Vitamin D Merging into Immune System-Skeletal Muscle Network: Effects on Human Health
Abstract
1. Introduction
2. Vitamin D Coordinates the Anti-Inflammatory Immune Response
3. Vitamin D Orchestrates Skeletal Muscle Integrity
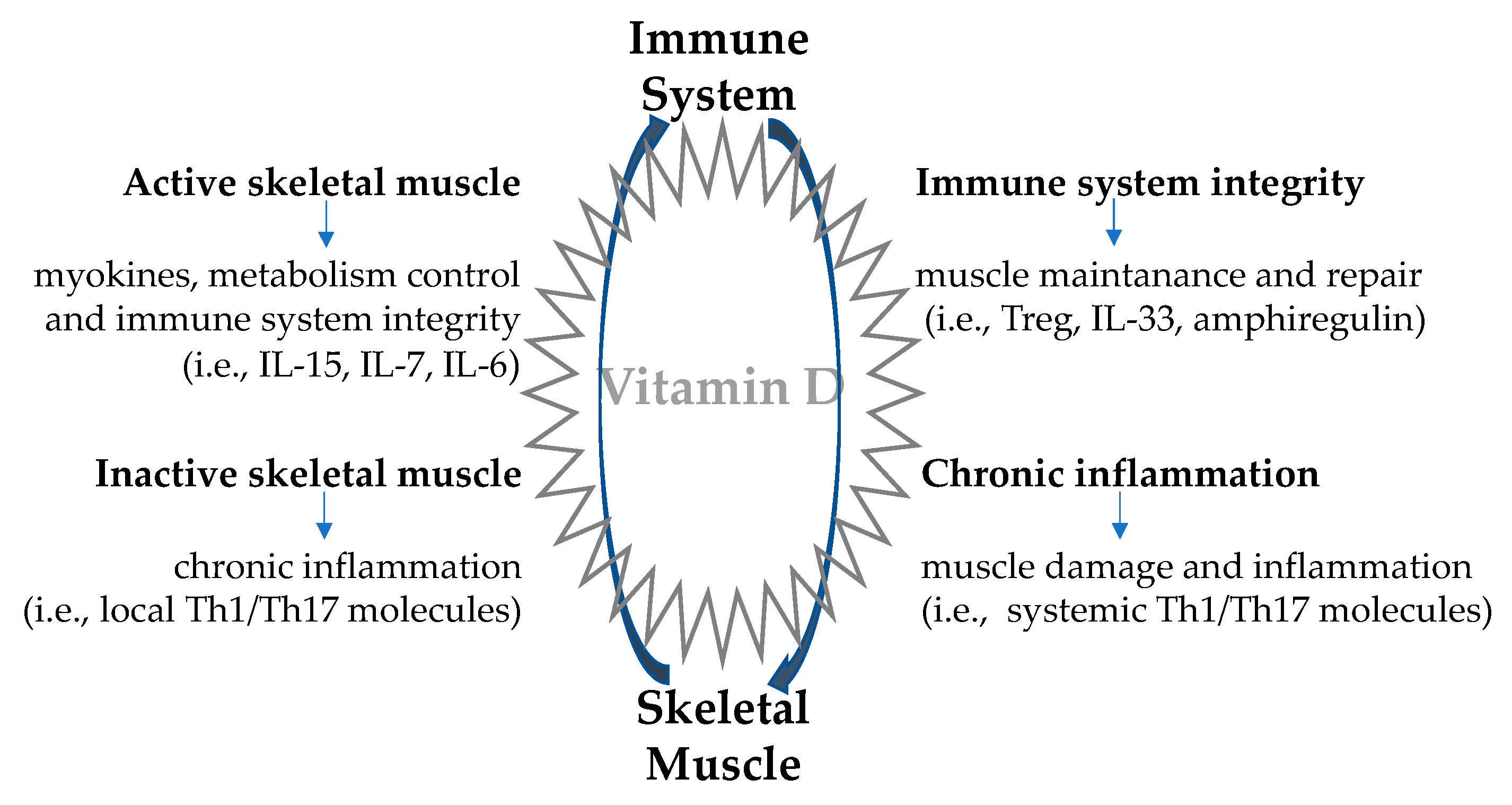
4. Skeletal Muscle-Immune System Interplay: A Two-Way Route
4.1. Immune Regulation of Skeletal Muscle Function
4.2. Skeletal Muscle Regulation of the Immune Function
5. Myokines, Immune Regulation, and Vitamin D: Converging Cell Signaling
6. Vitamin D Determination and Supplementation: Still Open Issues
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Holick, M.F.; MacLaughlin, J.A.; Doppelt, S.H. Regulation of cutaneous previtamin D3 photosynthesis in man: Skin pigment is not an essential regulator. Science 1981, 211, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.O.; Aspinall, R. Can We Translate Vitamin D Immunomodulating Effect on Innate and Adaptive Immunity to Vaccine Response? Nutrients 2015, 7, 2044–2060. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; White, J.H. The pleiotropic actions of vitamin D. Bioessays 2004, 26, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Haussler, M.R.; Norman, A.W. Chromosomal receptor for a vitamin D metabolite. Proc. Natl. Acad. Sci. USA 1969, 62, 155–162. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D: A millenium perspective. J. Cell Biochem. 2003, 88, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Mellanby, T. The part played by an “accessory factor” in the production of experimental rickets. J. Physiol. 1918, 52, 11–14. [Google Scholar]
- Holick, M.F. The cutaneous photosynthesis of previtamin D3: A unique photoendocrine system. J. Investig. Dermatol. 1981, 77, 51–58. [Google Scholar] [CrossRef]
- Grad, R. Cod and the consumptive: A brief history of cod-liver oil in the treatment of pulmonary tuberculosis. Pharm. Hist. 2004, 46, 106–120. [Google Scholar]
- Verstuyf, A.; Carmeliet, G.; Bouillon, R.; Mathieu, C. Vitamin D: A pleiotropic hormone. Kidney Int. 2010, 78, 140–145. [Google Scholar] [CrossRef]
- Wang, T.-T.; Tavera-Mendoza, L.E.; Laperriere, D.; Libby, E.; MacLeod, N.B.; Nagai, Y.; Bourdeau, V.; Konstorum, A.; Lallemant, B.; Zhang, R.; et al. Largescale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genes. Mol. Endocrinol. 2005, 19, 2685–2695. [Google Scholar] [CrossRef]
- Bikle, D. Nonclassic Actions of Vitamin D. JCEM 2009, 94, 26–34. [Google Scholar] [CrossRef] [PubMed]
- McGill, A.-T.; Stewart, J.M.; Lithander, F.E.; Strik, C.M.; Poppitt, S.D. Relationships of Low Serum Vitamin D3 With Anthropometry and Markers of the Metabolic Syndrome and Diabetes in Overweight and Obesity. Nutr. J. 2008, 28, 7. [Google Scholar] [CrossRef]
- Pittas, A.G.; Lau, J.; Hu, F.B.; Dawson-Hughes, B. The Role of Vitamin D and Calcium in Type 2 Diabetes. A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2007, 92, 2017–2029. [Google Scholar] [CrossRef] [PubMed]
- Kunadian, V.; Ford, G.A.; Bawamia, B.; Qiu, W.; Manson, J.E. Vitamin D deficiency and coronary artery disease: A review of the evidence. Am. Heart J. 2014, 167, 283–291. [Google Scholar] [CrossRef]
- Mozos, I.; Marginean, O. Links between Vitamin D Deficiency and Cardiovascular Diseases. Biomed. Res. Int. 2015, 2015, 109275. [Google Scholar] [CrossRef]
- Polly, P.; Tan, T.C. The role of vitamin D in skeletal and cardiac muscle function. Front. Physiol. 2014, 5, 145. [Google Scholar] [CrossRef]
- Illescas-Montes, R.; Melguizo-Rodríguez, L.; Ruiz, C.; Costela-Ruiz, V.J. Vitamin D and autoimmune diseases. Life Sci. 2019, 233, 116744. [Google Scholar] [CrossRef]
- Aranaw, C. Vitamin D and the Immune System. J. Investig. Med. 2011, 59, 881–886. [Google Scholar] [CrossRef]
- Crescioli, C.; Minisola, S. Vitamin D: Autoimmunity and Gender. Curr. Med. Chem. 2017, 24, 2671–2686. [Google Scholar] [CrossRef]
