Facile Synthesis of Highly Conductive Vanadium-Doped NiO Film for Transparent Conductive Oxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Methods
2.2. Characterization
2.3. Synthesis of Pristine and V-Doped NiO
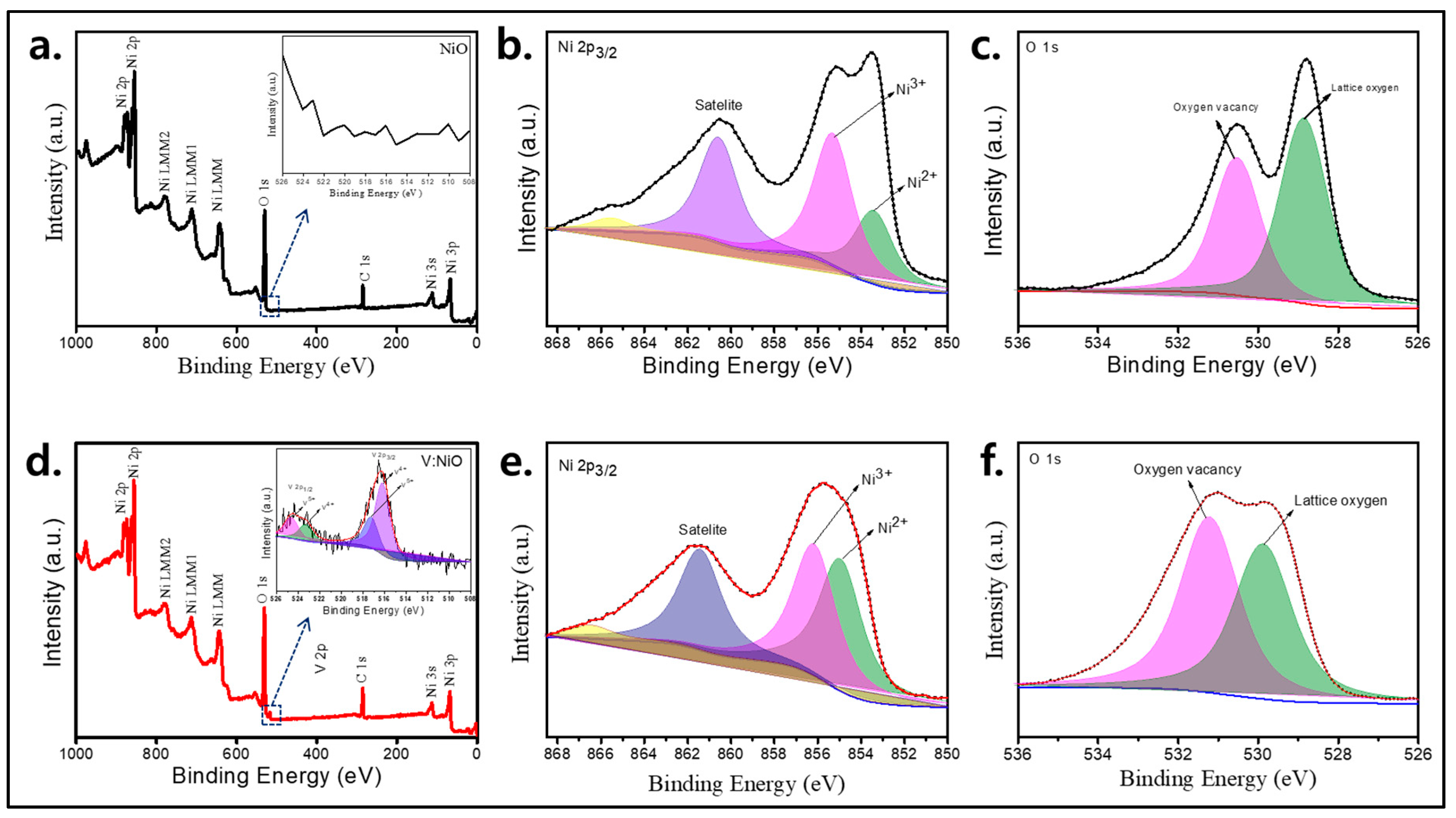
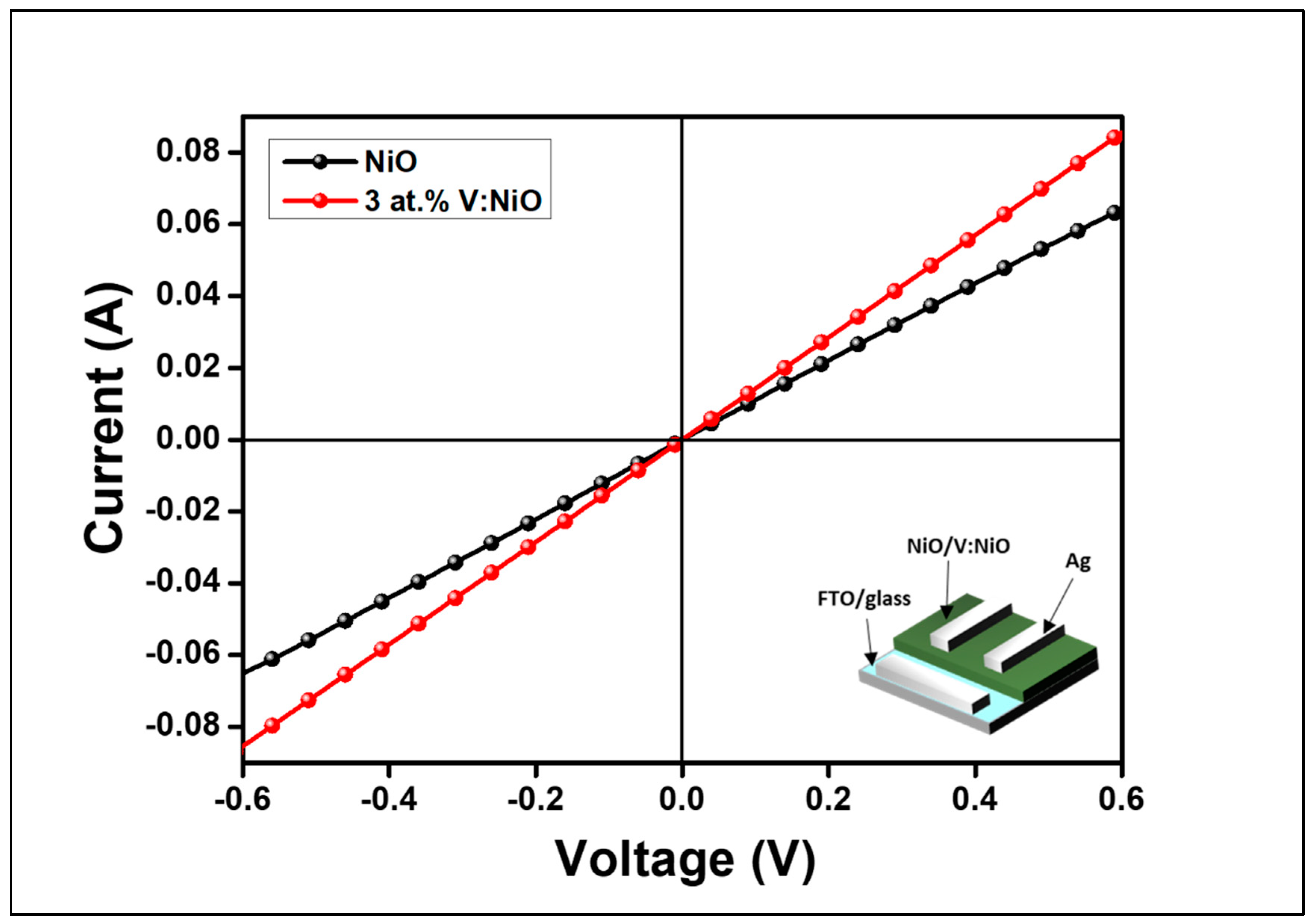
3. Results
3.1. Study of Material Properties
3.2. Study of Film Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zheng, J.; Hu, L.; Yun, J.S.; Zhang, M.; Lau, C.F.J.; Bing, J.; Deng, X.; Ma, Q.; Cho, Y.; Fu, W.; et al. Solution-Processed, Silver-Doped NiO x as Hole Transporting Layer for High-Efficiency Inverted Perovskite Solar Cells. ACS Appl. Energy Mater. 2018, 1, 561–570. [Google Scholar] [CrossRef]
- Hu, L.; Peng, J.; Wang, W.; Xia, Z.; Yuan, J.; Lu, J.; Huang, X.; Ma, W.; Song, H.; Chen, W.; et al. Sequential Deposition of CH3NH3PbI3 on Planar NiO Film for Efficient Planar Perovskite Solar Cells. ACS Photonics 2014, 1, 547–553. [Google Scholar] [CrossRef]
- Zhai, Z.; Huang, X.; Xu, M.; Yuan, J.; Peng, J.; Ma, W. Greatly Reduced Processing Temperature for a Solution-Processed NiO x Buffer Layer in Polymer Solar Cells. Adv. Energy Mater. 2013, 3, 1614–1622. [Google Scholar] [CrossRef]
