuper, C.; Pfurtscheller, G.; Babiloni, F. Human movement-related potentials vs. desynchronization of EEG alpha rhythm: A high-resolution EEG study. NeuroImage 1999, 10, 658–665. [Google Scholar]
- Pfurtscheller, G.; Neuper, C.; Krausz, G. Functional dissociation of lower and upper frequency mu rhythms in relation to voluntary limb movement. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2000, 111, 1873–1879. [Google Scholar] [CrossRef]
- Cassim, F.; Szurhaj, W.; Sediri, H.; Devos, D.; Bourriez, J.; Poirot, I.; Derambure, P.; Defebvre, L.; Guieu, J. Brief and sustained movements. Differences in event-related (de)synchronization (ERD/ERS) patterns. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2000, 111, 2032–2039. [Google Scholar] [CrossRef]
- Debener, S.; Minow, F.; Emkes, R.; Gandras, K.; de Vos, M. How about taking a low-cost, small, and wireless EEG for a walk? Psychophysiology 2012, 49, 1617–1621. [Google Scholar] [CrossRef] [PubMed]
- Böcker, K.B.E.; Brunia, C.H.M.; Cluitmans, P.J.M. A spatio-temporal dipole model of the readiness potential in humans. II. Foot movement. Electroencephalogr. Clin. Neurophysiol. 1994, 91, 286–294. [Google Scholar]
- Boschert, J.; Deecke, L. Cerebral potentials preceding voluntary toe, knee and hip movements and their vectors in human precentral gyrus. Brain Res. 1986, 376, 175–179. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Brunner, C.; Schlogl, A.; Lopes da Silva, F.H. Mu rhythm (de)synchronization and EEG single-trial classification of different motor imagery tasks. NeuroImage 2006, 31, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Justen, C.; Herbert, C. Snap your fingers! An ERP/sLORETA study investigating implicit processing of self-vs. other-related movement sounds using the passive oddball paradigm. Front. Hum. Neurosci. 2016, 10, 465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfurtscheller, G.; Lopes da Silva, F.H. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin. Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef]
- Neuper, C.; Klimesch, W. (Eds.) Advances in genetics: V. 159. In Event-Related Dynamics of Brain Oscillations; Academic Press: Amsterdam, The Netherlands; Boston, MA, USA, 2006. [Google Scholar]
- Beurskens, R.; Steinberg, F.; Antoniewicz, F.; Wolff, W.; Granacher, U. Neural Correlates of Dual-Task Walking: Effects of Cognitive versus Motor Interference in Young Adults. Neural Plast. 2016, 8032180. [Google Scholar] [CrossRef] [Green Version]
- Arad, E.; Bartsch, R.P.; Kantelhardt, J.W.; Plotnik, M. Performance-based approach for movement artifact removal from electroencephalographic data recorded during locomotion. PLoS ONE 2018, 13, e0197153. [Google Scholar] [CrossRef]
- Montero-Odasso, M.; Verghese, J.; Beauchet, O.; Hausdorff, J.M. Gait and cognition: A complementary approach to understanding brain function and the risk of falling. J. Am. Geriatr. Soc. 2012, 60, 2127–2136. [Google Scholar] [CrossRef] [Green Version]
- Woollacott, M.; Shumway-Cook, A. Attention and the control of posture and gait: A review of an emerging area of research. Gait Posture 2002, 16, 1–14. [Google Scholar] [CrossRef]
- Yogev-Seligmann, G.; Hausdorff, J.M.; Giladi, N. The role of executive function and attention in gait. Mov. Disord. Off. J. Mov. Disord. Soc. 2008, 23, 329–342. [Google Scholar] [CrossRef] [Green Version]
- Perry, J. Gait Analysis, 2nd ed.; SLACK Incorporated: Thorofare, NJ, USA, 2010. [Google Scholar]
- Whittle, M.W. Clinical gait analysis: A review. Hum. Mov. Sci. 1996, 15, 369–387. [Google Scholar] [CrossRef]
- Hochreiter, S.; Schmidhuber, J. Long short-term memory. Neural Comput. 1997, 9, 1735–1780. [Google Scholar] [CrossRef] [PubMed]
- Widmann, A.; Schröger, E.; Maess, B. Digital filter design for electrophysiological data—A practical approach. J. Neurosci. Methods 2015, 250, 34–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junghöfer, M.; Bradley, M.M.; Elbert, T.R.; Lang, P.J. Fleeting images: A new look at early emotion discrimination. Psychophysiology 2001, 38, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Herbert, C.; Junghofer, M.; Kissler, J. Event related potentials to emotional adjectives during reading. Psychophysiology 2008, 45, 487–498. [Google Scholar] [CrossRef]
- Saleh, K.; Hossny, M.; Nahavandi, S. Driving behavior classification based on sensor data fusion using LSTM recurrent neural networks. In Proceedings of the 2017 IEEE 20th International Conference on Intelligent Transportation Systems (ITSC), Yokohama, Japan, 16–19 October 2017; pp. 1–6. [Google Scholar]
- Fawaz, H.I.; Forestier, G.; Weber, J.; Idoumghar, L.; Muller, P.A. Deep learning for time series classification: A review. Data Min. Knowl. Discov. 2019, 33, 917–963. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Wang, H.; Dong, J.; Zhong, G.; Sun, X. Prediction of sea surface temperature using long short-term memory. IEEE Geosci. Remote Sens. Lett. 2017, 14, 1745–1749. [Google Scholar] [CrossRef] [Green Version]
- Alexander, L.D.; Black, S.E.; Patterson, K.K.; Gao, F.; Danells, C.J.; McIlroy, W.E. Association between gait asymmetry and brain lesion location in stroke patients. Stroke 2009, 40, 537–544. [Google Scholar] [CrossRef] [Green Version]
- Ptak, R. The frontoparietal attention network of the human brain: Action, saliency, and a priority map of the environment. Neuroscientist 2012, 18, 502–515. [Google Scholar] [CrossRef]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herbert, C.; Munz, M. Measuring Gait-Event-Related Brain Potentials (gERPs) during Instructed and Spontaneous Treadmill Walking: Technical Solutions and Automated Classification through Artificial Neural Networks. Appl. Sci. 2020, 10, 5405. https://doi.org/10.3390/app10165405
Herbert C, Munz M. Measuring Gait-Event-Related Brain Potentials (gERPs) during Instructed and Spontaneous Treadmill Walking: Technical Solutions and Automated Classification through Artificial Neural Networks. Applied Sciences. 2020; 10(16):5405. https://doi.org/10.3390/app10165405
Chicago/Turabian StyleHerbert, Cornelia, and Michael Munz. 2020. "Measuring Gait-Event-Related Brain Potentials (gERPs) during Instructed and Spontaneous Treadmill Walking: Technical Solutions and Automated Classification through Artificial Neural Networks" Applied Sciences 10, no. 16: 5405. https://doi.org/10.3390/app10165405