- Kamen, D.L.; Tangpricha, V.J. Vitamin D and molecular actions on the immune system: Modulation of innate and autoimmunity. Mol. Med. 2010, 88, 441–450. [Google Scholar] [CrossRef]
- Dzik, K.P.; Kaczor, J.J. Mechanisms of Vitamin D on Skeletal Muscle Function: Oxidative Stress, Energy Metabolism and Anabolic State. Eur. J. Appl. Physiol. 2019, 119, 825–839. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Adler, R.A.; Binkley, N.; Bollerslev, J.; Bouillon, R.; Dawson-Hughes, B.; Ebeling, P.R.; Feldman, D.; Formenti, A.M.; Lazaretti-Castro, M.; et al. Consensus Statement From 2nd International Conference on Controversies in Vitamin D. Rev. Endocr. Metab. Disord. 2020, 21, 89–116. [Google Scholar] [CrossRef] [PubMed]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the Immune System. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Provvedini, D.M.; Tsoukas, C.D.; Deftos, L.J.; Manolagas, S.C. 1,25-Dihydroxyvitamin D3 receptors in human leukocytes. Science 1983, 221, 1181–1183. [Google Scholar] [CrossRef]
- Takahashi, K.; Nakayama, Y.; Horiuchi, H.; Ohta, T.; Komoriya, K.; Ohmori, H.; Kamimura, T. Human neutrophils express messenger RNA of vitamin D receptor and respond to 1alpha,25-dihydroxyvitamin D3. Immunopharmacol. Immunotoxicol. 2002, 24, 335–347. [Google Scholar] [CrossRef]
- Hewison, M.; Freeman, L.; Hughes, S.V.; Evans, K.N.; Bland, R.; Eliopoulos, A.G.; Kilby, M.D.; Moss, P.A.; Chakraverty, R. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J. Immunol. 2003, 170, 5382–5390. [Google Scholar] [CrossRef]
- Kreutz, M.; Andreesen, R.; Krause, S.W.; Szabo, A.; Ritz, E.; Reichel, H. 1,25-dihydroxyvitamin D3 production and vitamin D3 receptor expression are developmentally regulated during differentiation of human monocytes into macrophages. Blood 1993, 82, 1300–1307. [Google Scholar] [CrossRef]
- Bikle, D.D. What is new in vitamin D: 2006–2007. Curr. Opin. Rheumatol. 2007, 19, 383–388. [Google Scholar] [CrossRef]
- Adorini, L.; Penna, G. Control of autoimmune diseases by the vitamin D endocrine system. Nat. Clin. Pract. Rheumatol. 2008, 4, 404–412. [Google Scholar] [CrossRef]
- Dong, X.; Bachman, L.A.; Kumar, R.; Griffin, M.D. Generation of antigen-specific interleukin -10-producingT-cells using dendritic cell stimulation and steroid hormone conditioning. Transpl. Immunol. 2003, 11, 323–333. [Google Scholar] [CrossRef]
- Almerighi, C.; Sinistro, A.; Cavazza, A.; Ciaprini, C.; Rocchi, G.; Bergamini, A. 1Alpha, 25-dihydroxyvitamin D3 inhibits CD40L-induced pro-inflammatory and immunomodulatory activity in human monocytes. Cytokine 2009, 45, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Penna, G.; Roncari, A.; Amuchastegui, S.; Daniel, K.C.; Berti, E.; Colonna, M.; Adorini, L. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1, 25dihydroxyvitamin D3. Blood 2005, 106, 3490–3497. [Google Scholar] [CrossRef]
- van der Aar, A.M.; Sibiryak, D.S.; Bakdash, G.; van Capel, T.M.; van der Kleij, H.P.; Opstelten, D.J.; Teunissen, M.B.; Kapsenberg, M.L.; de Jong, E.C. Vitamin D3 targets epidermal and dermal dendritic cells for induction of distinct regulatory T cells. J. Allergy Clin. Immunol. 2011, 127, 1532–1540. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.R.; Iwata, M.; VonAndrian, U.H. Vitamin effects on the immune system: Vitamins A and D take centre stage. Nat. Rev. Immunol. 2008, 8, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sims, G.P.; Chen, X.X.; Gu, Y.Y.; Chen, S.; Lipsky, P.E. Modulatory effects of 1, 25-dihydroxyvitamin D3 on human B cell differentiation. J. Immunol. 2007, 179, 1634–1647. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, H.A.; Borchers, M.; Gudat, F.; Duermueller, U.; Theiler, R.; Stähelin, H.B.; Dick, W. In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem. J. 2001, 33, 19–24. [Google Scholar] [CrossRef]