- Im, H.C.; Choo, D.C.; Kim, T.W.; Kim, J.H.; Seo, J.H.; Kim, Y.K. Highly efficient organic light-emitting diodes fabricated utilizing nickel-oxide buffer layers between the anodes and the hole transport layers. Thin Solid Films 2007, 515, 5099–5102. [Google Scholar] [CrossRef]
- Amin, S.; Tahira, A.; Solangi, A.; Mazzaro, R.; Ibupoto, Z.H.; Vomiero, A. A sensitive enzyme-free lactic acid sensor based on NiO nanoparticles for practical applications. Anal. Methods 2019, 11, 3578–3583. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Nagamuthu, S.; Muralidharan, G. Supercapacitor studies on NiO nanoflakes synthesized through a microwave route. ACS Appl. Mater. Interfaces 2013, 5, 2188–2196. [Google Scholar] [CrossRef]
- Seo, S.; Park, I.J.; Kim, M.; Lee, S.; Bae, C.; Jung, H.S.; Park, N.G.; Kim, J.Y.; Shin, H. An ultra-thin, un-doped NiO hole transporting layer of highly efficient (16.4%) organic-inorganic hybrid perovskite solar cells. Nanoscale 2016, 8, 11403–11412. [Google Scholar] [CrossRef]
- Natarajan, C.; Matsumoto, H.; Nogami, G. Improvement in electrochromic stability of electrodeposited nickel hydroxide thin film. J. Electrochem. Soc. 1997, 144. [Google Scholar] [CrossRef]