- Girgis, C.M.; Mokbel, N.; Cha, K.M.; Houweling, P.J.; Abboud, M.; Fraser, D.R.; Mason, R.S.; Clifton-Bligh, R.J.; Gunton, J.E. The vitamin D receptor (VDR) is expressed in skeletal muscle of male mice and modulates 25-hydroxyvitamin D (25OHD) uptake in myofibers. Endocrinology 2014, 155, 3227–3237. [Google Scholar] [CrossRef]
- Abboud, M.; Rybchyn, M.S.; Ning, Y.J.; Brennan-Speranza, T.C.; Girgis, C.M.; Gunton, J.E.; Fraser, D.R.; Mason, R.S. 1,25-Dihydroxycholecalciferol (calcitriol) modifies uptake and release of 25-hydroxycholecalciferol in skeletal muscle cells in culture. J. Steroid Biochem. Mol. Biol. 2018, 177, 109–115. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Lieberherr, M.; Fritsch, J.; Guillozo, H.; Alvarez, M.L.; Fitouri, Z.; Jehan, F.; Garabédian, M. The rapid effects of 1,25-dihydroxyvitamin D3 require the vitamin D receptor and influence 24-hydroxylase activity: Studies in human skin fibroblasts bearing vitamin D receptor mutations. Biol. Chem. 2004, 279, 7591–7597. [Google Scholar] [CrossRef]
- Tanaka, M.; Kishimoto, K.N.; Okuno, H.; Saito, H.; Itoi, E. Vitamin D receptor gene silencing effects on differentiation of myogenic cell lines. Muscle Nerve 2014, 49, 700–708. [Google Scholar] [CrossRef]
- Endo, I.; Inoue, D.; Mitsui, T.; Umaki, Y.; Akaike, M.; Yoshizawa, T.; Kato, S.; Matsumoto, T. Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myoregulatory transcription factors. Endocrinology 2003, 144, 5138–5144. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Borchers, M.; Gudat, F.; Dürmüller, U.; Stähelin, H.B.; Dick, W. Vitamin D receptor expression in human muscle tissue decreases with age. J. Bone Miner. Res. 2004, 19, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Koundourakis, N.E.; Margioris, A.N. Vitamin D and Physical Activity. In A Critical Evaluation of Vitamin D—Basic Overview; Gowder, S.J.T., Ed.; Qassim University: Buraydah, Saudi Arabia, 2017. [Google Scholar] [CrossRef]
- Marawan, A.; Nargiza Kurbanova, N.; Qayyum, R. Association between serum vitamin D levels and cardiorespiratory fitness in the adult population of the USA. Eur. J. Prev. Cardiol. 2018, 26, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Turinese, I.; Marinelli, P.; Bonini, M.; Rossetti, M.; Statuto, G.; Filardi, T.; Paris, A.; Lenzi, A.; Morano, S.; Palange, P.J. Metabolic and cardiovascular response to exercise in patients with type 1 diabetes. Endocrinol. Investig. 2017, 40, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Winzer, E.B.; Woitek, F.; Linke, A. Physical Activity in the Prevention and Treatment of Coronary Artery Disease. J. Am. Heart Assoc. 2018, 7, e007725. [Google Scholar] [CrossRef]
- Mason, R.S.; Rybchyn, M.S.; Abboud, M.; Brennan-Speranza, T.C.; Fraser, D.R. The Role of Skeletal Muscle in Maintaining Vitamin D Status in Winter. Curr. Dev. Nutr. 2019, 3, nzz087. [Google Scholar] [CrossRef]
- Antinozzi, C.; Corinaldesi, C.; Giordano, C.; Pisano, A.; Cerbelli, B.; Migliaccio, S.; Di Luigi, L.; Stefanantoni, K.; Vannelli, G.B.; Minisola, S.; et al. Potential role for the VDR agonist elocalcitol in metabolic control: Evidences in human skeletal muscle cells. J. Steroid Biochem. Mol. Biol. 2017, 167, 169–181. [Google Scholar] [CrossRef]
- Wang, Y.; DeLuca, H.F. Is the Vitamin D Receptor Found in Muscle? Endocrinology 2011, 152, 354–363. [Google Scholar] [CrossRef]
- Lepreux, S.; Hainfellner, J.A.; Vital, A. Idiopathic inflammatory myopathies overlapping with systemic diseases. Clin. Neuropathol. 2018, 37, 6–15. [Google Scholar] [CrossRef]
- Filardi, T.; Tavaglione, F.; Di Stasio, M.; Fazio, V.; Lenzi, A.; Morano, S. Impact of risk factors for gestational diabetes (GDM) on pregnancy outcomes in women with GDM. J. Endocrinol. Investig. 2018, 41, 671–676. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, S.; Gao, T.; Zhong, F.; Cai, J.; Sun, Y.; Ma, A. Association between Sarcopenia and Metabolic Syndrome in Middle-Aged and Older Non-Obese Adults: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 364. [Google Scholar] [CrossRef] [PubMed]