- Song, X.; Gao, L. Facile synthesis of polycrystalline NiO nanorods assisted by microwave heating. J. Am. Ceram. Soc. 2008, 91, 3465–3468. [Google Scholar] [CrossRef]
- Šurca, A.; Orel, B.; Pihlar, B.; Bukovec, P. Optical, spectroelectrochemical and structural properties of sol-gel derived Ni-oxide electrochromic film. J. Electroanal. Chem. 1996, 408, 83–100. [Google Scholar] [CrossRef]
- Wang, W.N.; Itoh, Y.; Lenggoro, I.W.; Okuyama, K. Nickel and nickel oxide nanoparticles prepared from nickel nitrate hexahydrate by a low pressure spray pyrolysis. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2004, 111, 69–76. [Google Scholar] [CrossRef]
- Qiu, Z.; Gong, H.; Zheng, G.; Yuan, S.; Zhang, H.; Zhu, X.; Zhou, H.; Cao, B. Enhanced physical properties of pulsed laser deposited NiO films via annealing and lithium doping for improving perovskite solar cell efficiency. J. Mater. Chem. C 2017, 5, 7084–7094. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, H.; Xiao, S.; Xue, Q.; Zhang, T.; Zhu, Z.; Li, Q.; Hu, C.; Yang, Y.; Hu, Z.; et al. Effects of a Molecular Monolayer Modification of NiO Nanocrystal Layer Surfaces on Perovskite Crystallization and Interface Contact toward Faster Hole Extraction and Higher Photovoltaic Performance. Adv. Funct. Mater. 2016, 26, 2950–2958. [Google Scholar] [CrossRef]