- Saini, J.; McPhee, J.S.; Al-Dabbagh, S.; Stewart, C.E.; Al-Shanti, N. Regenerative function of immune system: Modulation of muscle stem cells. Ageing Res. Rev. 2016, 27, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Kuswanto, W.; Burzyn, D.; Panduro, M.; Wang, K.K.; Jang, Y.C.; Wagers, A.J.; Christophe Benoist, C.; Mathis, D. Poor repair of skeletal muscle in aging mice reflects a defect in local, interleukin-33-dependent accumulation of regulatory T cells. Immunity 2016, 44, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Burzyn, D.; Kuswanto, W.; Kolodin, D.; Shadrach Jennifer, L.; Cerletti, M.; Jang, Y.; Sefik, E.; Tan, T.G.; Wagers, A.J.; Benoist, C.; et al. A special population of regulatory t cells potentiates muscle repair. Cell 2013, 155, 1282–1295. [Google Scholar] [CrossRef]
- Allenbach, Y.; Solly, S.; Gregoire, S.; Dubourg, O.; Salomon, B.; Butler-Browne, G.; Musset, L.; Herson, S.; Klatzmann, D.; Benveniste, O. Role of regulatory t cells in a new mouse model of experimental autoimmune myositis. Am. J. Pathol. 2009, 174, 989–998. [Google Scholar] [CrossRef]
- Murray, P.J. Macrophage polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Tonkin, J.; Temmerman, L.; Sampson, R.D.; Gallego-Colon, E.; Barberi, L.; Bilbao, D.; Schneider, M.D.; Musarò, A.; Rosenthal, N. Monocyte/Macrophage-derived IGF-1 orchestrates murine skeletal muscle regeneration and modulates autocrine polarization. Mol. Ther. 2015, 23, 1189–1200. [Google Scholar] [CrossRef]
- Junnila, R.K.; List, E.O.; Berryman, D.E.; Murrey, J.W.; Kopchick, J.J. The GH/IGF-1 axis in ageing and longevity. Nat. Rev. Endocrinol. 2013, 9, 366–376. [Google Scholar] [CrossRef]
- Reidy, P.T.; McKenzie, A.I.; Mahmassani, Z.S.; Petrocelli, J.J.; Nelson, D.B.; Lindsay, C.C.; Gardner, J.E.; Morrow, V.R.; Keefe, A.C.; Huffaker, T.B.; et al. Aging impairs mouse skeletal muscle macrophage polarization and muscle-specific abundance during recovery from disuse. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E85–E98. [Google Scholar] [CrossRef]
- Nelke, C.; Dziewas, R.; Minnerup, J.; Meuth, S.G.; Tobias Ruck, T. Skeletal Muscle as Potential Central Link Between Sarcopenia and Immune Senescence. EBioMedicine 2019, 49, 381–388. [Google Scholar] [CrossRef]
- Bano, G.; Trevisan, C.; Carraro, S.; Solmi, M.; Luchini, C.; Stubbs, B.; Manzato, E.; Sergi, G.; Veronese, N. Inflammation and sarcopenia: A systematic review and meta-analysis. Maturitas 2017, 96, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Dalle, S.; Rossmeislova, L.; Koppo, K. The role of inflammation in age-related sarcopenia. Front. Physiol. 2017, 8, 1045. [Google Scholar] [CrossRef] [PubMed]
- Alemán, H.; Esparza, J.; Ramirez, F.A.; Astiazaran, H.; Payette, H. Longitudinal evidence on the association between interleukin-6 and C-reactive protein with the loss of total appendicular skeletal muscle in free-living older men and women. Age Ageing 2011, 40, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Afzali, A.M.; Muntefering, T.; Wiendl, H.; Meuth, S.G.; Ruck, T. Skeletal muscle cells actively shape (auto)immune responses. Autoimmun. Rev. 2018, 17, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Cosquéric, G.; Sebag, A.; Ducolombier, C.; Thomas, C.; Piette, F.; Weill-Engerer, S. Sarcopenia is predictive of nosocomial infection in care of the elderly. Br. J. Nutr. 2007, 96, 895–901. [Google Scholar] [CrossRef]
- Altuna-Venegas, S.; Aliaga-Vega, R.; Maguina, J.L.; Parodi, J.F.; Runzer-Colmenares, F.M. Risk of community-acquired pneumonia in older adults with sarcopenia of a hospital from Callao, Peru 2010–2015. Arch. Gerontol. Geriatr. 2019, 82, 100–105. [Google Scholar] [CrossRef]
- Maeda, K.; Akagi, J. Muscle mass loss is a potential predictor of 90-day mortality in older adults with aspiration pneumonia. J. Am. Geriatr. Soc. 2017, 65, e18–e22. [Google Scholar] [CrossRef]