- Sun, J.; Lu, J.; Li, B.; Jiang, L.; Chesman, A.S.R.; Scully, A.D.; Gengenbach, T.R.; Cheng, Y.B.; Jasieniak, J.J. Inverted perovskite solar cells with high fill-factors featuring chemical bath deposited mesoporous NiO hole transporting layers. Nano Energy 2018, 49, 163–171. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, J.; Lin, F.; He, H.; Mao, J.; Wong, K.S.; Jen, A.K.Y.; Choy, W.C.H. Pinhole-free and surface-nanostructured niox film by room-Temperature solution process for high-performance flexible perovskite solar cells with good stability and reproducibility. ACS Nano 2016, 10, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Chen, P.; Que, M.; Xing, Y.; Que, W.; Niu, C.; Shao, J. Highly Efficient Flexible Perovskite Solar Cells Using Solution-Derived NiOx Hole Contacts. ACS Nano 2016, 10, 3630–3636. [Google Scholar] [CrossRef]
- Liu, S.; Liu, R.; Chen, Y.; Ho, S.; Kim, J.H.; So, F. Nickel oxide hole injection/transport layers for efficient solution-processed organic light-emitting diodes. Chem. Mater. 2014, 26, 4528–4534. [Google Scholar] [CrossRef]
- He, Q.; Yao, K.; Wang, X.; Xia, X.; Leng, S.; Li, F. Room-Temperature and Solution-Processable Cu-Doped Nickel Oxide Nanoparticles for Efficient Hole-Transport Layers of Flexible Large-Area Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 41887–41897. [Google Scholar] [CrossRef]
- Chen, W.; Liu, F.-Z.; Feng, X.-Y.; Djurišić, A.B.; Chan, W.K.; He, Z.-B. Cesium Doped NiO x as an Efficient Hole Extraction Layer for Inverted Planar Perovskite Solar Cells. Adv. Energy Mater. 2017, 7, 1700722. [Google Scholar] [CrossRef]
- Jung, J.W.; Chueh, C.C.; Jen, A.K.Y. A Low-Temperature, Solution-Processable, Cu-Doped Nickel Oxide Hole-Transporting Layer via the Combustion Method for High-Performance Thin-Film Perovskite Solar Cells. Adv. Mater. 2015, 27, 7874–7880. [Google Scholar] [CrossRef]
- Sun, X.; Li, B.; Metiu, H. Ethane activation by Nb-doped NiO. J. Phys. Chem. C 2013, 117, 23597–23608. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, D.; Yang, P.; Yang, L.; Qin, L.; Huang, Y.; Zhao, X. Sol-gel-processed yttrium-doped NiO as hole transport layer in inverted perovskite solar cells for enhanced performance. Appl. Surf. Sci. 2018, 441, 258–264. [Google Scholar] [CrossRef]
- Jun-Dar, H.; Wang-Ting, H.; Bo-Yu, L.; Yu-Ting, H.; Shr-Ting, W. Effect of Ag-doping on the characteristics of AgxNi1-xO transparent conducting oxide film and their applications in heterojunction diodes. J. Phys. D Appl. Phys. 2020, 53, 275107. [Google Scholar]
- Sharma, R.; Yadav, K. Effect of lattice defects on the structural and optical properties of Ni1—XAgXO (where X = 0.0, 0.01, 0.03, 0.05, 0.10 and 0.15) nanoparticles. Appl. Phys. A Mater. Sci. Process. 2018, 124. [Google Scholar] [CrossRef]
- Xie, Y.; Lu, K.; Duan, J.; Jiang, Y.; Hu, L.; Liu, T.; Zhou, Y.; Hu, B. Enhancing Photovoltaic Performance of Inverted Planar Perovskite Solar Cells by Cobalt-Doped Nickel Oxide Hole Transport Layer. ACS Appl. Mater. Interfaces 2018, 10, 14153–14159. [Google Scholar] [CrossRef]
- Keraudy, J.; Ferrec, A.; Richard-Plouet, M.; Hamon, J.; Goullet, A.; Jouan, P.Y. Nitrogen doping on NiO by reactive magnetron sputtering: A new pathway to dynamically tune the optical and electrical properties. Appl. Surf. Sci. 2017, 409, 77–84. [Google Scholar] [CrossRef]
- Chen, P.C.; Yang, S.H. Potassium-Doped Nickel Oxide as the Hole Transport Layer for Efficient and Stable Inverted Perovskite Solar Cells. ACS Appl. Energy Mater. 2019, 2, 6705–6713. [Google Scholar] [CrossRef]