- Kakanis, M.W.; Peake, J.; Brenu, E.W.; Simmonds, M.; Gray, B.; Hooper, S.L.; Marshall-Gradisnik, S.M. The open window of susceptibility to infection after acute exercise in healthy young male elite athletes. Exerc. Immunol. Rev. 2010, 16, 119–137. [Google Scholar] [CrossRef]
- Walsh, N.P. Recommendations to Maintain Immune Health in Athletes. Eur. J. Sport Sci. 2018, 18, 820–831. [Google Scholar] [CrossRef]
- Simpson, R.J.; Campbell, J.P.; Gleeson, M.; Karsten Krüger, K.; Nieman, D.C.; Pyne, D.B.; Turner, J.E.; Walsh, N.P. Can exercise affect immune function to increase susceptibility to infection? Exerc. Immunol. Rev. 2020, 26, 8–22. [Google Scholar]
- Pedersen, B.K. Muscle as a secretory organ. Compr. Physiol. 2013, 3, 1337–1362. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Junior, H.J.; Picca, A.; Calvani, R.; Uchida, M.C.; Marzetti, E. If my muscle could talk: Myokines as a biomarker of frailty. Exp. Gerontol. 2019, 127, 110715. [Google Scholar] [CrossRef] [PubMed]
- Giudice, J.; Taylor, J.M. Muscle as a paracrine and endocrine organ. Curr. Opin. Pharmacol. 2017, 34, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.; Febbraio, M. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Girard, D.; Paquet, M.E.; Paquin, R.; Beaulieu, A.D. Differential effects of interleukin-15 (IL-15) and IL-2 on human neutrophils: Modulation of phagocytosis, cytoskeleton rearrangement, gene expression, and apoptosis by IL-15. Blood 1996, 88, 3176. [Google Scholar] [CrossRef]
- Conlon, K.C.; Lugli, E.; Welles, H.C.; Rosenberg, S.A.; Fojo, A.T.; Morris, J.C.; Fleisher, T.A.; Dubois, S.P.; Perera, L.P.; Stewart, D.M.; et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 t cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J. Clin. Oncol. 2015, 33, 74–82. [Google Scholar] [CrossRef]
- Kennedy, M.K.; Glaccum, M.; Brown, S.N.; Butz, E.A.; Viney, J.L.; Embers, M.; Matsuki, N.; Charrier, K.; Sedger, L.; Willis, C.R.; et al. Reversible defects in natural killer and memory CD8 t cell lineages in interleukin 15-deficient mice. J. Exp. Med. 2000, 191, 771–780. [Google Scholar] [CrossRef]
- Duggal, N.A.; Pollock, R.D.; Lazarus, N.R.; Harridge, S.; Lord, J.M. Major features of immunesenescence, including reduced thymic output, are ameliorated by high levels of physical activity in adulthood. Aging Cell 2018, 17, e12750. [Google Scholar] [CrossRef]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef]
- Krutzik, S.R.; Hewison, M.; Liu, P.T.; Robles, J.A.; Stenger, S.; Adams, J.S.; Modlin, R.L. IL-15 Links TLR2/1-Induced Macrophage Differentiation to the Vitamin D-Dependent Antimicrobial Pathway. J. Immunol. 2008, 181, 7115–7120. [Google Scholar] [CrossRef]
- Gallagher, J.C.; Bikle, D. Vitamin D: Mechanisms of Action and Clinical Applications. Endocrinol. Metab. Clin. N. Am. 2017, 46, xvii–xviii. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.W.; Teles, R.M.B.; Haile, S.; Liu, P.T.; Modlin, R.L. Vitamin D status contributes to the antimicrobial activity of macrophages against Mycobacterium leprae. PLoS Negl. Trop. Dis. 2018, 12, e0006608. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Silwal, P.; Kim, I.; Modlin, R.L.; Jo, E.-K. Vitamin D-Cathelicidin Axis: At the Crossroads between Protective Immunity and Pathological Inflammation during Infection. Immune Netw. 2020, 20, e12. [Google Scholar] [CrossRef] [PubMed]
- Olmos-Ortiz, A.; García-Quiroz, J.; López-Marure, R.; González-Curiel, I.; Rivas-Santiago, B.; Olivares, A.; Avila, E.; Barrera, D.; Halhali, A.; Caldiño, F.; et al. Evidence of sexual dimorphism in placental vitamin D metabolism: Testosterone inhibits calcitriol-dependent cathelicidin expression. J. Steroid Biochem. Mol. Biol. 2016, 163, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Ingersoll, M.A. Sex differences shape the response to infectious diseases. PLoS Pathog. 2017, 13, e1006688. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Dalbeni, A.; Beatrice, G. Coronavirus disease 2019 (COVID-19): We don’t leave women alone. Int. J. Public Health 2020, 65, 235–236. [Google Scholar] [CrossRef] [PubMed]
- Haugen, F.; Norheim, F.; Lian, H.; Wensaas, A.J.; Dueland, S.; Berg, O.; Funderud, A.; Skålhegg, B.S.; Raastad, T.; Drevon, C.A. IL-7 is expressed and secreted by human skeletal muscle cells. Am. J. Physiol. Cell Physiol. 2010, 298, C807–C816. [Google Scholar] [CrossRef]
- Chien, Y.H.; Meyer, C.; Bonneville, M. γδ T cells: First line of defense and beyond. Annu. Rev. Immunol. 2014, 32, 121–155. [Google Scholar] [CrossRef]
- Bazdar, D.A.; Kalinowska, M.; Sieg, S.F. Interleukin-7 receptor signaling is deficient in CD4+ T cells from HIV-infected persons and is inversely associated with aging. J. Infect. Dis. 2009, 199, 1019–1028. [Google Scholar] [CrossRef]
- Aspinall, R. T cell development, ageing and interleukin-7. Mech. Ageing Dev. 2006, 127, 572–578. [Google Scholar] [CrossRef]
- Pellegrini, M.; Calzascia, T.; Toe, J.G.; Preston, S.P.; Lin, A.E.; Elford, A.R.; Shahinian, A.; Lang, P.A.; Lang, K.S.; Morre, M.; et al. IL-7 Engages Multiple Mechanisms to Overcome Chronic Viral Infection and Limit Organ Pathology. Cell 2011, 144, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Parish, I.A.; Kaech, S.M. IL-7 Knocks the Socs Off Chronic Viral Infection. Cell 2011, 144, 467–468. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bordon, Y. IL-7 goes antiviral. Nat. Rev. Immunol. 2011, 11, 159. [Google Scholar] [CrossRef] [PubMed]
- Capri, M.; Monti, D.; Salvioli, S.; Lescai, F.; Pierini, M.; Altilia, S.; Sevini, F.; Valensin, S.; Ostan, R.; Bucci, L.; et al. Complexity of Anti-immunosenescence Strategies in Humans. Artif. Organs 2006, 30, 730–742. [Google Scholar] [CrossRef] [PubMed]
- Aiello, A.; Farzaneh, F.; Candore, G.; Caruso, C.; Davinelli, S.; Gambino, C.M.; Ligotti, M.E.; Zareian, N.; Accardi, G. Immunosenescence and Its Hallmarks: How to Oppose Aging Strategically? A Review of Potential Options for Therapeutic Intervention. Front. Immunol. 2019, 10, 2247. [Google Scholar] [CrossRef] [PubMed]
- Dooms, H. Interleukin-7: Fuel for the autoimmune attack. J. Autoimmun. 2013, 45, 40–48. [Google Scholar] [CrossRef]
- Alkhedaide, A.Q.H.; Alshehri, Z.S.; Soliman, M.M.; Althumali, K.W.; Abu-Elzahab, H.S.; Baiomy, A.A.A. Vitamin D3 supplementation improves immune and inflammatory response in vitamin D deficient adults in Taif, Saudi Arabia. Biomed. Res. 2016, 27, 1049–1053. [Google Scholar]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Catoire, M.; Kersten, S. The search for exercise factors in humans. FASEB J. 2015, 29, 1615–1628. [Google Scholar] [CrossRef]
- Steensberg, A.; Fischer, C.P.; Keller, C.; Moller, K.; Pedersen, B.K. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E433–E437. [Google Scholar] [CrossRef]
- Fuster, J.J.; Walsh, K. The Good, the Bad, and the Ugly of interleukin-6 signaling. EMBO J. 2014, 33, 1425–1427. [Google Scholar] [CrossRef] [PubMed]
- Yasukawa, H.; Ohishi, M.; Mori, H.; Murakami, M.; Chinen, T.; Aki, D.; Hanada, T.; Takeda, K.; Akira, S.; Hoshijima, M.; et al. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat. Immunol. 2003, 4, 551–556. [Google Scholar] [CrossRef]
- Fischer, C.P. Interleukin-6 in acute exercise and training: What is the biological relevance? Exerc. Immunol. Rev. 2006, 12, 6–33. [Google Scholar] [PubMed]
- Pedersen, B.K. Muscular interleukin-6 and its role as an energy sensor. Med. Sci. Sports Exerc. 2012, 44, 392–396. [Google Scholar] [CrossRef]
- Steensberg, A.; Febbraio, M.A.; Osada, T.; Schjerling, P.; van Hall, G.; Saltin, B.; Pedersen, B.K. Interleukin-6 production in contracting human skeletal muscle is influenced by pre-exercise muscle glycogen content. J. Physiol. 2001, 537, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Crescioli, C. Targeting Age-Dependent Functional and Metabolic Decline of Human Skeletal Muscle: The Geroprotective Role of Exercise, Myokine IL-6, and Vitamin D. Int. J. Mol. Sci. 2020, 21, 1010. [Google Scholar] [CrossRef] [PubMed]