- Islam, M.B.; Yanagida, M.; Shirai, Y.; Nabetani, Y.; Miyano, K. NiO x Hole Transport Layer for Perovskite Solar Cells with Improved Stability and Reproducibility. ACS Omega 2017, 2, 2291–2299. [Google Scholar] [CrossRef] [Green Version]
- Kwon, U.; Kim, B.-G.; Nguyen, D.C.; Park, J.-H.; Ha, N.Y.; Kim, S.-J.; Ko, S.H.; Lee, S.; Lee, D.; Park, H.J. Solution-Processible Crystalline NiO Nanoparticles for High-Performance Planar Perovskite Photovoltaic Cells. Sci. Rep. 2016, 6, 30759. [Google Scholar] [CrossRef] [Green Version]
- AlFaify, S.; Shkir, M. A facile one pot synthesis of novel pure and Cd doped PbI2 nanostructures for electro-optic and radiation detection applications. Opt. Mater. 2019, 88, 417–423. [Google Scholar] [CrossRef]
- Geng, X.; Lahem, D.; Zhang, C.; Li, C.-J.; Olivier, M.-G.; Debliquy, M. Visible light enhanced black NiO sensors for ppb-level NO2 detection at room temperature. Ceram. Int. 2019, 45, 4253–4261. [Google Scholar] [CrossRef]
- Xia, X.; Jiang, Y.; Wan, Q.; Wang, X.; Wang, L.; Li, F. Lithium and Silver Co-Doped Nickel Oxide Hole-Transporting Layer Boosting the Efficiency and Stability of Inverted Planar Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 44501–44510. [Google Scholar] [CrossRef] [PubMed]
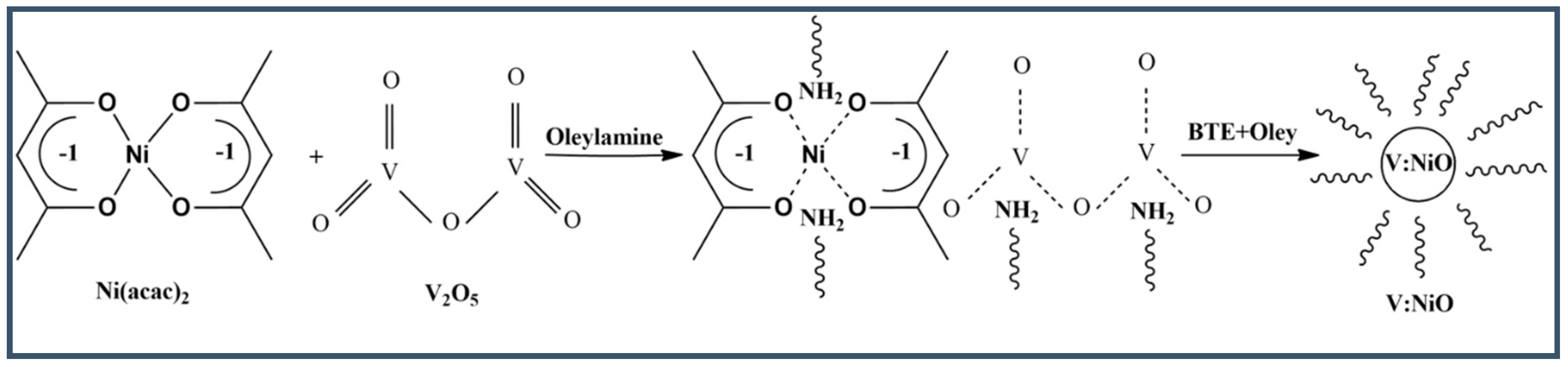