- Ashtary-Larky, D.; Kheirollah, A.; Bagheri, R.; Ghaffari, M.A.; Mard, S.A.; Hashemi, S.J.; Mir, I.; Wong, A. A single injection of vitamin D3 improves insulin sensitivity and β-cell function but not muscle damage or the inflammatory and cardiovascular responses to an acute bout of resistance exercise in vitamin D-deficient resistance-trained males. Br. J. Nutr. 2020, 123, 394–401. [Google Scholar] [CrossRef]
- Kim, H.; Baek, S.; Hong, S.M.; Lee, J.; Jung, S.M.; Lee, J.; Cho, M.L.; Kwok, S.K.; Park, S.H. 1,25-dihydroxy Vitamin D3 and Interleukin-6 Blockade Synergistically Regulate Rheumatoid Arthritis by Suppressing Interleukin-17 Production and Osteoclastogenesis. J. Korean Med. Sci. 2020, 35, e40. [Google Scholar] [CrossRef]
- Di Luigi, L.; Sottili, M.; Antinozzi, C.; Vannelli, G.B.; Romanelli, F.; Riccieri, V.; Valesini, G.; Lenzi, A.; Crescioli, C. The vitamin D receptor agonist BXL-01-0029 as a potential new pharmacological tool for the treatment of inflammatory myopathies. PLoS ONE 2013, 8, e77745. [Google Scholar] [CrossRef]
- Figarella-Branger, D.; Civatte, M.; Bartoli, C.; Pellissier, J.F. Cytokines, chemokines, and cell adhesion molecules in inflammatory myopathies. Muscle Nerve 2003, 28, 659–682. [Google Scholar] [CrossRef]
- Sottili, M.; Cosmi, L.; Borgogni, E.; Sarchielli, E.; Maggi, L.; Francalanci, M.; Vannelli, G.B.; Ronconi, E.; Adorini, L.; Annunziato, F.; et al. Immunomodulatory effects of BXL-01-0029, a less hypercalcemic vitamin D analogue, in human cardiomyocytes and T cells. Exp. Cell Res. 2009, 315, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Borgogni, E.; Sarchielli, E.; Sottili, M.; Santarlasci, V.; Cosmi, L.; Gelmini, S.; Lombardi, A.; Cantini, G.; Perigli, G.; Luconi, M.; et al. Elocalcitol inhibits inflammatory responses in human thyroid cells and T cells. Endocrinology 2008, 149, 3626–3634. [Google Scholar] [CrossRef]
- Scolletta, S.; Colletti, M.; Di Luigi, L.; Crescioli, C. Vitamin D receptor agonists target CXCL10: New therapeutic tools for resolution of inflammation. Mediat. Inflamm. 2013, 2013, 876319. [Google Scholar] [CrossRef] [PubMed]
- Crescioli, C.; Squecco, R.; Cosmi, L.; Sottili, M.; Gelmini, S.; Borgogni, E.; Sarchielli, E.; Scolletta, S.; Francini, F.; Annunziato, F.; et al. Immunosuppression in cardiac graft rejection: A human in vitro model to study the potential use of new immunomodulatory drugs. Exp. Cell Res. 2008, 314, 1337–1350. [Google Scholar] [CrossRef] [PubMed]
- Crescioli, C. Vitamin D receptor agonists: Suitable candidates as novel therapeutic options in autoimmune inflammatory myopathy. Biomed. Res. Int. 2014, 2014, 949730. [Google Scholar] [CrossRef] [PubMed]
- Jäpelt, R.B.; Jakobsen, J. Vitamin D in plants: A review of occurrence, analysis, and biosynthesis. Front. Plant Sci. 2013, 4, 136. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.S.; Whiting, S.J.; Barton, C.N. Vitamin D fortification in the United States and Canada: Current status and data needs. Am. J. Clin. Nutr. 2004, 80, 1710S–1716S. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Kunutsor, S.; Vitezova, A.; Oliver-Williams, C.; Chowdhury, S.; Kiefte-de-Jong, J.C.; Khan, H.; Baena, C.P.; Prabhakaran, D.; Hoshen, M.B.; et al. Vitamin D and risk of cause specific death: Systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ 2014, 348, g1903. [Google Scholar] [CrossRef]
- Wang, T.J. Vitamin D and cardiovascular disease. Annu. Rev. Med. 2016, 67, 261–272. [Google Scholar] [CrossRef]
- Grandi, N.C.; Breitling, L.P.; Vossen, C.Y.; Hahmann, H.; Wüsten, B.; März, W.; Rothenbacher, D.; Brenner, H. Serum vitamin D and risk of secondary cardiovascular disease events in patients with stable coronary heart disease. Am. Heart J. 2010, 159, 1044–1051. [Google Scholar] [CrossRef]
- Autier, P.; Mullie, P.; Macacu, A.; Dragomir, M.; Boniol, M.; Coppens, K.; Pizot, C.; Boniol, M. Effect of vitamin D supplementation on non-skeletal disorders: A systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017, 5, 986–1004. [Google Scholar] [CrossRef]
- Sempos, C.T.; Heijboer, A.C.; Bikle, D.D.; Bollerslev, J.; Bouillon, R.; Brannon, P.M.; DeLuca, H.F.; Jones, G.; Munns, C.F.; Bilezikian, J.P.; et al. Vitamin D assays and the definition of hypovitaminosis D: Results from the First International Conference on Controversies in Vitamin D. Br. J. Clin. Pharmacol. 2018, 84, 2194–2207. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef] [PubMed]
- Marcinowska-Suchowierska, E.; Kupisz-Urbańska, M.; Łukaszkiewicz, J.; Płudowski, P.; Jones, G. Vitamin D Toxicity-A Clinical Perspective. Front. Endocrinol. 2018, 9, 550. [Google Scholar] [CrossRef]
- Carter, G.D. 25-hydroxyvitamin D assays: The quest for accuracy. Clin. Chem. 2009, 55, 1300–1302. [Google Scholar] [CrossRef] [PubMed]
- Hollis, B.W. Editorial: The determination of circulating 25-hydroxyvitamin D: No easy task. J. Clin. Endocrinol. Metab. 2004, 89, 3149–3151. [Google Scholar] [CrossRef]
- Binkley, N.; Sempos, C.T.; Vitamin, D. Standardization Program (VDSP). Standardizing vitamin D assays: The way forward. J. Bone Miner. Res. 2014, 29, 1709–1714. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Tiberti, C.; Panimolle, F.; Bonamico, M.; Filardi, T.; Pallotta, L.; Nenna, R.; Pontone, S.; Dotta, F.; Pugliese, G.; Lenzi, A.; et al. Long-standing type 1 diabetes: Patients with adult-onset develop celiac-specific immunoreactivity more frequently than patients with childhood-onset diabetes, in a disease duration-dependent manner. Acta Diabetol. 2014, 51, 675–678. [Google Scholar] [CrossRef]
- Cranney, C.; Horsely, T.; O’Donnell, S.; Weiler, H.; Puil, L.; Ooi, D.; Atkinson, S.; Ward, L.; Moher, D.; Hanley, D.; et al. Effectiveness and Safety of Vitamin D; Evidence Report/Technology Assessment No. 158 Prepared by the University of Ottawa Evidence-Based Practice Center under Contract No. 290-02.0021; AHRQ Publication No. 07-E013; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2007.

| Myokines | Immune Regulation | Vitamin D |
|---|---|---|
| IL-15 | -macrophage differentiation -NK cell activation -CD8+T cell homeostasis -neutrophils migration -naïve T-cells survival -B cell proliferation | -enhancement of IL-15-induced monocyte to macrophage conversion -enhancement of anti-microbial activity |
| IL-7 | -thymic/extrathymic lymphocyte (γδ T cells) development and maturation -immune competence -first-line immune defense | -enhancement of IL-7 immune competence for protection against infections -reduction of IL-7 defective signaling |
| IL-6 | -IL-1ra and IL-10 modulation -M2-like macrophage profile -anti-inflammatory/metabolic balance | -enhancement of anti-IL-6 antibody action (tocilizumab) -improvement of IL-6-dependent metabolic/anti-inflammatory control |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crescioli, C. Vitamin D Merging into Immune System-Skeletal Muscle Network: Effects on Human Health. Appl. Sci. 2020, 10, 5592. https://doi.org/10.3390/app10165592
Crescioli C. Vitamin D Merging into Immune System-Skeletal Muscle Network: Effects on Human Health. Applied Sciences. 2020; 10(16):5592. https://doi.org/10.3390/app10165592
Chicago/Turabian StyleCrescioli, Clara. 2020. "Vitamin D Merging into Immune System-Skeletal Muscle Network: Effects on Human Health" Applied Sciences 10, no. 16: 5592. https://doi.org/10.3390/app10165592
APA StyleCrescioli, C. (2020). Vitamin D Merging into Immune System-Skeletal Muscle Network: Effects on Human Health. Applied Sciences, 10(16), 5592. https://doi.org/10.3390/app10165592