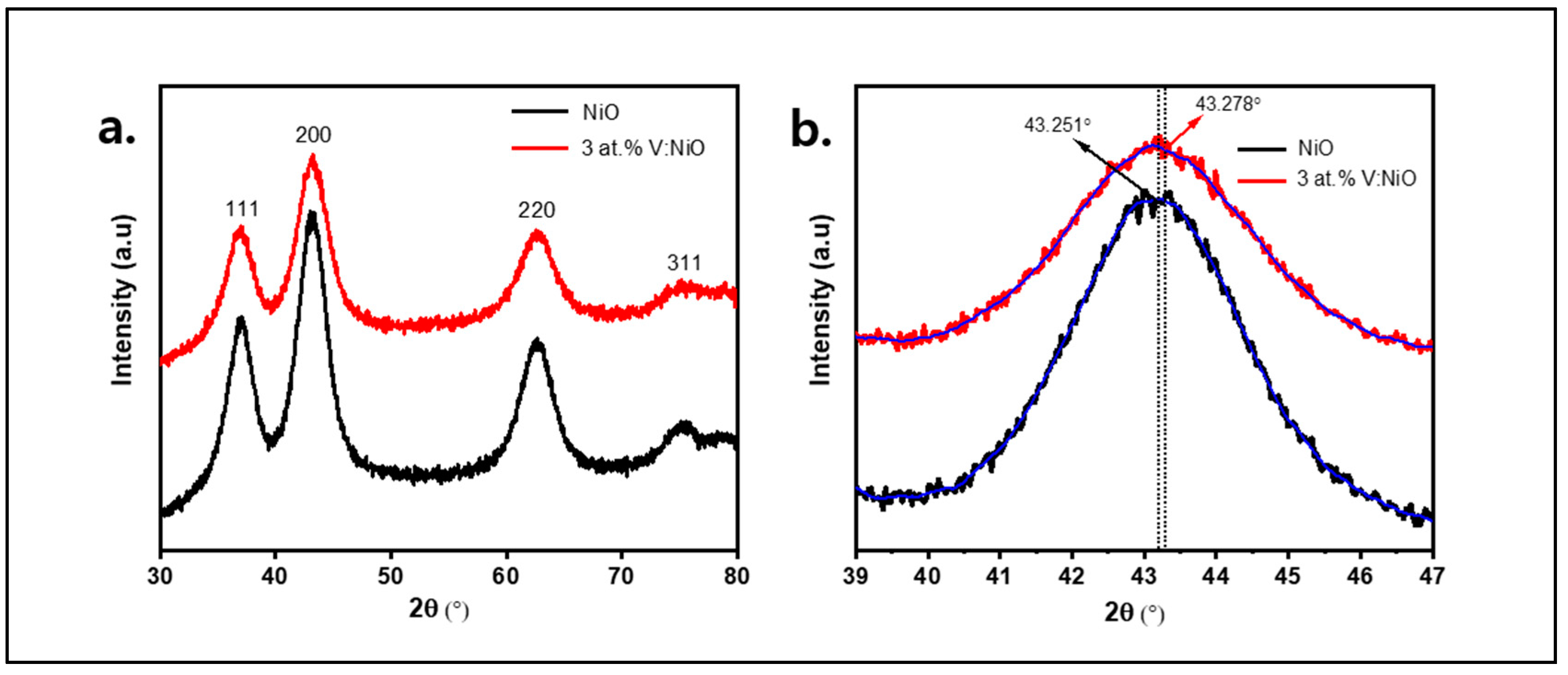
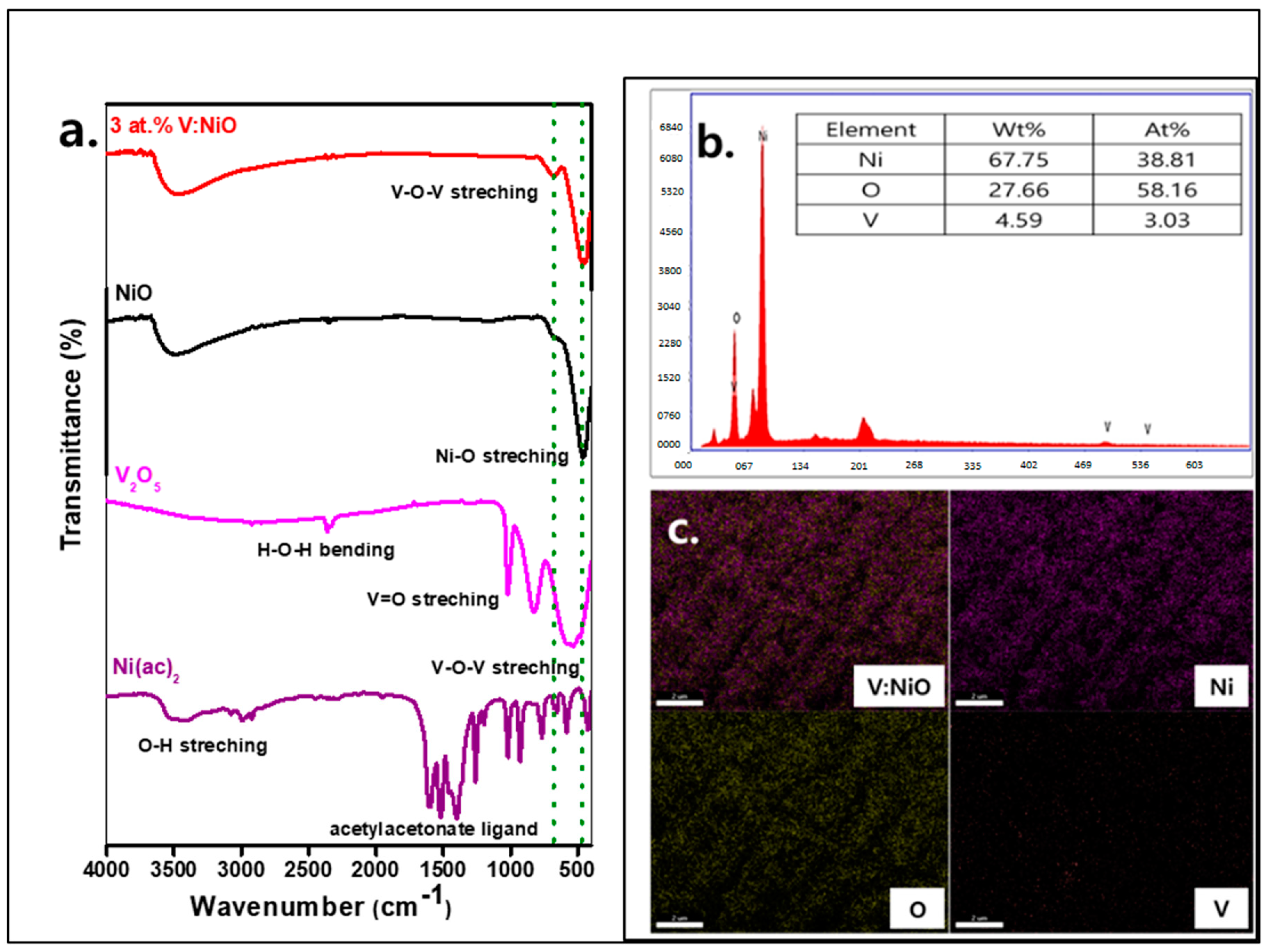

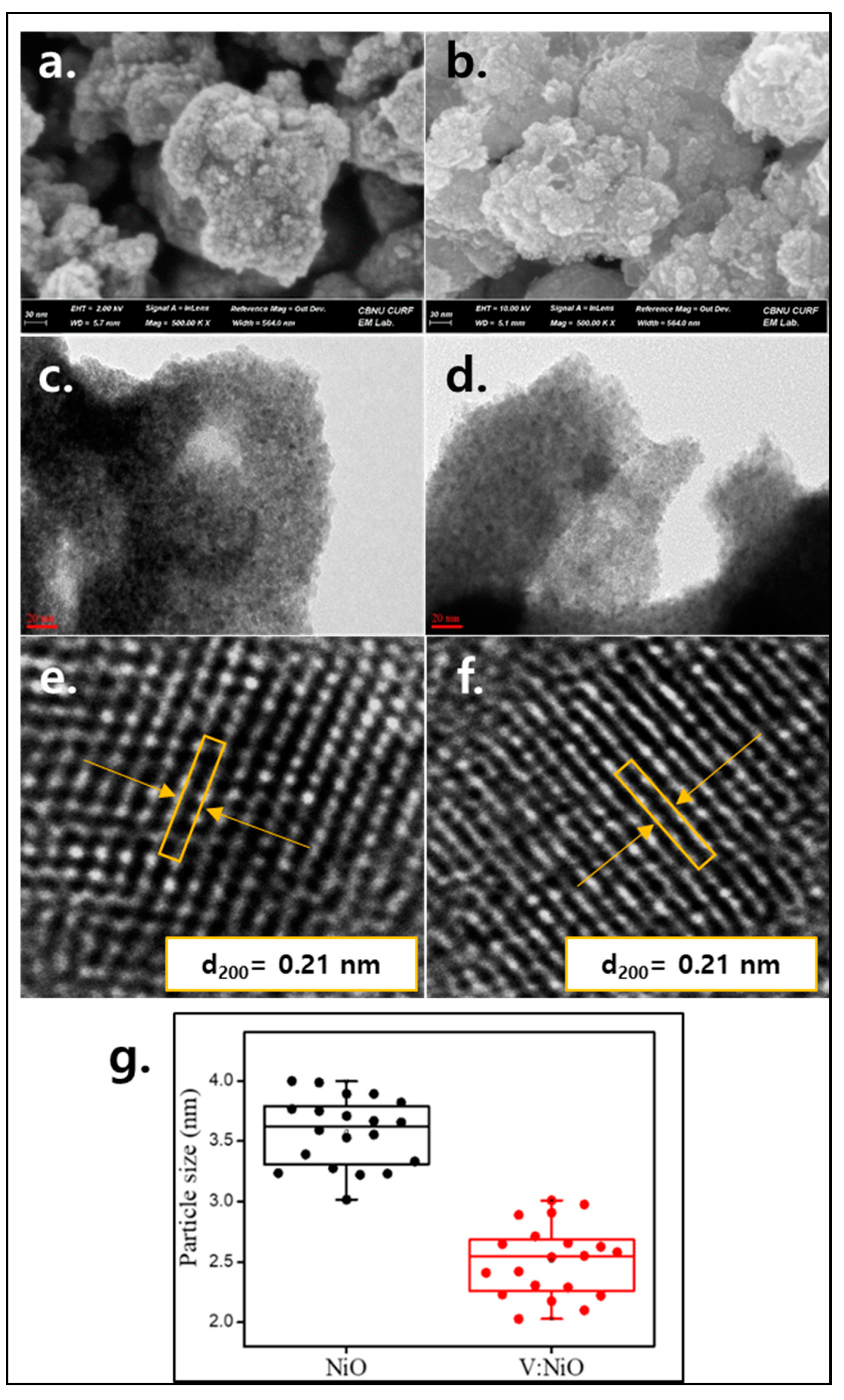

| Samples | 2θ (°) | hkl | FWHM | dsp (A°) | Dp (nm) | δ (nm−1) | ε × 10−2 |
|---|---|---|---|---|---|---|---|
| NiO | 43.251 | 200 | 2.94 | 2.095 | 3.04 | 0.1082 | 0.955 |
| 3 at.%V:NiO | 43.278 | 200 | 3.17 | 2.094 | 2.82 | 0.1257 | 1.054 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotta, A.; Seo, H.K. Facile Synthesis of Highly Conductive Vanadium-Doped NiO Film for Transparent Conductive Oxide. Appl. Sci. 2020, 10, 5415. https://doi.org/10.3390/app10165415
Kotta A, Seo HK. Facile Synthesis of Highly Conductive Vanadium-Doped NiO Film for Transparent Conductive Oxide. Applied Sciences. 2020; 10(16):5415. https://doi.org/10.3390/app10165415
Chicago/Turabian StyleKotta, Ashique, and Hyung Kee Seo. 2020. "Facile Synthesis of Highly Conductive Vanadium-Doped NiO Film for Transparent Conductive Oxide" Applied Sciences 10, no. 16: 5415. https://doi.org/10.3390/app10165415
APA StyleKotta, A., & Seo, H. K. (2020). Facile Synthesis of Highly Conductive Vanadium-Doped NiO Film for Transparent Conductive Oxide. Applied Sciences, 10(16), 5415. https://doi.org/10.3390/app10165415






